Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation and Drying Experiments
2.2. Sampling
2.3. Analyses
2.3.1. Measurement of Temperature
2.3.2. Enumeration of Yeasts, Lactic Acid Bacteria, and Acetic Acid Bacteria
2.3.3. Measurement of pH of Cotyledon and Pulp
2.3.4. Determination of Sugars and Organic Acids in Dried Cocoa Beans
2.3.5. Cut-Test of Dried Beans
2.3.6. Sensory Assessment
2.4. Statistics
3. Results
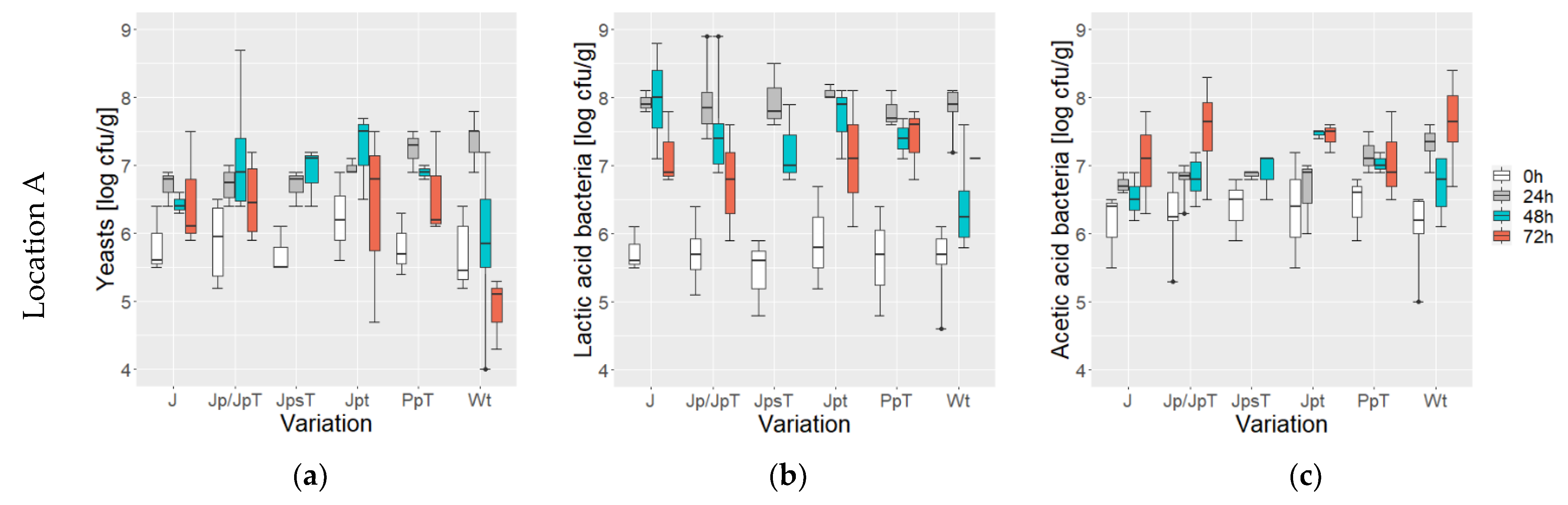
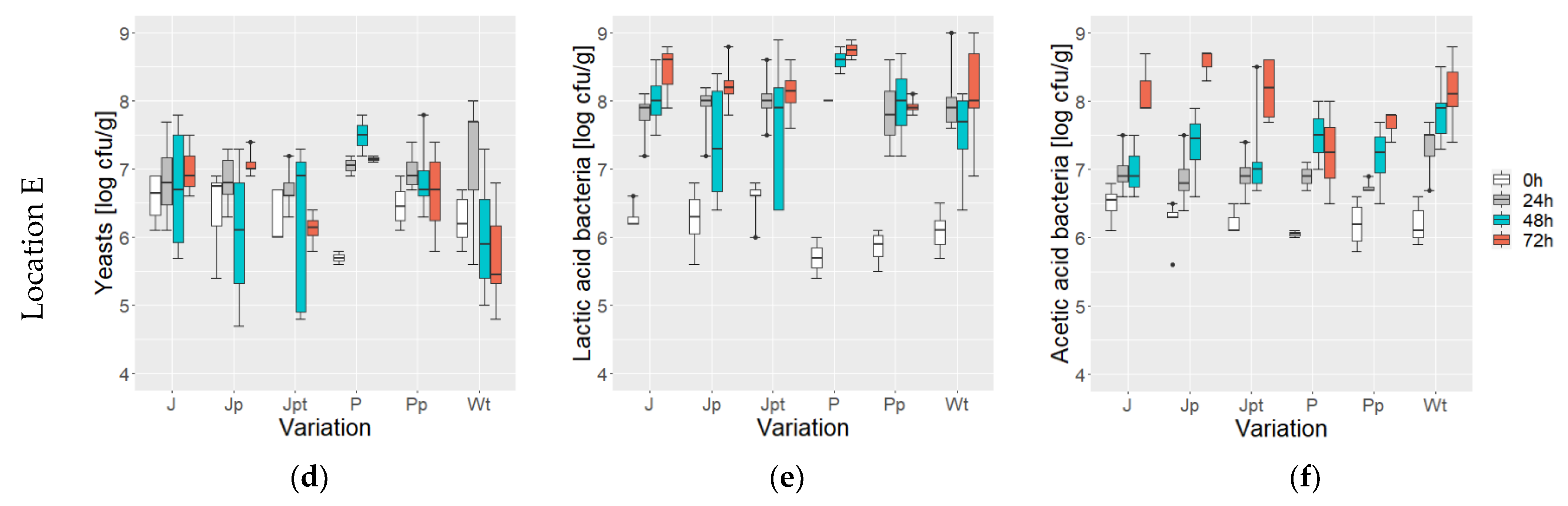
3.1. Microbial Counts during Fermentation
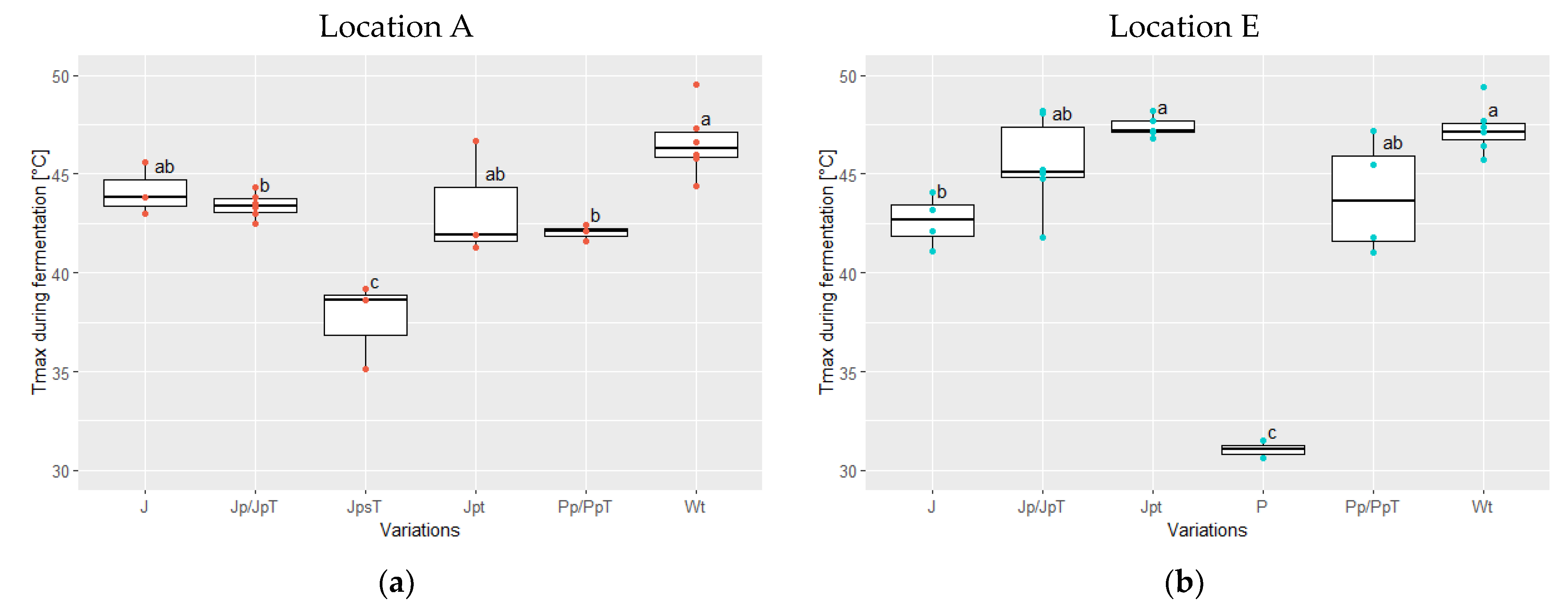
3.2. Temperature Development during Fermentation and Drying Temperatures
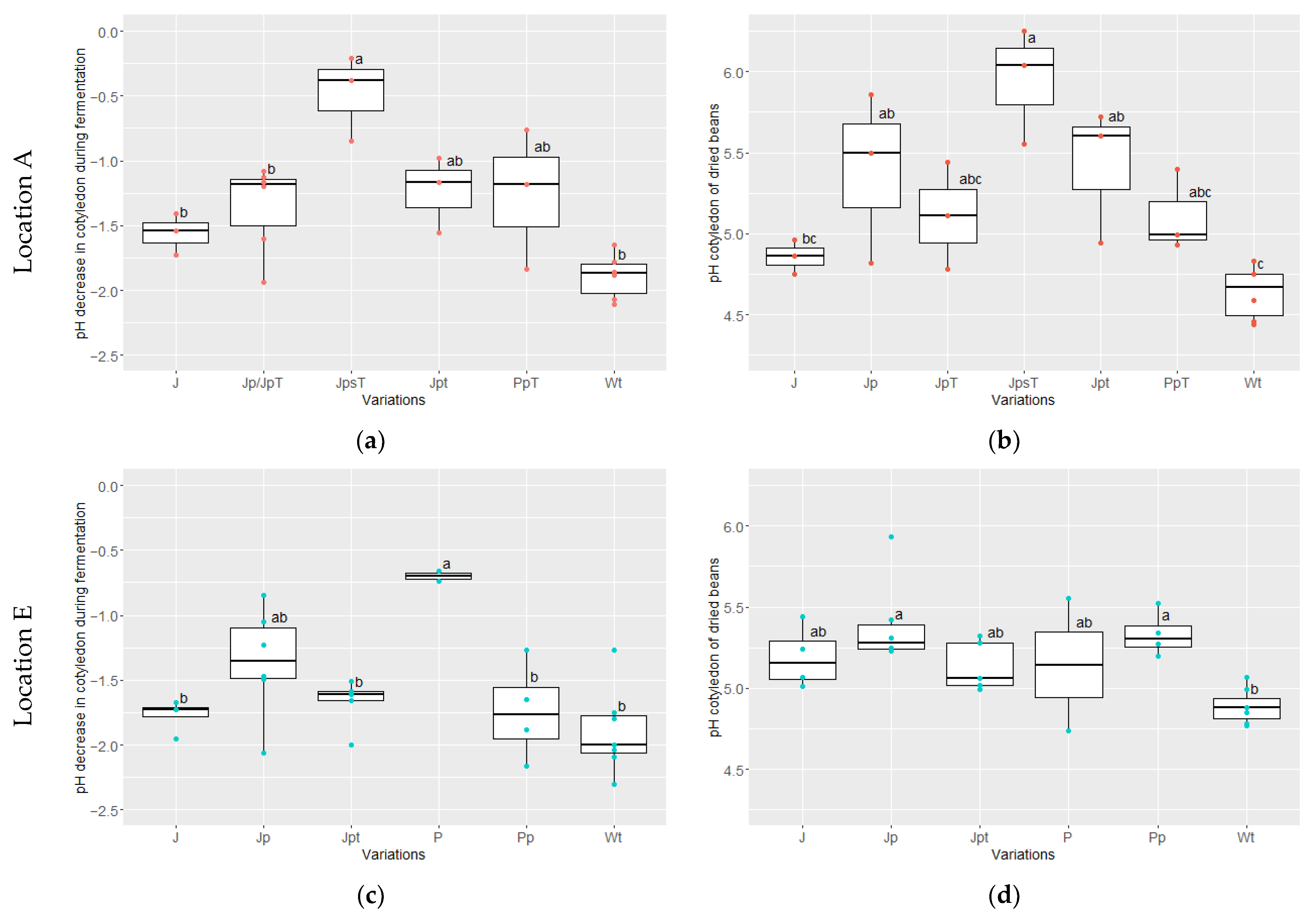
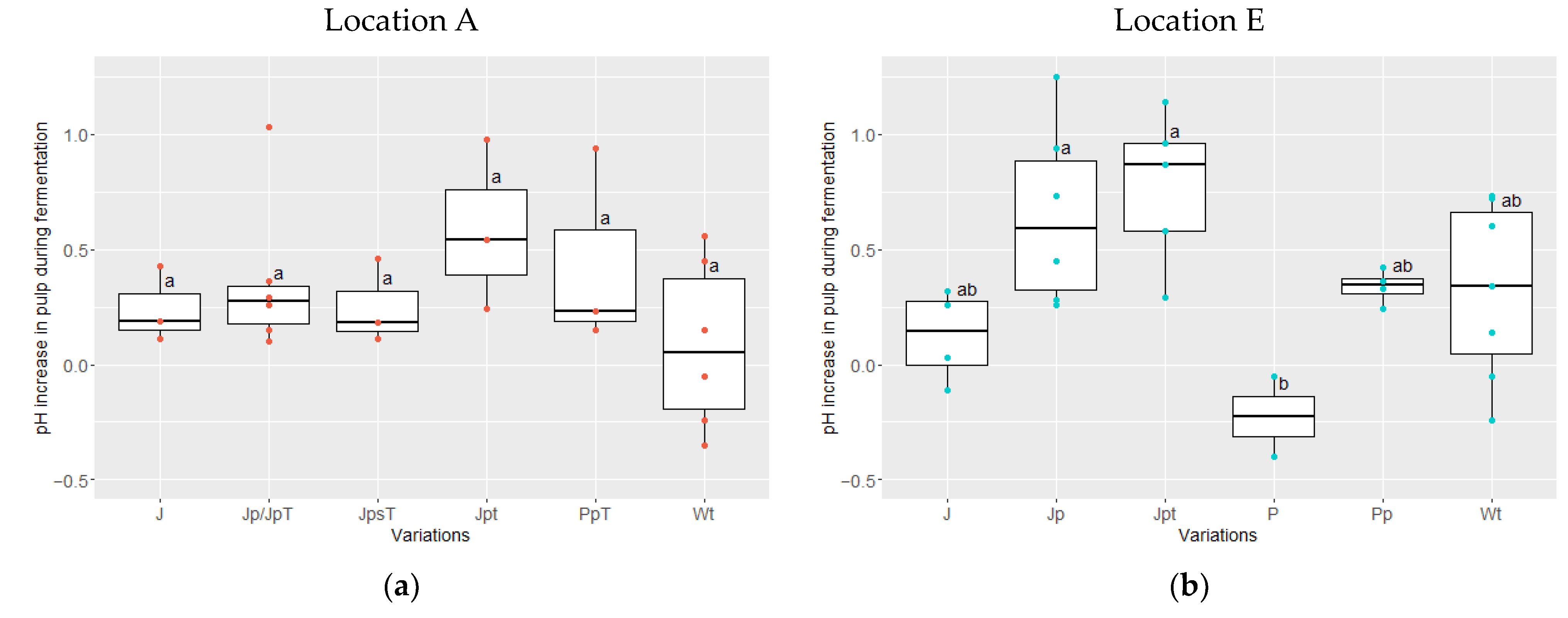
3.3. Pulp and Cotyledon pH
3.4. Sugars and Organic Acids in Dried Cocoa Beans
3.5. Cut-Test of Dried Beans
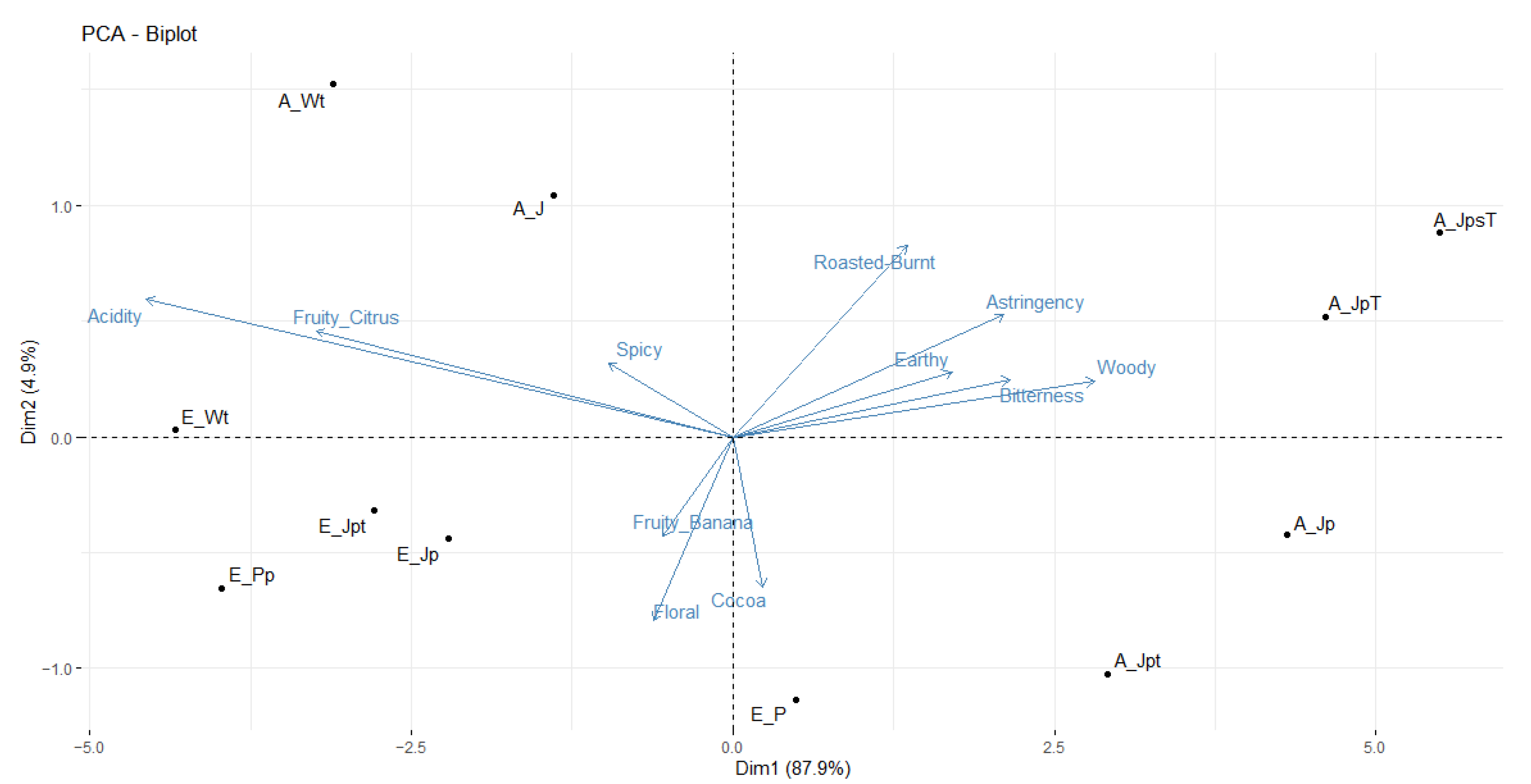
3.6. Sensory Description of Cocoa Bean Mass
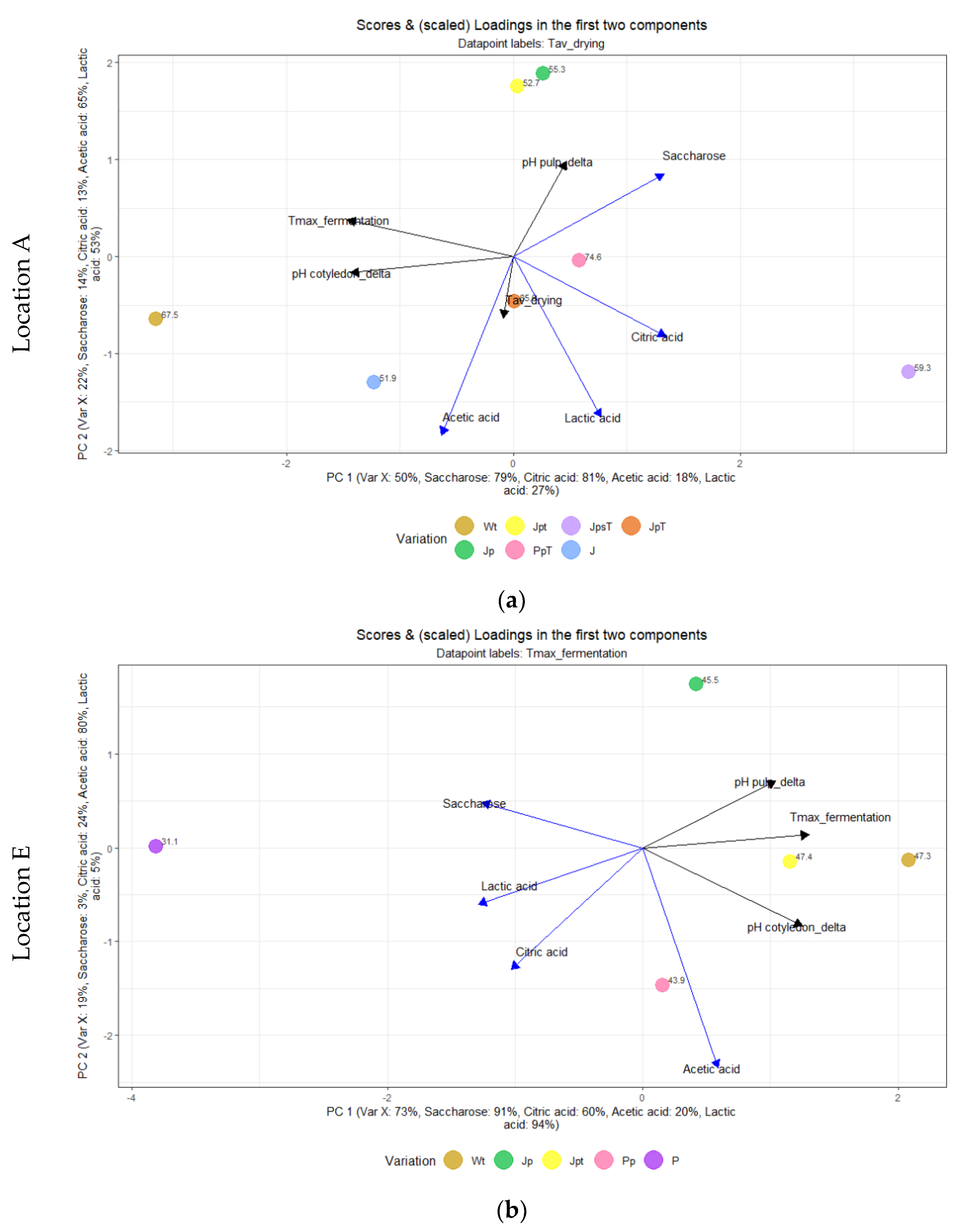
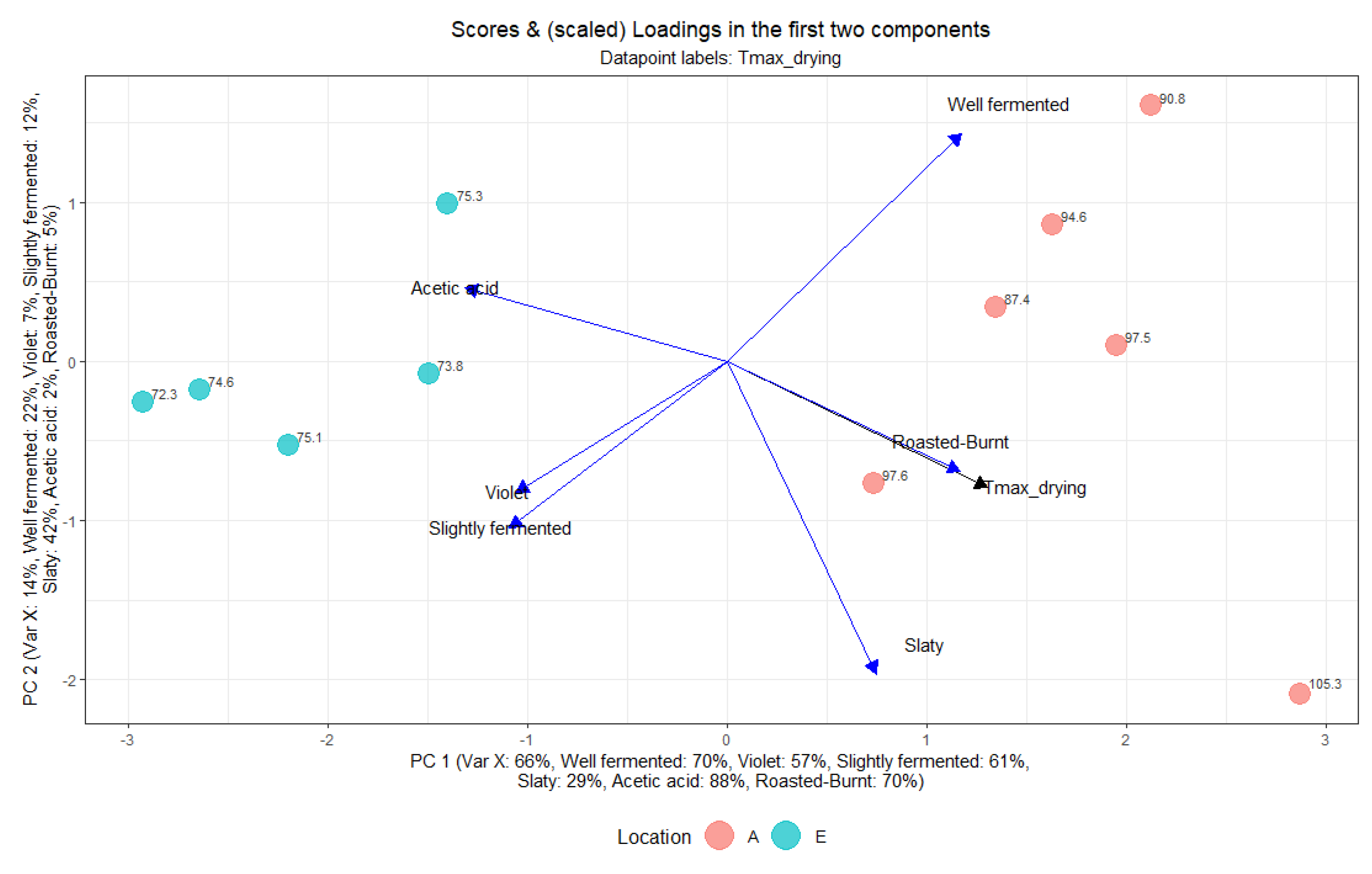
3.7. Multivariate Analysis Using Partial Least Squares (PLS)
3.7.1. Effect of Post-Harvesting Parameters on Selected Quality Parameters
3.7.2. Effect of Drying Temperature on Cut-Test and Acetic Acid per Location
4. Discussion
4.1. Different Microbiological and Physicochemical Dynamics and Quality Depending on Fermentation Variation
