Cytotoxic and Phytotoxic Activities of Native Brazilian Forest Gabiroba (Campomanesia xanthocarpa Berg.), Fruits, and Flour against Shrimp (Artemia salina L.) and Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.1.1. Process of Elaboration of WGF Extract
2.1.2. Physical Chemical Compositions of GF and WGF
2.1.3. Antioxidant Activity of GF and WGF
2.1.4. Acute In Vitro Cytotoxicity of WGF Extract
2.1.5. Phytotoxicity Bioassay of WGF Extract
Germination Test of WGF Extract
Growth Test of WGF on Seedlings
2.1.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of GF
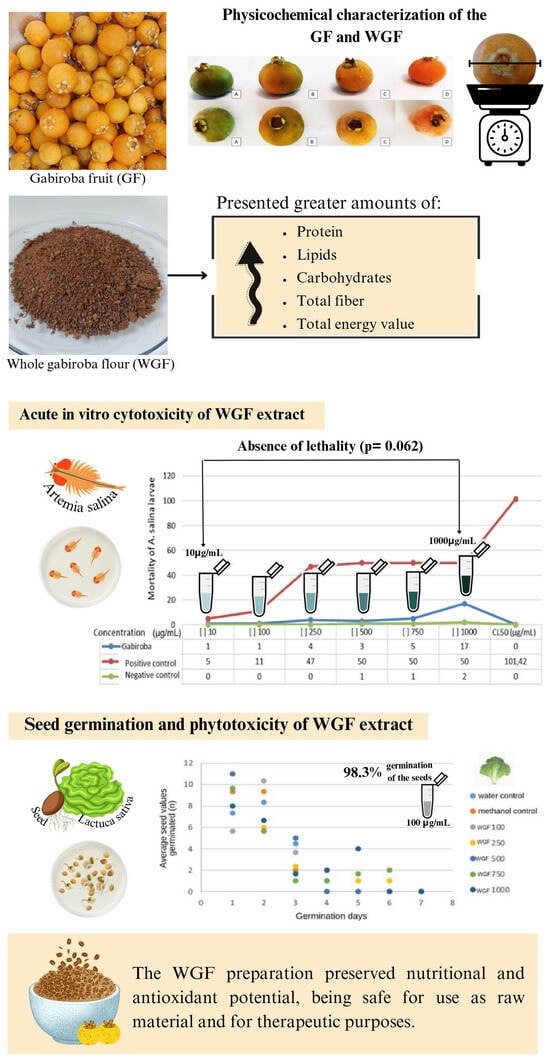
3.2. Physicochemical Characterization of the GF and WGF
3.3. Antioxidant Activities of WGF and GF
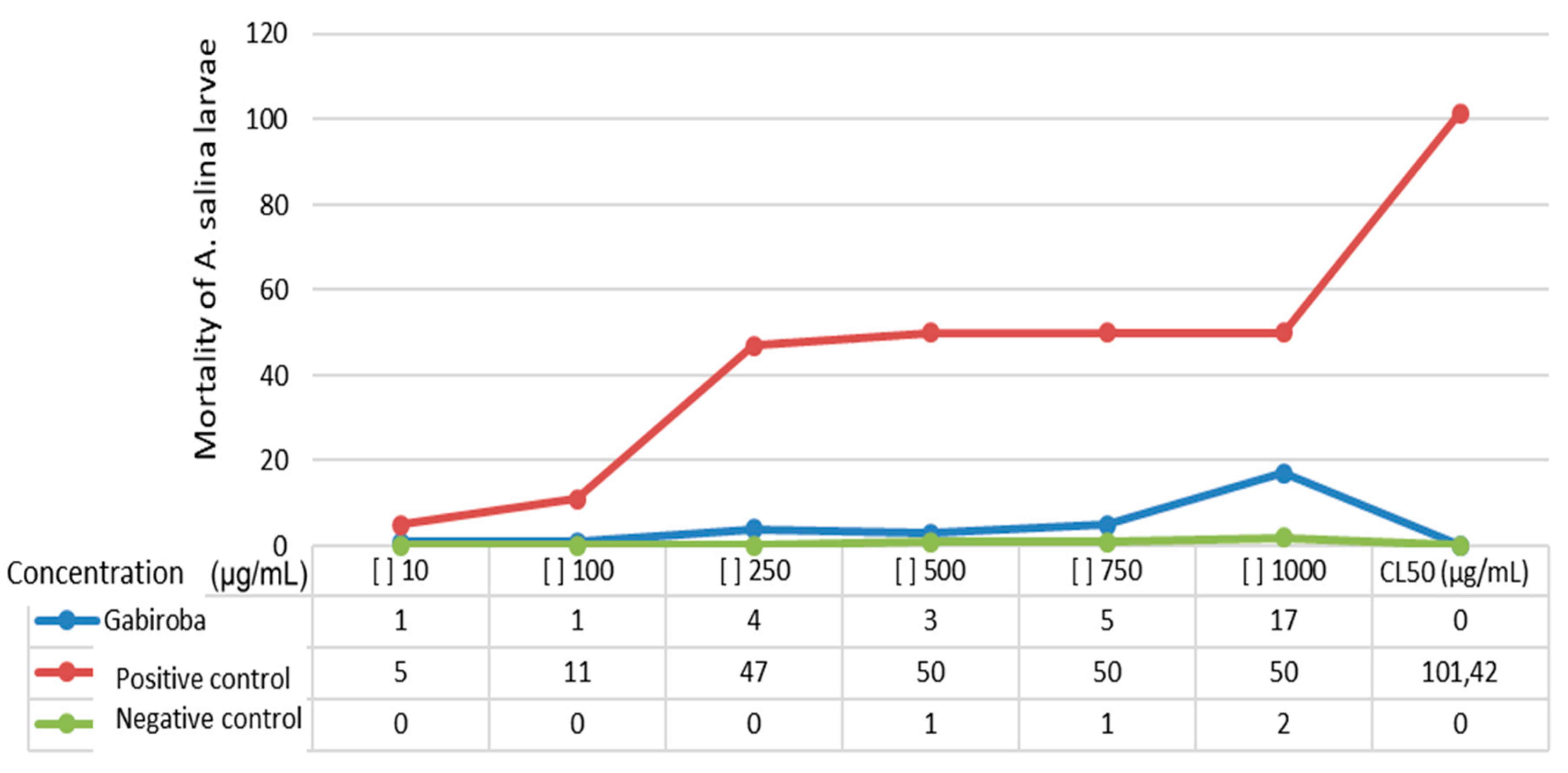
3.4. Acute In Vitro Cytotoxicity of WGF Extract
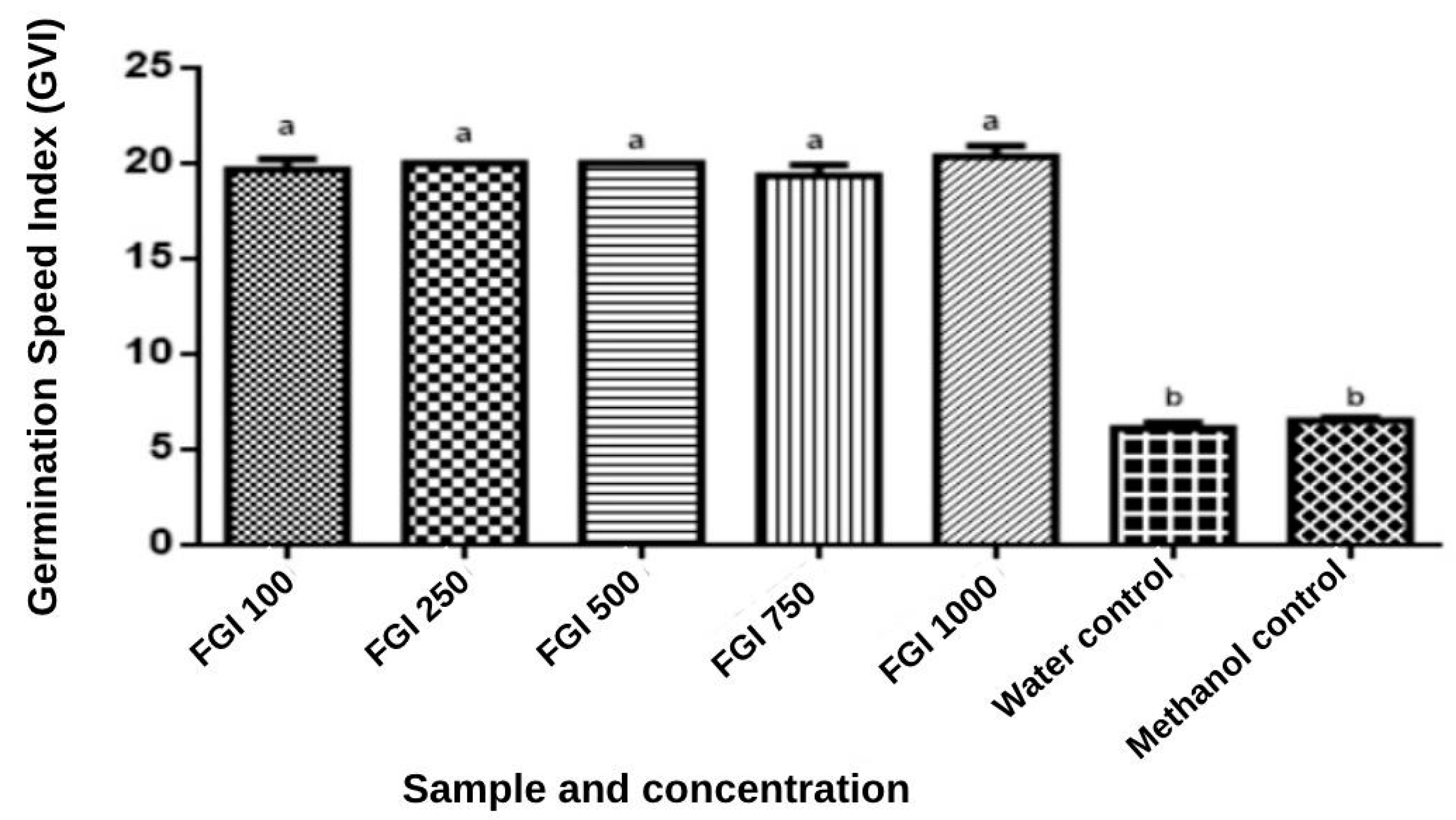
3.5. Seed Germination and Phytotoxicity of WGF Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sereno, A.B.; Gibbert, L.; Bertin, R.L.; Krüger, C.C.H. Cultivo do Maná-Cubiu (Solanum sessiliflorum Dunal) no litoral do Paraná e sua contextualização com a segurança alimentar e nutricional. Divers@! 2017, 10, 123–132. [Google Scholar] [CrossRef]
- Romanelli, J.P.; Meli, P.; Santos, J.P.B.; Jacob, I.N.; Souza, L.R.; Rodrigues, A.V.; Trevisan, D.P.; Huang, C.; Almeida, D.R.A.; Silva, L.G.M.; et al. Biodiversity responses to restoration across the Brazilian Atlantic Forest. Sci. Total Environ. 2022, 821, 153403. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Nobre, C.A.; Chies, J.A.B. Brazilian Biodiversity as a Source of Power, and Sustainable Development: A Neglected Opportunity. Sustainability 2022, 15, 482. [Google Scholar] [CrossRef]
- Embrapa, Valor Nutricional da Guabiroba–Folder. Empresa Brasileira de Pesquisa Agropecuária–Embrapa Florestas. Colombo/PR. 2015. Available online: https://www.embrapa.br/florestas/busca-de-publicacoes/-publicacao/1027135/valor-nutricional-daguabiroba (accessed on 29 March 2022).
