Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
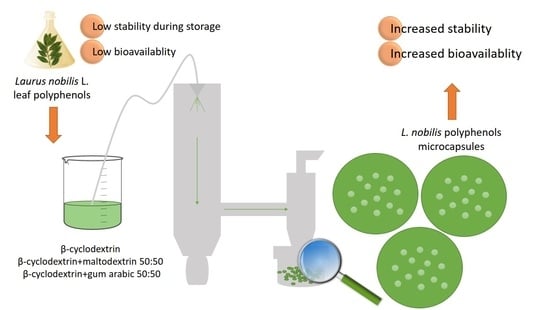
2.2. Plant Material
2.3. Microwave-Assisted Extraction (MAE)
2.4. Microencapsulation by Spray Drying
2.5. Microcapsules’ Characterization
2.5.1. Process Yield
2.5.2. Moisture Content
2.5.3. Solubility
2.5.4. Hygroscopicity
2.5.5. Encapsulation Efficiency and Capacity
2.5.6. SEM Analysis
2.5.7. UPLC-MS2 Analysis
2.5.8. Antioxidant Capacity
2.5.9. Bioaccessibility of Polyphenols
2.6. Statistical Analysis
3. Results and Discussion
3.1. Process Yield
3.2. Moisture Content
3.3. Solubility
3.4. Hygroscopicity
3.5. Encapsulation Efficiency and Capacity
3.6. Morphology of the Microcapsules
3.7. Individual Polyphenolic Content
3.8. Antioxidant Capacity
3.9. Bioaccessibility of Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of laurel tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Tran, T.T.A.; Nguyen, H.V.H. Effects of spray-drying temperatures and carriers on physical and antioxidant properties of lemongrass leaf extract powder. Beverages 2018, 4, 84. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Jusoh, Y.M.M.; Nawi, N.M.; Aziz, A.A.; Padzil, A.M.; Lian, H.L. Advanced Natural Food Colorant Encapsulation Methods: Anthocyanin Plant Pigment. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 495–526. [Google Scholar]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef]
- Fadlelmoula, A.A. Dietary Gum Arabic as Animal Feed Additive. In Gum Arabic; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–267. [Google Scholar]
- Chaumun, M.; Goëlo, V.; Ribeiro, A.M.; Rocha, F.; Estevinho, B.N. In vitro evaluation of microparticles with Laurus nobilis L. extract prepared by spray-drying for application in food and pharmaceutical products. Food Bioprod. Process. 2020, 122, 124–135. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- López-Caamal, A.; Reyes-Chilpa, R. The New World Bays (Litsea, Lauraceae). A Botanical, Chemical, Pharmacological and Ecological Review in Relation to their Traditional and Potential Applications as Phytomedicines. Bot. Rev. 2021, 87, 392–420. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting. Supplement; AOAC: Rockville, MD, USA, 1990; Volume 15, Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 12 February 2023).