4.2. Different Microbiological and Physicochemical Dynamics and Quality Depending on Location
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardhana, M.; Fleet, G.H. The Microbial Ecology of Cocoa Bean Fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Lopez-Yerena, A.; Vallverdú-Queralt, A. Traceability, Authenticity and Sustainability of Cocoa and Chocolate Products: A Challenge for the Chocolate Industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 475–489. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors Influencing Quality Variation in Cocoa (Theobroma Cacao) Bean Flavour Profile—A Review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Miescher Schwenninger, S. Monitoring of Cocoa Post-Harvest Process Practices on a Small-Farm Level at Five Locations in Ecuador. Heliyon 2022, 8, e09628. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical Composition and Physical Quality Characteristics of Ghanaian Cocoa Beans as Affected by Pulp Pre-Conditioning and Fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef]
- Biehl, B.; Meyer, B.; Said, M.B.; Samarakoddy, R.J. Bean Spreading: A Method for Pulp Preconditioning to Impair Strong Nib Acidification during Cocoa Fermentation in Malaysia. J. Sci. Food Agric. 1990, 51, 35–45. [Google Scholar] [CrossRef]
- Biehl, B.; Meyer, B.; Crone, G.; Pollmann, L.; Said, M.B. Chemical and Physical Changes in the Pulp during Ripening and Post-Harvest Storage of Cocoa Pods. J. Sci. Food Agric. 1989, 48, 189–208. [Google Scholar] [CrossRef]
- Fernández, R.D.R.; Gallo, F.W.M.; Cedeño, Á.M.G.; Mercedes, M.; Galeas, P.; Quinteros, H.N.M.; Ferrín, L.M.C.; Álvarez, A.E.B.; Morante, P.E.N. Efecto del Tipo y Tiempo de Fermentación en la Calidad Física y Química del Cacao (Theobroma cacao L.) Tipo Nacional. Cienc. Tecnol. 2012, 5, 7–12. [Google Scholar] [CrossRef]
- Camu, N.; González, Á.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of Turning and Environmental Contamination on the Dynamics of Populations of Lactic Acid and Acetic Acid Bacteria Involved in Spontaneous Cocoa Bean Heap Fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the Influence of Pod Storage on Sugar and Free Amino Acid Profiles and the Implications on Some Maillard Reaction Related Flavor Volatiles in Forastero Cocoa Beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Ganeswari, I.; Bariah, K.S.; Amizi, M.A.; Sim, K.Y.K. Effects of Different Fermentation Approaches on the Microbiological and Physicochemical Changes during Cocoa Bean Fermentation. Int. Food Res. J. 2015, 22, 70–76. [Google Scholar]
- Bariah, S.K.; Tajul, A.Y. Effect of Cocoa Pods Storage on the Temperature and Physicochemical Changes during Shallow Box Fermentation. IJISET-Int. J. Innov. Sci. Eng. Technol. 2017, 4, 197–203. [Google Scholar]