- Barbieri, S.F.; De Oliveira Petkowicz, C.L.; De Godoy, R.C.B.; De Azeredo, H.C.M.; Franco, C.R.C.; Silveira, J.L.M. Pulp, and jam of gabiroba (Campomanesia xanthocarpa Berg): Characterization, and rheological properties. Food Chem. 2018, 263, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. O que há de novo em biopotencial de subprodutos de frutas e vegetais aplicados na indústria de processamento de alimentos. Tendências Ciência Tecnol. Aliment. 2017, 67, 150–159. [Google Scholar]
- Pereira Barbosa, J.; Dos Santos Lima, M.; Amaral Souza Tette, P. Prebiotic potential of Puçá, and Gabiroba fruit by-products from Cerrado Savannah. Food Biotechnol. 2022, 36, 371–393. [Google Scholar] [CrossRef]
- Morais, I.B.D.M.; Silva, D.B.; Carollo, C.A.; Ferreira-Neto, M.L.; Fidelis-De-Oliveira, P.; Bispo-Da-Silva, L.B. Hypotensive activity of Campomanesia xanthocarpa leaf extract: Beyond angiotensin II type 1 receptor blockage. Nat. Prod. Res. 2021, 35, 4798–4802. [Google Scholar] [CrossRef]
- Sant’anna, L.S.; Merlugo, L.; Ehle, C.S.; Limberger, J.; Fernandes, M.B.; Santos, M.C.; Mendez, A.S.L.; Paula, F.R.; Moreira, C.M. Chemical composition, and hypotensive effect of Campomanesia xanthocarpa. Evid.-Based Complement. Altern. Med. 2017, 2017, 1591762. [Google Scholar] [CrossRef]
- Viecili, P.R.; Borges, D.O.; Kirsten, K.; Malheiros, J.; Viecili, E.; Melo, R.D.; Trevisan, G.; Da Silva, M.A.; Bochi, G.V.; Moresco, R.N.; et al. Effects of Campomanesia xanthocarpa on inflammatory processes, oxidative stress, endothelial dysfunction, and lipid biomarkers in hypercholesterolemic individuals. Atherosclerosis 2014, 234, 85–92. [Google Scholar] [CrossRef]
- Regginato, A.; Cunico, L.; Bertoncello, K.T.; Schindler, M.S.Z.; Chitolina, R.; Marins, K.; Zanatta, A.P.; Calisto, J.F.; Oliveira, J.V.; Magro, J.D.; et al. Antidiabetic, and hypolipidemic potential of Campomanesia xanthocarpa seed extract obtained by supercritical CO2. Braz. J. Biol. 2021, 81, 621–631. [Google Scholar] [CrossRef]
- Capeletto, C.; Conterato, G.; Scapinello, J.; Rodrigues, F.S.; Copini, M.S.; Kuhn, F.; Tres, M.V.; Magro, J.D.; Oliveira, J.V. Composition, antioxidant, and antimicrobial activity of guavirova (Campomanesia xanthocarpa Berg) seed extracts obtained by supercritical CO2 and compressed n-butane. J. Supercrit. Fluids 2016, 110, 32–38. [Google Scholar] [CrossRef]
