Comparative Evaluation of Quality Attributes of the Dried Cherry Blossom Subjected to Different Drying Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Drying Experiments
2.2.1. Hot-Air Drying (HAD)
2.2.2. Infrared Hot-Air Drying (IHAD)
2.2.3. Catalytic Infrared Drying (CID)
2.2.4. Relative Humidity Drying (RHD)
2.2.5. Pulsed Vacuum Drying (PVD)
2.2.6. Microwave Vacuum Drying (MVD)
2.2.7. Vacuum Freeze Drying (VFD)
2.3. Preparation of Tea Infusions
2.4. Determination of the Bioactive Compounds
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Total Anthocyanin Content (TAC)
2.5. Antioxidant Activities Assay
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. α-Glucosidase Inhibitory Ability (GIA) Assay
2.7. Flavor and Taste Analysis of Tea Infusion
2.8. Sensory Evaluation by Fuzzy Mathematics with Assessors
2.9. Statistical Analysis
3. Results and Discussion
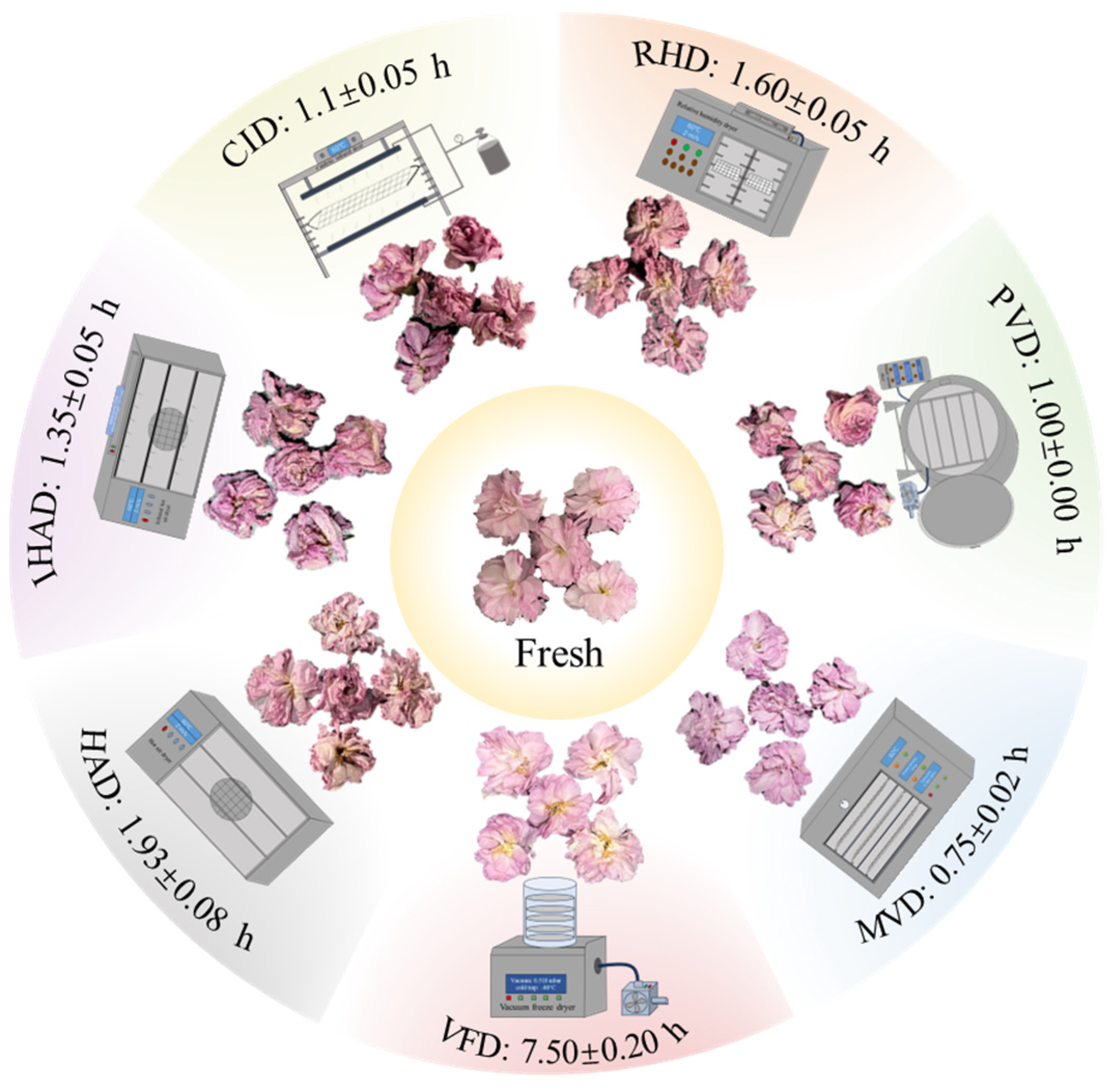
3.1. Impact of Drying Techniques on Drying Time and Appearance of Cherry Blossoms
3.2. Impact of Drying Techniques on the Bioactive Compounds of Petals
3.3. Impact of Drying Techniques on Antioxidant Activities of Petals
3.4. Impact of Drying Techniques on the GIA of Petals
3.5. Impact of Drying Techniques on the Quality Attributes of Tea Infusions
3.5.1. Bioactive Compounds, Antioxidant Activities, and GIA
3.5.2. Flavor and Taste
3.5.3. Fuzzy Mathematical Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, Y.A.; Lee, J.Y.; Lee, P.; Shin, H.S.; Kim, J.E. Inhibition of Solar UV-Induced Matrix Metalloproteinase (MMP)-1 Expression by Non-Enzymatic Softening Cherry Blossom (Prunus yedoensis) Extract. Plants 2021, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Sun, S.F.; Liu, Y.A.; Wu, Z.F.; Zhang, X. Anti-Aging Effect of Guanshan Cherry Blossom Extract by Regulating Intestinal Microbiota and Activating Glial Cells. Food Biosci. 2023, 55, 103051. [Google Scholar] [CrossRef]
- Zhao, L.L.; Fan, H.Z.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible Flowers: Review of Flower Processing and Extraction of Bioactive Compounds by Novel Technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef] [PubMed]
- Baeghbali, V.; Ngadi, M.; Niakousari, M. Effects of Ultrasound and Infrared Assisted Conductive Hydro-Drying, Freeze-Drying and Oven Drying on Physicochemical Properties of Okra Slices. Innov. Food Sci. Emerg. Technol. 2020, 63, 102313. [Google Scholar] [CrossRef]