- Sulaiman, K.B.; Yang, T.A. Color Characteristics of Dried Cocoa Using Shallow Box Fermentation Technique. World Acad. Sci. Eng. Technol. 2015, 9, 1281–1285. [Google Scholar]
- Quevedo Guerrero, J.N.; Romero López, J.A.; Tuz Guncay, I.G. Calidad físico química y sensorial de granos y licor de cacao (Theobroma cacao l.) Usando cinco métodos de fermentación. Rev. Científica Agroecosiste-Mas 2018, 6, 115–127. [Google Scholar]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of Turning, Pod Storage and Fermentation Time on Microbial Ecology and Volatile Composition of Cocoa Beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Guehi, T.S.; Dadie, A.T.; Koffi, K.P.B.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, K.D.; Nemlin, G.J. Performance of Different Fermentation Methods and the Effect of Their Duration on the Quality of Raw Cocoa Beans: Fermentation’s Effect on the Raw Cocoa Quality. Int. J. Food Sci. Technol. 2010, 45, 2508–2514. [Google Scholar] [CrossRef]
- Guehi, T.S.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, D.K.; Zahouli, I.B. Effect of Turning Beans and Fermentation Method on the Acidity and Physical Quality of Raw Cocoa Beans. Adv. J. Food Sci. Technol. 2010, 2, 163–171. [Google Scholar]