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar] [CrossRef]
- Cegledi, E.; Elez Garofulić, I.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. [Google Scholar] [CrossRef] [PubMed]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Bigović, D.; Janković, T. Application of gum Arabic in the production of spray-dried chokeberry polyphenols, microparticles characterisation and in vitro digestion method. Lek. Sirovine 2018, 38, 9–16. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Šeparović, J.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules 2022, 27, 5099. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Crooks, R.; Howes, T.; Rigby, S. A semi-empirical approach to optimise the quantity of drying aids required to spray dry sugar-rich foods. Dry. Technol. 1997, 15, 2509–2525. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Tonon, R.V.; Freitas, S.S.; Hubinger, M.D. Spray drying of acai (Euterpe Oleraceae Mart.) juice: Effect of inlet air temperature and type of carrier agent. J. Food Process. Preserv. 2011, 35, 691–700. [Google Scholar] [CrossRef]
- Wang, R.; Ding, B.; Liang, G. Interaction poses, intermolecular forces, dynamic preferences between flavonoids and maltosyl-β-cyclodextrin. J. Mol. Liq. 2022, 346, 117068. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and delivery of bioactive citrus pomace polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Pui, L.P.; Saleena, L.A.K. Effects of spray-drying parameters on physicochemical properties of powdered fruits. Foods Raw Mater. 2022, 10, 235–251. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.E. The morphology of spray-dried particles a qualitative view. Dry. Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Jiménez-Aparicio, A.R.; Gutiérrez-Lopez, G.F. Description of morphological changes of particles along spray drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Recovery, structure and physicochemical properties of an aggregate-rich fraction from Acacia senegal gum. Food Hydrocoll. 2019, 89, 864–873. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, J.; Fonseca, F.; Ferreira-da-Silva, F.; Mateus, N.; Vincken, J.-P.; de Freitas, V. Molecular binding between anthocyanins and pectic polysaccharides–Unveiling the role of pectic polysaccharides structure. Food Hydrocoll. 2020, 102, 105625. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Xiao, J. Recent advances on the stability of dietary polyphenols. eFood 2022, 3, e21. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tian, M.; Cheng, Y.; Ji, C.; Hu, S.; Liu, H.; Lu, J.; Ren, J. Effects of simulated in vitro gastrointestinal digestion on antioxidant activities and potential bioaccessibility of phenolic compounds from K. coccinea fruits. Front. Nutr. 2022, 9, 1024651. [Google Scholar] [CrossRef] [PubMed]