- Cao, X.H.; Zhang, M.; Mujumdar, A.S.; Zhong, Q.F.; Wang, Z.S. Effect of Microwave Freeze Drying on Quality and Energy Supply in Drying of Barley Grass. J. Sci. Food Agric. 2018, 98, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Sinha, A.; Gupta, R.; Sablani, S.S. Drying of Selected Major Spices: Characteristics and Influencing Parameters, Drying Technologies, Quality Retention and Energy Saving, and Mathematical Models. Food Bioprocess Technol. 2021, 14, 1028–1054. [Google Scholar] [CrossRef]
- Fathi, F.; Ebrahimi, S.N.; Matos, L.C.; Oliveira, M.; Alves, R.C. Emerging Drying Techniques for Food Safety and Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1125–1160. [Google Scholar] [CrossRef]
- Feng, Y.B.; Xu, B.G.; Yagoub, A.E.A.; Ma, H.L.; Sun, Y.H.; Xu, X.; Yu, X.J.; Zhou, C.S. Role of Drying Techniques on Physical, Rehydration, Flavor, Bioactive Compounds and Antioxidant Characteristics of Garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An Overview of Encapsulation of Active Compounds Used in Food Products by Drying Technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Shofinita, D.; Lestari, D.; Aliwarga, L.; Sumampouw, G.A.; Ambarwati, S.A.; Gunawan, K.C.; Achmadi, A.B. Drying Methods of Coffee Extracts and Their Effects on Physicochemical Properties: A Review. Food Bioprocess Technol. 2023, 26. [Google Scholar] [CrossRef]
- Juhari, N.H.; Martens, H.J.; Petersen, M.A. Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx During Different Drying Methods. Molecules 2021, 26, 6260. [Google Scholar] [CrossRef] [PubMed]
- Li, B.R.; Lin, J.Y.; Zheng, Z.A.; Duan, H.; Li, D.; Wu, M. Effects of Different Drying Methods on Drying Kinetics and Physicochemical Properties of Chrysanthemum morifolium Ramat. Int. J. Agric. Biol. Eng. 2019, 12, 187–193. [Google Scholar] [CrossRef]
- Cheng, K.; Dong, W.J.; Long, Y.Z.; Zhao, J.P.; Hu, R.S.; Zhang, Y.J.; Zhu, K.X. Evaluation of the Impact of Different Drying Methods on the Phenolic Compounds, Antioxidant Activity, and In Vitro Digestion of Green Coffee Beans. Food Sci. Nutr. 2019, 7, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Li, X.Y.; Xu, X.H.; Xing, A.Q.; Xu, Y.Q.; Yang, X.F.; Sun, Y.; Zhu, J.Y.; Wang, Y.H. Quality Components Identification and Formation Analysis of Tea (Camellia Sinensis L.) Flower Beverages from Three Cultivars. LWT 2023, 181, 114739. [Google Scholar] [CrossRef]