- Portillo, E.; Labarca, M.; Grazziani, L.; Cros, E.; Assemat, S.; Davrieux, F.; Boulager, R. Influencia de la condiciones del tratamiento poscosecha sobre la temperatura y acidez en granos de cacao Criollo (Theobroma cacao L.). Rev. Fac. Agron. 2011, 28, 646–660. [Google Scholar]
- Dulce, V.-R.; Anne, G.; Manuel, K.; Carlos, A.-A.; Jacobo, R.-C.; Sergio de Jesús, C.-E.; Eugenia, L.-C. Cocoa Bean Turning as a Method for Redirecting the Aroma Compound Profile in Artisanal Cocoa Fermentation. Heliyon 2021, 7, e07694. [Google Scholar] [CrossRef] [PubMed]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Yao, A.K.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod Storage Time and Spontaneous Fermentation Treatments and Their Impact on the Generation of Cocoa Flavour Precursor Compounds. Int. J. Food Sci. Technol. 2021, 56, 2516–2529. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of Starter Cultures and Fermentation Techniques on the Volatile Aroma and Sensory Profile of Chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Amores, F.M.; Solórzano, E.G.; Rodríguez, G.A.; La Mantia, A.; Blasi, P.; Loor, R.G. Differentiation of Ecuadorian National and CCN-51 Cocoa Beans and Their Mixtures by Computer Vision: Differentiation of Ecuadorian Cocoa. J. Sci. Food Agric. 