- Prestes, A.A.; Verruck, S.; Vargas, M.O.; Canella, M.H.M.; Silva, C.C.; Da Silva Barros, E.L.; Dantas, A.; De Oliveira, L.V.A.; Maran, B.M.; Matos, M.; et al. Influence of Guabiroba Pulp (Campomanesia xanthocarpa O. Berg) Added to Fermented Milk on Probiotic Survival Under In vitro Simulated Gastrointestinal Conditions. Food Res. Int. 2021, 141, 110135. [Google Scholar] [CrossRef] [PubMed]
- Salmazzo, G.R.; Verdan, M.H.; Silva, F.; Cicarelli, R.M.; Mota, J.D.S.; Salvador, M.J.; De Carvalho, J.E.; Cardoso, C.A.L. Chemical composition, and antiproliferative, antioxidant and trypanocidal activities of the fruits from Campomanesia xanthocarpa (Mart.) O. Berg (Myrtaceae). Nat. Prod. Res. 2021, 35, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.T.; Carvalho, T.C.; Lucchesi, L.A.C. Relações cálcio e magnésio presentes no solo e teores foliares de macronutrientes. Rev. Acadêmica Ciência Anim. 2011, 9, 27–32. [Google Scholar] [CrossRef]
- Sereno, A.B.; Bampi, M.; Dos Santos, I.E.; Ferreira, S.M.R.; Bertin, R.L.; Krüger, C.C.H. Mineral profile, carotenoids and composition of cocona (Solanum sessiliflorum Dunal), a wild Brazilian fruit. J. Food Compos. Anal. 2018, 72, 32–38. [Google Scholar] [CrossRef]
- Álvarez-Alarcón, N.; Osorio-Méndez, J.J.; Ayala-Fajardo, A.; Garzón-Méndez, W.F.; Garavito-Aguilar, Z.V. Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity. Toxicol. Rep. 2021, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sereno, A.B.; Gibbert, L.; De Andrade, M.T.P.; Pinto, C.D.; Da Silva, M.A.B.; Dias, J.F.G.; Miguel, O.B.; Krüger, C.C.H.; De Messias-Reason, I.J. Segurança Alimentar e Toxicidade Preliminar do Araçá Amarelo (Psidium cattleianum) Alimentos, Nutrição e Saúde, 1st ed.; Atena: Ponta Grossa, Brazil, 2021; pp. 1–30. [Google Scholar]
- Cândido, A.C.S.; Scalon, S.P.Q.; Silva, C.B.; Simionatto, E.; Morel, A.F.; Stüker, C.Z.; Matos, M.F.C.; Peres, M.T.L.P. Composição química e fitotoxicidade dos óleos essenciais de Croton doctoris S. Moore (Euphorbiaceae). Braz. J. Biol. 2022, 82, e231957. [Google Scholar] [CrossRef] [PubMed]
- Molisch, H. Der Einfluss Einer Pflanze auf Die, Andere-Alelopatia; Fischer: Jena, Germany, 1937. [Google Scholar]