- Yigit, R.; Çoklar, H.; Akbulut, M. Some Physicochemical and Phytochemical Properties of Syringa vulgaris L. Flower Tea: Influence of Flower Drying Technique, Brewing Method and Brewing Time. J. Food Meas. Charact. 2022, 16, 4185–4197. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Xia, Q.L.; Lu, S.M. Study on Drying Methods and Their Influences on Effective Components of Loquat Flower Tea. LWT Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, Y.; Zhang, S.R.; Luo, L.Y.; Zeng, L. Effect of Brewing Conditions on Phytochemicals and Sensory Profiles of Black Tea Infusions: A Primary Study on the Effects of Geraniol and β-Ionone on Taste Perception of Black Tea Infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef]
- Vidinamo, F.; Fawzia, S.; Karim, M.A. Investigation of the Effect of Drying Conditions on Phytochemical Content and Antioxidant Activity in Pineapple (Ananas comosus). Food Bioprocess Technol. 2022, 15, 72–81. [Google Scholar] [CrossRef]
- Mounir, S.; Ghandour, A.; Tellez-Perez, C.; Aly, A.A.; Mujumdar, A.S.; Allaf, K. Phytochemicals, Chlorophyll Pigments, Antioxidant Activity, Relative Expansion Ratio, and Microstructure of Dried Okra Pods: Swell-Drying by Instant Controlled Pressure Drop Versus Conventional Shade Drying. Dry. Technol. 2021, 39, 2145–2159. [Google Scholar] [CrossRef]
- Xu, B.G.; Chen, J.N.; Tiliwa, E.S.; Yan, W.Q.; Azam, S.M.R.; Yuan, J.; Wei, B.X.; Zhou, C.S.; Ma, H.L. Effect of Multi-Mode Dual-Frequency Ultrasound Pretreatment on the Vacuum Freeze-Drying Process and Quality Attributes of the Strawberry Slices. Ultrason. Sonochemistry 2021, 78, 105714. [Google Scholar] [CrossRef]
- Ismail, B.B.; Liu, D.; Pu, Y.; He, Q.; Guo, M. High-Intensity Ultrasound Processing of Baobab Fruit Pulp: Effect on Quality, Bioactive Compounds, and Inhibitory Potential on the Activity of α-Amylase and α-Glucosidase. Food Chem. 2021, 361, 130144. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.B.; Suo, K.; Chen, L.Q.; Wahia, H.; Zhou, C.S.; Yang, Z.F.; Chang, X.H.; Shi, L.Y.; Chen, W.; Song, C.B.; et al. Improving the Hot Air Drying of Garlic Slices by Perforation Synergistic Alcohol Pretreatment. Dry. Technol. 2023, 41, 2077–2087. [Google Scholar] [CrossRef]
- Wei, Y.; Li, T.; Xu, S.; Ni, T.; Deng, W.W.; Ning, J. The Profile of Dynamic Changes in Yellow Tea Quality and Chemical Composition during Yellowing Process. LWT 2021, 139, 110792. [Google Scholar] [CrossRef]
- ISO 5496; International Organization for Standardization, Sensory Analysis—Methodology—Initiation and Training of Assessors in the Detection and Recognition of Odours. 2nd ed, ISO: Geneva, Switzerland, 2006.