2018, 98, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.S.; Miller, K.B.; Lopez, A.S. Cocoa and Coffee. In Food Microbiology: Fundamentals and Frontiers, 2nd ed; Doyle, M.P., Beuchat, L.R., Montville, T.J., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 721–733. [Google Scholar]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Chichester, UK, 2010; ISBN 978-1-4051-9906-3. [Google Scholar]
- Ruíz Pinargote, M.; Mera Morán, O.; Prado Cedeño, Á.; Cedeño Guzmán, W. Influencia de la época de cosecha en la calidad del licor de cacao tipo nacional. Rev. Espamciencia 2014, 5, 79–87. [Google Scholar]
- Garcia-Armisen, T.; Papalexandratou, Z.; Hendryckx, H.; Camu, N.; Vrancken, G.; De Vuyst, L.; Cornelis, P. Diversity of the Total Bacterial Community Associated with Ghanaian and Brazilian Cocoa Bean Fermentation Samples as Revealed by a 16 S rRNA Gene Clone Library. Appl. Microbiol. Biotechnol. 2010, 87, 2281–2292. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of Fermentation Level and Geographical Origin on Cocoa Bean Oligopeptide Pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef]
- Avadí, A.; Temple, L.; Blockeel, J.; Salgado, V.; Molina, G.; Andrade, D. Análisis de La Cadena de Valor Del Cacao En Ecuador. Available online: https://capacity4dev.europa.eu/projects/value-chain-analysis-for-development-vca4d/info/235-ecuador-cocoa_en (accessed on 13 October 2023).