| Sample | Inlet Temperature | Carrier | Sample:Carrier Ratio | Moisture Content % | Process Yield % | Solubility % | Hygroscopicity mg/100 g | Encapsulation Efficiency % | Encapsulation Capacity % | OEE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 120 | β-CD | 1:1 | 2.69 ± 0.13 | 77.77 ± 0.55 | 28.02 ± 0.20 | 17.23 ± 0.12 | 84.71 ± 0.28 | 42.55 ± 2.06 | 0.36 ± 0.02 |
| 2 | β-CD + MD 50:50 | 3.54 ± 0.08 | 75.69 ± 0.53 | 57.83 ± 0.41 | 17.82 ± 0.13 | 69.07 ± 1.13 | 42.20 ± 0.63 | 0.29 ± 0.00 | ||
| 3 | β-CD + GA 50:50 | 3.53 ± 0.35 | 75.50 ± 0.53 | 53.86 ± 0.38 | 21.80 ± 0.15 | 71.26 ± 0.92 | 53.22 ± 0.00 | 0.38 ± 0.00 | ||
| 4 | β-CD | 1:2 | 4.58 ± 0.14 | 78.91 ± 0.56 | 44.85 ± 0.32 | 12.70 ± 0.09 | 82.20 ± 1.19 | 55.46 ± 0.99 | 0.46± 0.01 | |
| 5 | β-CD + MD 50:50 | 4.54 ± 0.24 | 75.99 ± 0.53 | 56.20 ± 0.40 | 15.16 ± 0.11 | 78.50 ± 1.72 | 69.46 ± 0.67 | 0.55 ± 0.01 | ||
| 6 | β-CD + GA 50:50 | 4.72 ± 0.08 | 71.69 ± 0.50 | 53.01 ± 0.37 | 18.30 ± 0.13 | 81.98 ± 2.61 | 76.67 ± 1.70 | 0.63 ± 0.03 | ||
| 7 | β-CD | 1:3 | 5.71 ± 0.02 | 73.70 ± 0.52 | 45.67 ± 0.32 | 9.94 ± 0.07 | 72.40 ± 1.59 | 38.55 ± 3.49 | 0.28 ± 0.03 | |
| 8 | β-CD + MD 50:50 | 5.44 ± 0.21 | 70.36 ± 0.50 | 46.04 ± 0.32 | 11.83 ± 0.08 | 53.09 ± 0.86 | 56.79 ± 1.07 | 0.30 ± 0.01 | ||
| 9 | β-CD + GA 50:50 | 4.39 ± 0.35 | 84.46 ± 0.59 | 47.33 ± 0.33 | 17.59 ± 0.12 | 50.20 ± 0.71 | 62.70 ± 1.39 | 0.31 ± 0.00 | ||
| 10 | 150 | β-CD | 1:1 | 3.70 ± 0.22 | 72.09 ± 0.51 | 40.47 ± 0.28 | 18.02 ± 0.13 | 63.48 ± 0.29 | 34.26 ± 0.19 | 0.22± 0.00 |
| 11 | β-CD + MD 50:50 | 3.95 ± 0.15 | 77.28 ± 0.54 | 58.90 ± 0.41 | 16.35 ± 0.12 | 61.91 ± 0.79 | 35.91 ± 2.09 | 0.22 ± 0.01 | ||
| 12 | β-CD + GA 50:50 | 2.70 ± 0.16 | 73.71 ± 0.52 | 54.77 ± 0.39 | 19.46 ± 0.14 | 60.34 ± 0.84 | 47.22 ± 3.02 | 0.29 ± 0.02 | ||
| 13 | β-CD | 1:2 | 3.66 ± 0.16 | 73.73 ± 0.52 | 45.00 ± 0.32 | 12.08 ± 0.08 | 88.67 ± 1.60 | 62.33 ± 1.47 | 0.55 ± 0.00 | |
| 14 | β-CD + MD 50:50 | 2.80 ± 0.17 | 69.87 ± 0.49 | 46.03 ± 0.32 | 15.31 ± 0.11 | 69.87 ± 5.61 | 61.65 ± 1.50 | 0.43 ± 0.05 | ||
| 15 | β-CD + GA 50:50 | 3.84 ± 0.10 | 67.91 ± 0.48 | 33.03 ± 0.23 | 19.80 ± 0.14 | 83.01 ± 3.39 | 67.29 ± 0.57 | 0.56 ± 0.02 | ||
| 16 | β-CD | 1:3 | 3.37 ± 0.16 | 66.20 ± 0.47 | 47.35 ± 0.33 | 10.70 ± 0.08 | 81.84 ± 0.42 | 41.99 ± 3.18 | 0.34 ± 0.02 | |