- Ferreira, E.G.B.D.S.; Matos, V.P.; Sena, L.H.D.M.; Sales, A.G.D.F.A. Efeito alelopático do extrato aquoso de sabiá na germinação de sementes de fava. Rev. Ciência Agronômica 2010, 41, 463–467. [Google Scholar] [CrossRef]
- Bursal, E.; Gülçin, İ. Polyphenol contents, and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Maria Do Socorro, M.R.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds, and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Belém, I.D.P.B.; Monteiro, B.N.; Dias, A.C. A desinfecção da água: Um estudo usando insumos vegetais que possuem psoraleno em tsa The disinfection of water: A study using plant inputs that contain psoralene in tsa. Braz. J. Dev. 2022, 8, 4728–4740. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemistis). Official Methods of Analysis of the AOAC, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC (Association of Official Analytical Chemistry). Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Millar, K.A.; Gallagher, E.; Burke, R.; Mccarthy, S.; Barry-Ryan, C. Proximate composition, and anti-nutritional factors of fava-bean (Vicia faba), green-pea, and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Arnous, A.; Markis, D.; Kefalas, P. Correlation of pigment, and flavonol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Atwater, W.O.; Bryant, A.P. 12th Annual Report (1899) of the Storrs CT Agricultural Experimental Station; University of Connecticut: Storrs, CT, USA, 1900. [Google Scholar]
- Atwater, W.O.; Bryant, A.P. Annual Report (1899) of the Storrs, CT Agricultural Experimental Station. 1890. Available online: https://archive.org/details/annualreportofst12stor/page/n3/mode/2up (accessed on 11 December 2023).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; Mclaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.U.; Jang, H.G.; Kim, D.K.; Kim, Y.M.; Boo, H.O.; Kim, Y.J. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic. 2005, 106, 309–317. [Google Scholar] [CrossRef]
- Dias, J.F.G.; Círio, G.M.; Miguel, M.D.; Miguel, O.G. Contribuição ao estudo alelopático de Maytenus ilicifolia Mart. ex Reiss., Celastraceae. Rev. Bras. Farmacogn. 2005, 15, 220–223. [Google Scholar] [CrossRef]