- ISO 3972; International Organization for Standardization, Sensory Analysis—Methodology—Method of Investigating Sensitivity of Taste. 3rd ed. ISO: Geneva, Switzerland, 2011.
- ISO 11036; International Organization for Standardization, Sensory Analysis—Methodology—Texture Profile. 2nd ed. ISO: Geneva, Switzerland, 2020.
- ISO 8589; International Organization for Standardization, Sensory Analysis—General Guidance for the Design of Test Rooms. 2nd ed. ISO: Geneva, Switzerland, 2007.
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standards Press of China: Beijing, China, 2018.
- Xue, S.; He, L. Optimization of Adding Polysaccharides from Chicory Root Based on Fuzzy Mathematics to Improve Physicochemical Properties of Silver Carp Surimi Balls during Storage. J. Food Process. Preserv. 2021, 45, e15307. [Google Scholar] [CrossRef]
- Feng, M.; Chitrakar, B.; Zhou, C.S.; Xu, B.G.; Wei, B.X.; Wang, B.; Ma, H.L.; Chang, L.; Mujumdar, A.S.; Duan, X.; et al. Comparative Evaluation of Chemical Substances and Sensory Properties of Postharvest Rose (Rosa rugosa cv. Plena) and Tea Infusion Prepared by Five Drying Techniques. Dry. Technol. 2023, 41, 523–535. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Xiao, Y.D.; Lagnika, C.; Li, D.J.; Liu, C.Q.; Jiang, N.; Song, J.F.; Zhang, M.A. Comparative Evaluation of Nutritional Properties, Antioxidant Capacity and Physical Characteristics of Cabbage (Brassica oleracea var. Capitate var L.) Subjected to Different Drying Methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef]
- Li, C.Y.; Ren, G.Y.; Zhang, L.D.; Duan, X.; Wang, Z.; Ren, X.; Chu, Q.Q.; He, T. Effects of Different Drying Methods on the Drying Characteristics and Drying Quality of Cistanche deserticola. LWT 2023, 184, 115000. [Google Scholar] [CrossRef]
- Liu, C.Y.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Effects of Pulsed Electric Fields on Vacuum Drying and Quality Characteristics of Dried Carrot. Food Bioprocess Technol. 2020, 13, 45–52. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Feng, Y.B.; Zhou, C.S.; Yagoub, A.E.A.; Wahia, H.; Ma, H.L.; Zhang, J.; Sun, Y.H. Ultrasound Freeze-Thawing Style Pretreatment to Improve the Efficiency of the Vacuum Freeze-Drying of Okra (Abelmoschus esculentus (L.) Moench) and the Quality Characteristics of the Dried Product. Ultrason. Sonochem. 2021, 70, 105300. [Google Scholar] [CrossRef]
- Xu, B.G.; Feng, M.; Chitrakar, B.; Wei, B.X.; Wang, B.; Zhou, C.S.; Ma, H.L.; Wang, B.; Chang, L.; Ren, G.Y.; et al. Selection of Drying Techniques for Pingyin Rose on the Basis of Physicochemical Properties and Volatile Compounds Retention. Food Chem. 2022, 385, 132539. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Prieto, M.A.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of Different Drying Techniques on the Quality and Bioactive Compounds of Plant-Based Products: A Critical Review on Current Trends. Dry. Technol. 2022, 40, 1539–1561. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of Different Drying Methods on Chinese Ginger (Zingiber officinale Roscoe): Changes in Volatiles, Chemical Profile, Antioxidant Properties, and Microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Rahman, M.S. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as A Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Fu, J.X.; Yang, D. In Situ Stability of Anthocyanins in Lycium ruthenicum Murray. Molecules 2021, 26, 7073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Staniszewska, I.; Liu, Z.L.; Zielinska, D.; Xiao, H.W.; Pan, Z.L.; Nowak, K.W.; Zielinska, M. Microwave-Vacuum-Assisted Drying of Pretreated Cranberries: Drying Kinetics, Bioactive Compounds and Antioxidant Activity. LWT 2021, 146, 111464. [Google Scholar] [CrossRef]
- Xu, G.B.; Yin, H.; He, X.; Wang, D.F.; Zhao, Y.Y.; Yue, J. Optimization of Microwave Vacuum Drying of Okra and the Study of the Product Quality. J. Food Process Eng. 2020, 43, 13337. [Google Scholar] [CrossRef]
- Gümüşay, Ö.A.; Borazan, A.A.; Ercal, N.; Demirkol, O. Drying Effects on the Antioxidant Properties of Tomatoes and Ginger. Food Chem. 2015, 173, 156–162. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Lech, K.; Tkacz, K.; Nowicka, P. Influence of Different Drying Methods on the Quality of Japanese Quince Fruit. LWT 2019, 114, 108416. [Google Scholar] [CrossRef]
- Li, M.Q.; Bao, X.; Zhang, X.T.; Ren, H.B.; Cai, S.B.; Hu, X.S.; Yi, J.J. Exploring the Phytochemicals and Inhibitory Effects against α-Glucosidase and Dipeptidyl Peptidase-IV in Chinese Pickled Chili Pepper: Insights into Mechanisms by Molecular Docking Analysis. LWT 2022, 162, 113467. [Google Scholar] [CrossRef]
- Wongsa, P.; Bhuyar, P.; Tongkoom, K.; Spreer, W.; Muller, J. Influence of Hot-Air Drying Methods on the Phenolic Compounds/Allicin Content, Antioxidant Activity and α-Amylase/α-Glucosidase Inhibition of Garlic (Allium sativum L.). Eur. Food Res. Technol. 2023, 249, 523–535. [Google Scholar] [CrossRef]
- Wu, H.R.; Xu, B.J. Inhibitory Effects of Onion against α-Glucosidase Activity and its Correlation with Phenolic Antioxidants. Int. J. Food Prop. 2014, 17, 599–609. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Li, J.W.; Fan, L.P.; Wang, L. Influence of Ultrasound-Assisted Vacuum Drying on Physicochemical Characteristics, Antioxidant Activity, and α-Glucosidase Inhibition Activity of Flos Sophorae Immaturus. Foods 2023, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.L.; Liu, D.; Jin, Y.; Zhao, J.; Zhao, J.; Li, H.Y.; Li, L.W.; Zhang, H.; Wang, H. In Vitro and In Vivo Inhibitory Effect of Anthocyanin-Rich Bilberry Extract on α-Glucosidase and α-Amylase. LWT 2021, 145, 111484. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Nofitasari, D.; Rahmawati, A.; Eryanti, A.Y.; Rosmiati, M. Effects of Brewing Conditions on Total Phenolic Content, Antioxidant Activity and Sensory Properties of Cascara. Food Chem. Adv. 2023, 2, 100183. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J. α-Glucosidase Inhibitory Effect of Mulberry (Morus alba) Leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of Different Drying Techniques on Bioactive Components, Fatty Acid Composition, and Volatile Profile of Robusta Coffee Beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Liu, C.; Lu, X.S.; Fang, D.L.; Hu, Q.H.; Zhang, Y.Y.; Zhao, L.Y. Characterization of Flavor Frame in Shiitake Mushrooms (Lentinula edodes) Detected by HS-GC-IMS Coupled with Electronic Tongue and Sensory Analysis: Influence of Drying Techniques. LWT 2021, 146, 111402. [Google Scholar] [CrossRef]
- Djekic, I.; Lorenzo, J.M.; Munekata, P.E.S.; Gagaoua, M.; Tomasevic, I. Review on Characteristics of Trained Sensory Panels in Food Science. J. Texture Stud. 2021, 52, 501–509. [Google Scholar] [CrossRef]
- Vivek, K.; Subbarao, K.V.; Routray, W.; Kamini, N.R.; Dash, K.K. Application of Fuzzy Logic in Sensory Evaluation of Food Products: A Comprehensive Study. Food Bioprocess Technol. 2020, 13, 1–29. [Google Scholar] [CrossRef]




| Var (Flavor) | PC1 | PC2 | Var (Taste) | PC1 | PC2 |
|---|---|---|---|---|---|
| W1C | −0.792 | 0.491 | Sourness | 0.954 | −0.208 |
| W5S | 0.953 | 0.148 | Bitterness | −0.063 | 0.684 |
| W3C | −0.836 | 0.506 | Astringency | 0.421 | 0.799 |
| W6S | 0.832 | 0.439 | Aftertaste-B | 0.299 | 0.764 |
| W5C | −0.797 | 0.436 | Aftertaste-A | 0.615 | 0.633 |
| W1S | 0.964 | 0.201 | Umami | −0.978 | 0.175 |
| W1W | 0.989 | −0.045 | Richness | −0.333 | 0.765 |
| W2S | 0.989 | 0.066 | Saltiness | −0.919 | 0.312 |
| W2W | 0.994 | 0.050 | |||
| W3S | 0.467 | 0.834 |
| Drying Techniques | Yn Comprehensive Evaluation Set | Comprehensive Score |
|---|---|---|
| HAD | Y1 = {0.190, 0.220, 0.315, 0.275} | 2.325 |
| IHAD | Y2 = {0.335, 0.350, 0.285, 0.030} | 2.990 |
| CID | Y3 = {0.445, 0.335, 0.165, 0.045} | 3.160 |
| RHD | Y4 = {0.525, 0.360, 0.115, 0.000} | 3.410 |
| PVD | Y5 = {0.665, 0.315, 0.030, 0.000} | 3.665 |
| MVD | Y6 = {0.690, 0.295, 0.015, 0.000} | 3.675 |
| VFD | Y7 = {0.790, 0.210, 0.000, 0.000} | 3.790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, K.; Feng, Y.; Zhang, Y.; Yang, Z.; Zhou, C.; Chen, W.; Shi, L.; Yan, C. Comparative Evaluation of Quality Attributes of the Dried Cherry Blossom Subjected to Different Drying Techniques. Foods 2024, 13, 104. https://doi.org/10.3390/foods13010104
Suo K, Feng Y, Zhang Y, Yang Z, Zhou C, Chen W, Shi L, Yan C. Comparative Evaluation of Quality Attributes of the Dried Cherry Blossom Subjected to Different Drying Techniques. Foods. 2024; 13(1):104. https://doi.org/10.3390/foods13010104
Chicago/Turabian StyleSuo, Kui, Yabin Feng, Yang Zhang, Zhenfeng Yang, Cunshan Zhou, Wei Chen, Liyu Shi, and Chunfeng Yan. 2024. "Comparative Evaluation of Quality Attributes of the Dried Cherry Blossom Subjected to Different Drying Techniques" Foods 13, no. 1: 104. https://doi.org/10.3390/foods13010104
APA StyleSuo, K., Feng, Y., Zhang, Y., Yang, Z., Zhou, C., Chen, W., Shi, L., & Yan, C. (2024). Comparative Evaluation of Quality Attributes of the Dried Cherry Blossom Subjected to Different Drying Techniques. Foods, 13(1), 104. https://doi.org/10.3390/foods13010104