| 17 | β-CD + MD 50:50 | 2.26 ± 0.17 | 81.58 ± 0.57 | 52.36 ± 0.37 | 13.55 ± 0.10 | 78.76 ± 1.12 | 69.67 ± 2.60 | 0.55 ± 0.03 | ||
| 18 | β-CD + GA 50:50 | 2.72 ± 0.18 | 80.28 ± 0.56 | 52.66 ± 0.37 | 18.08 ± 0.13 | 74.95 ± 2.93 | 59.67 ± 3.22 | 0.45 ± 0.04 | ||
| 19 | 180 | β-CD | 1:1 | 3.65 ± 0.12 | 68.96 ± 0.49 | 39.81 ± 0.28 | 20.15 ± 0.14 | 81.44 ± 1.72 | 62.46 ± 1.92 | 0.51 ± 0.03 |
| 20 | β-CD + MD 50:50 | 3.73 ± 0.24 | 75.14 ± 0.53 | 58.91 ± 0.41 | 18.46 ± 0.13 | 71.18 ± 2.04 | 59.82 ± 0.92 | 0.43 ± 0.02 | ||
| 21 | β-CD + GA 50:50 | 3.83 ± 0.22 | 73.10 ± 0.51 | 52.63 ± 0.37 | 21.27 ± 0.15 | 67.26 ± 0.29 | 55.88 ± 1.59 | 0.38± 0.01 | ||
| 22 | β-CD | 1:2 | 3.42 ± 0.16 | 74.69 ± 0.53 | 47.45 ± 0.33 | 14.28 ± 0.10 | 89.83 ± 1.03 | 67.96 ± 1.46 | 0.61 ± 0.02 | |
| 23 | β-CD + MD 50:50 | 2.51 ± 0.26 | 78.96 ± 0.56 | 59.47 ± 0.42 | 14.13 ± 0.10 | 74.41 ± 3.39 | 80.23 ± 1.70 | 0.60 ± 0.04 | ||
| 24 | β-CD + GA 50:50 | 3.49 ± 0.27 | 75.67 ± 0.53 | 52.88 ± 0.37 | 17.99 ± 0.13 | 75.38 ± 0.62 | 69.44 ± 0.94 | 0.52 ± 0.01 | ||
| 25 | β-CD | 1:3 | 3.50 ± 0.04 | 74.38 ± 0.52 | 45.88 ± 0.32 | 12.00 ± 0.08 | 92.07 ± 0.57 | 58.58 ± 0.86 | 0.54 ± 0.00 | |
| 26 | β-CD + MD 50:50 | 3.66 ± 0.17 | 76.39 ± 0.54 | 60.15 ± 0.42 | 15.24 ± 0.11 | 45.30 ± 2.15 | 59.17 ± 1.31 | 0.27 ± 0.01 | ||
| 27 | β-CD + GA 50:50 | 4.39 ± 0.19 | 73.35 ± 0.52 | 46.12 ± 0.32 | 16.41 ± 0.12 | 82.63 ± 0.85 | 75.89 ± 1.71 | 0.63 ± 0.02 | ||
| Average | 3.72 | 74.72 | 49.14 | 16.14 | 73.54 | 58.04 | 0.43 | |||
| Source of Variation | N | Process Yield % | Moisture Content % | Solubility % | Hygroscopicity g/100 g | EE % | EC % | OEE |
|---|---|---|---|---|---|---|---|---|
| Inlet temperature | p = 0.27 ‡ | p < 0.01 † | p = 0.31 ‡ | p = 0.70 ‡ | p = 0.59 ‡ | p < 0.05 † | p < 0.05 † | |
| 120 °C | 18 | 76.01 ± 0.95 a | 4.35 ± 0.22 b | 48.09 ± 2.04 a | 15.82 ± 0.77 a | 71.49 ± 2.86 a | 55.29 ± 2.97 a | 0.40 ± 0.03 a |
| 150 °C | 18 | 73.63 ± 1.22 a | 3.22 ± 0.14 a | 47.84 ± 1.81 a | 15.93 ± 0.77 a | 73.65 ± 2.38 a | 53.33 ± 3.14 a | 0.40 ± 0.03 a |
| 180 °C | 18 | 74.52 ± 0.63 a | 3.58 ± 0.12 a | 51.48 ± 1.64 a | 16.66 ± 0.77 a | 75.50 ± 3.21 a | 65.49 ± 1.94 b | 0.50 ± 0.03 b |
| Carrier | p = 0.22 ‡ | p = 0.76 | p < 0.01 † | p < 0.01 † | p < 0.01† | p < 0.05 † | p = 0.38 ‡ | |
| β-CD | 18 | 73.38 ± 0.96 a | 3.81 ± 0.02 a | 42.72 ± 1.41 a | 14.12 ± 0.81 a | 81.84 ± 2.07 b | 51.57 ± 2.83 a | 0.43 ± 0.03 a |
| β-CD + MD 50:50 | 18 | 75.70 ± 0.96 a | 3.60 ± 0.02 a | 55.10 ± 1.29 b | 15.32 ± 0.47 a | 66.90 ± 2.65 a | 59.43 ± 3.14 a | 0.40 ± 0.03 a |