- Macías, F.A.; Castellano, D.; Molinillo, J.M. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
- Adegas, F.S.; Voll, E.; Prete, C.E.C. Embebição e germinação de sementes de picão-preto (Bidens pilosa). Planta Daninha 2003, 21, 21–25. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination-aid in selection evaluation for seedling emergence, and vigour. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; De Araujo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Distribution of nutrients, and functional potential in fractions of Eugenia pyriformis: An underutilized native Brazilian fruit. Food Res. Int. 2020, 137, 109522. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F. Manual do Sistema Sisvar para Análises Estatísticas; Universidade Federal de Lavras Departamento de Ciências Exatas: Lavras, Brazil, 2000. [Google Scholar]
- Etgeton, S.A.P.; Ávila, S.; Silva, A.C.R.; De Lima, J.J.; Rodrigues, A.D.D.P.S.; Beux, M.R.; Kruger, C.C.H. Nutritional Composition, Simulated Digestion and Biological Activities of Campomanesia xanthocarpa Fruit. Plant Foods Hum. Nutr. 2023, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.R.; De Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; De Fgiueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps, and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Jacometti, G.A.; Mello, L.R.; Nascimento, P.H.; Sueiro, A.C.; Yamashita, F.; Mali, S. The physicochemical properties of fibrous residues from the agro industry. LWT-Food Sci. Technol. 2015, 62, 138–143. [Google Scholar] [CrossRef]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic, and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.L.; Feitoza, G.S.; Barbosa, J.; Maria, L.; Araújo, K.S.; Silva, M.V.; Correia, M.T.S.; De Souza, M.P.; Carneiro-Da-Cunha, M.D.G. Nutritional, and technological potential of Umbu (Spondias tuberosa Arr. Cam.) processing by-product flour. An. Acad. Bras. Ciências 2022, 94, e20200940. [Google Scholar] [CrossRef]
- FAO/OMS. Human Vitamin, and Mineral Requirements. In Report 7th Joint FAO/OMS Expert Consultation. Bangkok, Thail, and, XXII + 286. 2001. Available online: https://www.fao.org/3/y2809e/y2809e.pdf (accessed on 11 December 2023).