- Managanta, A.A.; Sumardjo; Sadono, D.; Tjitropranoto, P. Strategy to Increase Farmers’ Productivity Cocoa Using Structural Equation Modeling. IOP Conf. Ser. Earth Environ. Sci. 2022, 1107, 1–10. [Google Scholar] [CrossRef]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef]
- Sandhya, M.V.S.; Yallappa, B.S.; Varadaraj, M.C.; Puranaik, J.; Rao, L.J.; Janardhan, P.; Murthy, P.S. Inoculum of the Starter Consortia and Interactive Metabolic Process in Enhancing Quality of Cocoa Bean (Theobroma cacao) Fermentation. LWT-Food Sci. Technol. 2016, 65, 731–738. [Google Scholar] [CrossRef]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing Chocolate Production through Traceability: A Review of the Influence of Farming Practices on Cocoa Bean Quality. Food Control. 2013, 29, 167–187. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Capa, M.C.; Vargas Tierras, Y.B.; Burbano Cachiguango, R.A.; Calero Cárdenas, A.S.; Ramírez Romero, C.A. Evaluación del grano de cacao (Theobroma cacao L.), usando dos fermentadores, provincias Orellana y Sucumbíos, Ecuador. Green World J. 2020, 3, 1–9. [Google Scholar]
- Erazo Solorzano, C.Y.; Disca, V.; Muñoz-Redondo, J.M.; Tuárez García, D.A.; Sánchez-Parra, M.; Carrilo Zenteno, M.D.; Moreno-Rojas, J.M.; Rodríguez-Solana, R. Effect of Drying Technique on the Volatile Content of Ecuadorian Bulk and Fine-Flavor Cocoa. Foods 2023, 12, 1065. [Google Scholar] [CrossRef]
- Solórzano Chavez, E.; Nicklin, C.; Amores Puyutaxi, F.; Jiménez Barragan, J.; Barzola Miranda, S. Comparación sensorial del cacao (Theobroma cacao L.) Nacional fino de aroma cultivado en diferentes zonas del Ecuador. Cienc. Tecnol. 2015, 8, 37–47. [Google Scholar] [CrossRef]
- Amores Puyutaxi, F.M.; Saltos Rivera, E.A. Influencia Del Presecado de Las Almendras Sobre La Evolución Del pH y Porcentajes de Fermentación Durante La Época Seca En Las Variedades de Cacao Ccn-51 y Nacional. Quevedo, Ecuador: INIAP, Estación Experimental Tropical Pichilingue, Programa Nacional de Cacao y Café. 2005. 1p. Available online: http://repositorio.iniap.gob.ec/handle/41000/3492 (accessed on 13 October 2023).
- Neira Mosquera, J.A.; Revilla Escobar, K.Y.; Aldas Morejon, J.P.; Sánchez Llaguno, S.N. Métodos de fermentación del cacao nacional (Theobroma cacao) y su influencia en las características físico-químicas, contenido de cadmio y perfiles sensoriales. Alternativas 2020, 21, 42–48. [Google Scholar] [CrossRef]
- Romanens, E.; Näf, R.; Lobmaier, T.; Pedan, V.; Leischtfeld, S.F.; Meile, L.; Schwenninger, S.M. A Lab-Scale Model System for Cocoa Bean Fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 3349–3362. [Google Scholar] [CrossRef]
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs. International Organization for Standardization: Vernier, Switzerland, 2007.
- Romanens, E.; Pedan, V.; Meile, L.; Miescher Schwenninger, S. Influence of Two Anti-Fungal Lactobacillus Fermentum-Saccharomyces Cerevisiae Co-Cultures on Cocoa Bean Fermentation and Final Bean Quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef]
- Müller, D.C.; Schipali, S.; Näf, P.; Kinner, M.; Miescher Schwenninger, S.; Schönlechner, R. Potential of a Techno-Functional Sourdough and Its Application in Sugar-Reduced Soft Buns. Fermentation 2022, 8, 42. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-6487-8. [Google Scholar]
- ISO 8586:2012; Sensory analysis. International Organization for Standardization: Vernier, Switzerland, 2014.