| β-CD + GA 50:50 | 18 | 75.08 ± 0.96 a | 3.73 ± 0.02 a | 49.59 ± 1.57 b | 18.97 ± 0.40 b | 71.89 ± 2.56 a | 63.10 ± 2.33 b | 0.46 ± 0.03 a |
| Sample:Carrier Ratio | p = 0.79 ‡ | p = 0.61 ‡ | p = 0.67 ‡ | p < 0.01 † | p < 0.01 † | p < 0.01 † | p < 0.01 † | |
| 1:1 | 18 | 74.36 ± 0.63 a | 3.50 ± 0.11 a | 49.47 ± 2.48 a | 18.95 ± 0.42 b | 70.07 ± 1.92 a | 48.17 ± 2.35 a | 0.34 ± 0.02 a |
| 1:2 | 18 | 74.16 ± 0.87 a | 3.73 ± 0.18 a | 48.66 ± 1.79 a | 15.53 ± 0.60 a | 80.43 ± 1.57 b | 67.83 ± 1.75 b | 0.54 ± 0.02 b |
| 1:3 | 18 | 75.63 ± 1.31 a | 3.94 ± 0.27 a | 49.28 ± 1.12 a | 13.93 ± 0.69 a | 70.14 ± 3.78 a | 58.11 ± 2.73 a | 0.41 ± 0.03 a |
| Total | 54 |
| Compound Number | Retention Time | Tentative Identification | Concentration (mg 100 g−1 Powder) | ||
|---|---|---|---|---|---|
| β-CD | β-CD + MD 50:50 | β-CD + GA 50:50 | |||
| Phenolic acids | |||||
| 1 | 1.679 | Gallic acid * | 0.26 ± 0.01 b | 0.18 ± 0.01 a | 0.34 ± 0.01 c |
| 2 | 2.313 | 3,4-dihydrobenzoic acid hexoside | 0.17 ± 0.00 b | 0.17 ± 0.00 b | 0.13 ± 0.00 a |
| 3 | 3.488 | Syringic acid * | 5.42 ± 0.15 b | 5.39 ± 0.15 b | 4.35 ± 0.12 a |
| 4 | 3.508 | Protocatechuic acid * | 0.69 ± 0.02 b | 0.60 ± 0.02 a | 0.54 ± 0.02 a |
| 5 | 4.259 | Rosmarinic acid * | 0.55 ± 0.02 b | 0.78 ± 0.02 c | 0.44 ± 0.01 a |
| 6 | 4.813 | p-hydroxybenzoic acid | 0.45 ± 0.01 a | 0.49 ± 0.01 a | 0.44 ± 0.01 a |
| 7 | 5.043 | Chlorogenic acid * | 0.38 ± 0.01 b | 0.35 ± 0.01 b | 0.27 ± 0.01 a |
| 8 | 5.711 | Caffeic acid * | 0.52 ± 0.01 b | 0.19 ± 0.01 a | 0.80 ± 0.02 c |
| 9 | 7.28 | p-coumaric acid * | 0.62 ± 0.02 b | 0.55 ± 0.02 a | 0.50 ± 0.01 a |
| 10 | 8.587 | Ferulic acid * | 0.79 ± 0.02 b | 0.66 ± 0.02 a | 0.61 ± 0.02 a |
| ∑ Phenolic acids | 9.84 ± 0.28 b | 9.36 ± 0.26 b | 8.44 ± 0.24 a | ||
| Flavones | |||||
| 11 | 2.755 | Apigenin-6-C-(O-deoxyhexosyl)-hexoside | 0.00 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 12 | 6.938 | Luteolin-6-C-glucoside | 0.54 ± 0.02 b | 0.49 ± 0.01 b | 0.38 ± 0.01 a |
| 13 | 8.29 | Apigenin * | 0.07 ± 0.00 b | 0.06 ± 0.00 a | 0.05 ± 0.00 a |
| 14 | 9.849 | Luteolin * | 23.22 ± 0.66 b | 22.94 ± 0.65 b | 16.35 ± 0.46 a |
| ∑ Flavones | 23.84 ± 0.67 b | 23.50 ± 0.66 b | 16.79 ± 0.47 a | ||
| Flavan-3-ols | |||||
| 15 | 5.93 | Catechin * | 124.96 ± 3.53 b | 118.19 ± 3.34 b | 103.76 ± 2.93 a |
| 16 | 5.937 | Epicatechin | 123.25 ± 3.49 b | 116.71 ± 3.30 b | 102.97 ± 2.91 a |
| 17 | 6.02 | Epigallocatechin gallate * | 0.04 ± 0.00 a | 0.08 ± 0.00 b | 0.04 ± 0.00 a |
| 18 | 7.905 | Epicatechin gallate * | 0.22 ± 0.01 c | 0.10 ± 0.00 b | 0.07 ± 0.00 a |