- Khusun, H.; Februhartanty, J.; Anggraini, R.; Mognard, E.; Alem, Y.; Noor, M.; Karim, N.; Laporte, C.; Poulain, J.P.; Monsivais, P.; et al. As fontes alimentas de proteína animal e vegetal na Indonésia diferem entre os grupos sociodemográficos: Pesquisa sociocultural na transição de proteínas na Indonésia e na Malásia. Front. Nutr. 2022, 9, 762459. [Google Scholar] [CrossRef]
- Tonheim, L.E.; Groufh-Jacobsen, S.; Stea, T.H.; Henjum, S. Nutritional Impact of Replacing Meat and Dairy Products with Plant-Based Substitutes. Preprint 2022. [Google Scholar] [CrossRef]
- Kraak, V.I. Perspective: Unpacking the wicked challenges for alternative proteins in the United States: Can highly processed plant-based, and cell-cultured food, and beverage products support healthy, and sustainable diets, and food systems? Adv. Nutr. 2022, 13, 38–47. [Google Scholar] [CrossRef]
- Anvisa. Resolução-Rdc N° 54, de 12 de novembro de 2012. Agência Nacional de Vigilância Sanitária. Dispõe sobre o Regulamento Técnico sobre Informação Nutricional Complementar. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0054_12_11_2012.html (accessed on 11 December 2023).
- De Oliveira Raphaelli, C.; Vinholes, J.R.; Ribeiro, J.A.; Fiorentini, Â.M.; Nora, L.; Vizzotto, M. Fruto de Eugenia uniflora L.: Uma revisão sobre sua composição química e bioatividade. Nat. Prod. J. 2022, 12, 42–59. [Google Scholar]
- Cain, J.P.; Silva, A.D.; Soares, J.M.; Santos, M.M.R.; Amaral, L.D.; Santos, E.D.; Novello, D. Adição de farinha de resíduos de guavira em barra de cereais: Aceitabilidade sensorial e caracterização físico-química. Conexão Ci 2019, 14, 18–26. [Google Scholar]
- Vallilo, M.I.; Moreno, P.R.; Oliveira, E.D.; Lamardo, L.C.; Garbelotti, M.L. Chemical composition of Campomanesia xanthocarpa Berg-Myrtaceae fruit. Food Sci. Technol. 2008, 28, 231–237. [Google Scholar] [CrossRef]
- Pereira, M.F.M.C.; Steffens, R.S.; Jablonski, A.; Hertz, P.F.; Rios, A.O.; Vizzotto, M.; Flôres, S.H. Characterization, and Antioxidant Potential of Brazilian Fruits from the Myrtaceae Family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E. Sugars and starch in the nutritional management of diabetes mellitus. Am. J. Clin. Nutr. 2003, 78, 858S–864S. [Google Scholar] [CrossRef] [PubMed]
- Tabela Brasileira De Composição De Alimentos (TBCA). Universidade de São Paulo (USP). Food Research Center (FoRC). Versão 7.1. São Paulo. 2020. Available online: http://www.fcf.usp.br/tbca (accessed on 1 November 2022).
- Biavatti, M.W.; Farias, C.; Curtius, F.; Brasil, L.M.; Hort, S.; Schuster, L.; Leite, S.N.; Prado, S.R.T. Preliminary studies on Campomanesia xanthocarpa (Berg.), and Cuphea carthagenensis (Jacq.) JF Macbr. Aqueous extract: Weight control and biochemical parameters. J. Ethnopharmacol. 2004, 93, 385–389. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Mcmackin, E.; Dean, M.; Woodside, J.V.; Mckinley, M.C. Whole grains and health: Attitudes to whole grains against a prevailing background of increased marketing and promotion. Public Health Nutr. 2013, 16, 743–751. [Google Scholar] [CrossRef]
- Sereno, A.B.; Dos Santos, I.E.; Hauser, A.B.; Gibbert, L.; Bampi, M.; Pinto, C.D.; Bertin, R.L.; Kruger, C.C.H. Development, and acceptability of breads added with cocona flour (Solanum sessiliflorum Dunal): Impact on the glycemic index. Res. Soc. Dev. 2022, 11, 3. [Google Scholar] [CrossRef]
- Ramos, B.F.M. Composição nutricional de pães com farinha de trigo integral e refinada, comercializados em hipermercados de Salvador, BA. Hig. Aliment. 2017, 31, 266–267. [Google Scholar]