| ∑ Flavan-3-ols | 248.48 ± 7.03 b | 235.09 ± 6.65 b | 206.85 ± 5.85 a | ||
| Proanthocyanidins | |||||
| 19 | 6.249 | Procyandinin trimer | 78.67 ± 2.23 a,b | 80.41 ± 2.27 b | 71.30 ± 2.02 a |
| ∑ Proanthocyanidins | 78.67 ± 2.23 b | 80.41 ± 2.27 b | 71.30 ± 2.02 a | ||
| Flavonols | |||||
| 20 | 7.692 | Rutin * | 136.91 ± 3.87 a | 125.99 ± 3.56 a | 123.21 ± 3.48 a |
| 21 | 7.969 | Quercetin-3-glucoside | 362.32 ± 10.25 b | 358.52 ± 10.14 b | 268.95 ± 7.61 a |
| 22 | 8.48 | Kaempferol-3-rutinoside | 46.60 ± 1.32 b | 47.44 ± 1.34 b | 33.24 ± 0.94 a |
| 23 | 8.51 | Kaempferol-3-hexoside | 85.64 ± 2.42 a | 82.03 ± 2.32 a | 85.67 ± 2.42 a |
| 24 | 8.52 | Quercetin-3-pentoside | 84.33 ± 2.39 a | 82.73 ± 2.34 a | 81.42 ± 2.30 a |
| 25 | 8.877 | Isorhamnetin-3-hexoside | 125.78 ± 3.56 b | 122.43 ± 3.46 b | 86.69 ± 2.45 a |
| 26 | 8.897 | Quercetin-3-rhamnoside | 162.36 ± 4.59 b | 160.58 ± 4.54 b | 133.26 ± 3.77 a |
| 27 | 9.178 | Kaempferol-3-O-pentoside | 38.03 ± 1.08 a | 35.19 ± 1.00 a | 35.07 ± 0.99 a |
| 28 | 9.825 | Kaempferol-3-O-deoxyhexoside | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.08 ± 0.00 a |
| 29 | 12.137 | Myricetin * | 0.19 ± 0.01 a | 0.19 ± 0.01 a | 0.22 ± 0.01 b |
| ∑ Flavonols | 1042.25 ± 29.48 b | 1015.19 ± 28.71 b | 847.81 ± 23.98 a | ||
| Total | 1403.07 ± 39.68 b | 1363.54 ± 38.57 b | 1151.19 ± 32.56 a | ||
| Carrier | DPPH μmol TE g−1 Powder | FRAP μmol TE g−1 Powder | ORAC μmol TE g−1 Powder |
|---|---|---|---|
| β-CD | 162.18 ± 4.83 a | 210.00 ± 9.06 a | 88.59 ± 1.84 a |
| β-CD + MD 50:50 | 201.43 ± 3.85 b | 267.18 ± 1.93 b | 157.92 ± 3.28 c |
| β-CD + GA 50:50 | 159.30 ± 1.80 a | 196.15 ± 16.77 a | 99.43± 2.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Roje, M.; Dragović-Uzelac, V. Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying. Foods 2023, 12, 1923. https://doi.org/10.3390/foods12091923
Dobroslavić E, Elez Garofulić I, Zorić Z, Pedisić S, Roje M, Dragović-Uzelac V. Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying. Foods. 2023; 12(9):1923. https://doi.org/10.3390/foods12091923
Chicago/Turabian StyleDobroslavić, Erika, Ivona Elez Garofulić, Zoran Zorić, Sandra Pedisić, Marin Roje, and Verica Dragović-Uzelac. 2023. "Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying" Foods 12, no. 9: 1923. https://doi.org/10.3390/foods12091923
APA StyleDobroslavić, E., Elez Garofulić, I., Zorić, Z., Pedisić, S., Roje, M., & Dragović-Uzelac, V. (2023). Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying. Foods, 12(9), 1923. https://doi.org/10.3390/foods12091923