- Gibbert, L.; Sereno, A.B.; De Andrade, M.T.P.; Da Silva, M.A.B.; Miguel, M.D.; Montrucchio, D.P.; De Messias-Reason, I.J.; Dantas, A.M.; Borges, G.S.C.; Miguel, O.G.; et al. Nutritional composition, antioxidant activity and anticancer potential of Syzygium cumini (L.), and Syzygium malaccense (L.) fruits. Res. Soc. Dev. 2021, 10, 4. [Google Scholar] [CrossRef]
- Amarante, C.B.D.; Müller, A.H.; Póvoa, M.M.; Dolabela, M.F. Estudo fitoquímico biomonitorado pelos ensaios de toxicidade frente à Artemia salina e de atividade antiplasmódica do caule de aninga (Montrichardia linifera). Acta Amaz. 2011, 41, 431–434. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. A review of the traditional use of Southern African medicinal plants for the treatment of inflammation and inflammatory pain. J. Ethnopharmacol. 2022, 283, 114436. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Grosso, C.; Valentão, P.; Andrade, P.B. α-Glucosidase and α-amylase inhibitors from Myrcia spp.: A stronger alternative to acarbose? J. Pharm. Biomed. Anal. 2016, 118, 322–327. [Google Scholar] [CrossRef] [PubMed]
- da Silva, É.R.; Salmazzo, G.R.; da Silva Arrigo, J.; Oliveira, R.J.; Kassuya, C.A.; Cardoso, C.A. Anti-inflammatory Evaluation and Toxicological Analysis of Campomanesia xanthocarpa Berg. Inflammation 2016, 39, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Markman, B.E.; Bacchi, E.M.; Kato, E.T.M. Antiulcerogenic effects of Campomanesia xanthocarpa. J. Ethnopharmacol. 2004, 94, 55–57. [Google Scholar] [CrossRef]
- Pastori, T.; Flores, F.C.; Boligon, A.A.; Athayde, M.L.; Da Silva, C.D.E.B.; Do Canto-Dorow, T.S.; Tedesco, S.B. Genotoxic effects of Campomanesia xanthocarpa extracts on Allium cepa vegetal system. Pharm. Biol. 2013, 51, 1249–1255. [Google Scholar] [CrossRef]
- Klafke, J.Z.; Da Silva, M.A.; Panigas, T.F.; Belli, K.C.; De Oliveira, M.F.; Barichello, M.M.; Rigo, F.K.; Rossato, M.F.; Dos Santos, A.R.S.; Pizzolatti, M.G.; et al. Effects of Campomanesia xanthocarpa on biochemical, hematological and oxidative stress parameters in hypercholesterolemic patients. J. Ethnopharmacol. 2010, 127, 299–305. [Google Scholar] [CrossRef]
- Rosado, L.D.S.; Rodrigues, H.C.A.; Pinto, J.E.B.P.; Custódio, T.N.; Pinto, L.B.B.; Bertolucci, S.K.V. Alelopatia do extrato aquoso e do óleo essencial de folhas do manjericão “Maria Bonita” na germinação de alface, tomate e melissa. Rev. Bras. Plantas Med. 2009, 11, 422–428. [Google Scholar] [CrossRef]
- Gómez, C.; Contento, L.; Carsen, A. Toxicity test to assess pollutants removal during wastewater treatment, and the quality of receiving waters in Argentina. Environ. Toxicol. 2001, 16, 217–224. [Google Scholar] [CrossRef]
- Lima, L.M.; Pedroza, L.S.; Osório, M.I.C.; Souza, J.C.; Nunez, C.V. Fitotoxicidade de extratos de plantas de Vismia japurensis cultivadas in vivo e in vitro. Braz. J. Biol. 2022, 82. [Google Scholar] [CrossRef] [PubMed]
- Muniz, F.R.; Cardoso, M.D.G.; Von Pinho, É.V.R.; Vilela, M. Qualidade fisiológica de sementes de milho, feijão, soja e alface na presença de extrato de tiririca. Rev. Bras. Sementes 2007, 29, 195–204. [Google Scholar] [CrossRef]
- Aouad, A.; Baaziz, M.; Mergoum, M. Quantitative aspects of peroxidases in some moroccan cereal varieties. Actes Prem. Journées L’arbre. 1998, pp. 1–7. Available online: https://www.cabidigitallibrary.org/action/doSearch?AllField=Quantitative+aspects+of+peroxidases+in+some+moroccan+cereal+varieties%0D%0A (accessed on 11 December 2023).
- Souza Filho, A.P.S.; Rodrigues, L.R.A.; Rodrigues, T.J.D. Efeito de extratos aquosos de assa-peixe sobre a germinação de três espécies de braquiária. Planta Daninha 1996, 14, 93–100. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: London, UK, 1984; p. 422. [Google Scholar]
- Radouane, L.; Rhim, T. Allelopathic interaction of pepper (Capsicum annuum), and pearl millet (Pennisetum glaucum) intercropped. Int. J. Environ. 2014, 3, 32–40. [Google Scholar] [CrossRef]
- Silva, B.A.; De Castro, T.L.A.; Viana, L.F.; Santos, M.D.S.M.; Cardoso, C.A.L. Effect of the peel extracts from two Campomanesia (Myrtaceae) species on Allium cepa L. (Amaryllidaceae). Rev. Agric. Neotrop. 2022, 9, 6831. [Google Scholar] [CrossRef]
- Auharek, S.A.; Do Carmo Vieira, M.; Cardoso, C.A.L.; Oliveira, R.J.; Cunha-Laura, A.L. Reproductive toxicity of Campomanesia xanthocarpa (Berg.) in female Wistar rats. J. Ethnopharmacol. 2013, 148, 341–343. [Google Scholar] [CrossRef]
- Barbieri, S.F.; Da Costa Amaral, S.; Ruthes, A.C.; De Oliveira Petkowicz, C.L.; Kerkhoven, N.C.; Da Silva, E.R.A.; Silveira, J.L.M. Pectins from the pulp of gabiroba (Campomanesia xanthocarpa Berg.): Structural characterization, and rheological behavior. Carbohydr. Polym. 2019, 214, 250–258. [Google Scholar] [CrossRef]





| Gabiroba Fruit (GF) 1 | Whole Gabiroba Flour (WGF) 2 | |
|---|---|---|
| Moisture (g/100 g) | 81.18 ± 0.39 a | 12.33 ± 0.21 b |
| Ashes (g/100 g) | 0.29 ± 0.07 a | 3.03 ± 0.15 b |
| pH | 3.72 ± 0.02 a | 3.49 ± 0.02 b |
| Protein (g/100 g) | 0.81 ± 0.64 b | 4.75 ± 0.05 a |
| Lipids (g/100 g) | 2.67 ± 0.04 b | 8.73 ± 0.52 a |
| Carbohydrates (g/100 g) | 8.63 ± 1.14 b | 54.27 ± 0.93 a |
| Soluble fiber (g/100 g) | 1.52 ± 0.74 b | 7.15 ± 1.87 a |
| Insoluble fiber (g/100 g) | 4.90 ± 4.37 b | 9.74 ± 1.52 a |
| Total fiber (g/100 g) | 6.42 ± 5.11 b | 16.89 ± 3.39 a |
| TEV 3 (Kcal) | 60.79 ± 13.18 b | 314.57 ± 8.66 a |
| Fraction | DPPH (µmol TEAC.100 g−1) | ORAC (µmol TEAC.100 g−1) | FRAP (µmol TEAC.100 g−1) |
|---|---|---|---|
| GF 1 | 380.64 ± 43.62 a | 2824.00 ± 11.79 | 126.97 ± 25.57 |
| WGF 2 | 681.72 ± 12.35 b | NA 3 | NA 3 |
| p-value | 0.0003 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereno, A.B.; Pinto, C.D.; Gibbert, L.; de Andrade, M.T.P.; da Silva, M.A.B.; Etgeton, S.A.P.; Miguel, O.G.; Dias, J.d.F.G.; Krüger, C.C.H.; de Messias Reason, I.J. Cytotoxic and Phytotoxic Activities of Native Brazilian Forest Gabiroba (Campomanesia xanthocarpa Berg.), Fruits, and Flour against Shrimp (Artemia salina L.) and Lettuce (Lactuca sativa L.). Foods 2024, 13, 123. https://doi.org/10.3390/foods13010123
Sereno AB, Pinto CD, Gibbert L, de Andrade MTP, da Silva MAB, Etgeton SAP, Miguel OG, Dias JdFG, Krüger CCH, de Messias Reason IJ. Cytotoxic and Phytotoxic Activities of Native Brazilian Forest Gabiroba (Campomanesia xanthocarpa Berg.), Fruits, and Flour against Shrimp (Artemia salina L.) and Lettuce (Lactuca sativa L.). Foods. 2024; 13(1):123. https://doi.org/10.3390/foods13010123
Chicago/Turabian StyleSereno, Aiane Benevide, Carla Dayane Pinto, Luciana Gibbert, Marina Talamini Piltz de Andrade, Michelli Aparecida Bertolazo da Silva, Schaina Andriela Pontarollo Etgeton, Obdulio Gomes Miguel, Josiane de Fátima Gaspari Dias, Claudia Carneiro Hecke Krüger, and Iara José de Messias Reason. 2024. "Cytotoxic and Phytotoxic Activities of Native Brazilian Forest Gabiroba (Campomanesia xanthocarpa Berg.), Fruits, and Flour against Shrimp (Artemia salina L.) and Lettuce (Lactuca sativa L.)" Foods 13, no. 1: 123. https://doi.org/10.3390/foods13010123