- Lê, S.; Lê, T.M.; Cadoret, M. Napping and Sorted Napping as a Sensory Profiling Technique. In Rapid Sensory Profiling Techniques; Woodhead Publishing: Cambridge, UK, 2015; pp. 197–213. ISBN 978-1-78242-248-8. [Google Scholar]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Crafack, M.; Jespersen, L.; Jakobsen, M. The Microbiology of Cocoa Fermentation. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 7, pp. 39–60. [Google Scholar]
- Papalexandratou, Z.; De Vuyst, L. Assessment of the Yeast Species Composition of Cocoa Bean Fermentations in Different Cocoa-Producing Regions Using Denaturing Gradient Gel Electrophoresis. FEMS Yeast Res. 2011, 11, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing Cocoa Flavour Using Pichia Kluyveri and Kluyveromyces Marxianus in a Defined Mixed Starter Culture for Cocoa Fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.S. Cocoa Beans: From Tree to Factory. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 10–47. [Google Scholar]
- Schwan, R.F.; Pereira, G.V.d.M.; Fleet, G.H. Microbial Activities during Cocoa Fermentation. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 129–192. [Google Scholar]
- Hashim, P.; Selamat, J.; Muhammad, S.K.S.; Ali, A. Changes in Free Amino Acid, Peptide-N, Sugar and Pyrazine Concentration during Cocoa Fermentation. J. Sci. Food Agric. 1998, 78, 543–550. [Google Scholar] [CrossRef]
- Tigrero-Vaca, J.; Maridueña-Zavala, M.G.; Liao, H.-L.; Prado-Lince, M.; Zambrano-Vera, C.S.; Monserrate-Maggi, B.; Cevallos-Cevallos, J.M. Microbial Diversity and Contribution to the Formation of Volatile Compounds during Fine-Flavor Cacao Bean Fermentation. Foods 2022, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, C.; Van De Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of Cocoa Beans: Fine-Scale Monitoring of the Starter Cultures Applied and Metabolomics of the Fermentation and Drying Steps. Front. Microbiol. 2021, 11, 616875. [Google Scholar] [CrossRef]
- Jinap, S.; Wan Rosli, W.I.; Russly, A.R.; Nordin, L.M. Effect of Roasting Time and Temperature on Volatile Component Profiles during Nib Roasting of Cocoa Beans (Theobroma cacao). J. Sci. Food Agric. 1998, 77, 441–448. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Pacheco-Montealegre, M.E.; Dávila-Mora, L.L.; Botero-Rute, L.M.; Reyes, A.; Caro-Quintero, A. Fine Resolution Analysis of Microbial Communities Provides Insights Into the Variability of Cocoa Bean Fermentation. Front. Microbiol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Lasisi, D. A Comparative Study of Effects of Drying Methods on Quality of Cocoa Beans. Int. J. Eng. Res. 2014, 3, 991–996. [Google Scholar]
- Jinap, S.; Thien, J.; Yap, T.N. Effect of Drying on Acidity and Volatile Fatty Acids Content of Cocoa Beans. J. Sci. Food Agric. 1994, 65, 67–75. [Google Scholar] [CrossRef]
- Wood, G.A.; Lass, R.A. Cocoa, 4th ed; Blackwell: Oxford, UK, 2008; ISBN 978-0-632-06398-7. [Google Scholar]
- Frauendorfer, F.; Schieberle, P. Key Aroma Compounds in Fermented Forastero Cocoa Beans and Changes Induced by Roasting. Eur. Food Res. Technol. 2019, 245, 1907–1915. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Changes in Key Aroma Compounds of Criollo Cocoa Beans during Roasting. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef]
- Ozturk, G.; Young, G.M. Food Evolution: The Impact of Society and Science on the Fermentation of Cocoa Beans. Compr. Rev. Food Sci. Food Saf. 2017, 16, 431–455. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]


| Fermentation Variation | Total Runs 1 per Location | Device 2 | Pre-Drying on Day 1 | Turning on Day 2 | Drying Temperature 3 | Fermentation Time 4 |
|---|---|---|---|---|---|---|
| Wt (control) | A: n = 6, E: n = 7 | Wooden Box | without | with 5 | low | d1–d4 |
| J | A: n = 3, E: n = 4 | Plastic + Jute | without | without | low | d1–d4 |
| Jp | A: n = 3, E: n = 6 | Plastic + Jute | with | without | low | d1–d4 |
| JpT | A: n = 3 | Plastic + Jute | with | without | high | d1–d4 |
| JpsT | A: n = 3 | Plastic + Jute | with | without | high | d1–d3 |
| Jpt | A: n = 3, E: n = 5 | Plastic + Jute | with | with | low | d1–d4 |
| P | E: n = 2 | Plastic + Plastic | without | without | low | d1–d4 |
| Pp | E: n = 4 | Plastic + Plastic | with | without | low | d1–d4 |
| PpT | A: n = 3 | Plastic + Plastic | with | without | high | d1–d4 |
| Attribute | Definition | Scale |
|---|---|---|
| Acidic | Basic taste, triggered, e.g., by citric, acetic, or lactic acid solution | Very weak–very strong |
| Bitter | Basic taste, triggered, e.g., by caffeine solution | |
| Fruity-Citrus | Aroma reminiscent of citrus fruits such as lemon or orange | |
| Fruity-Banana | Aroma reminiscent of banana (dried) | |
| Floral | Aroma reminiscent of dried flowers (hay flower), tea (orange blossom), or orange blossom soap | |
| Nutty | Aroma reminiscent of unroasted nuts with or without skins as well as raw cocoa bean (without pungent acidity) | |
| Woody | Aroma and mouthfeel reminiscent of wet wood (wooden ice cream sticks) | |
| Spicy | Aroma reminiscent of pepper | |
| Cocoa | Aroma reminiscent of dark chocolate | |
| Roasted-burnt | Aroma reminiscent of toasted/slightly burnt cacao nibs or burnt bread | Not roasted–roasted-burnt |
| Earthy | Aroma that is musty, reminiscent of wet earth | Very weak–very strong |
| Astringent | Dry, rough, or furry mouthfeel on the tongue and palate, triggered, e.g., by alum or tannin solution |
| Location/Samples | Tmax during Drying 1 | Tav during Drying 2 |
|---|---|---|
| A | 95.2 ± 13.7 °C | 64.3 ± 14.7 °C |
| E | 74.7 ± 6.5 °C | 48.0 ± 4.2 °C |
| A_Wt, J, Jp, Jpt | 94.5 ± 15.3 °C | 59.0 ± 12.8 °C |
| A_PpT, JpT, JpsT | 96.3 ± 11.3 °C | 73.2 ± 13.8 °C |
| Location | Variation | Saccharose | Glucose | Fructose | Citric Acid | Lactic Acid | Acetic Acid |
|---|---|---|---|---|---|---|---|
| A | Wt | 6.1 ± 2.9 | 2.4 ± 0.8 | 7.5 ± 2.6 | 3.3 ± 0.2 | 1 ± 0.1 | 14.8 ± 3.4 |
| J | 10.1 ± 0.6 | 2.5 ± 0.8 | 5.5 ± 1 | 3.5 ± 0.0 | 2 ± 0.7 | 15.7 ± 1.6 | |
| Jp | 17.4 | 0.9 | 1.2 | 3.5 | 1.2 | 3.3 | |
| Jpt | 15.3 ± 1.0 | 2.3 ± 1.1 | 5.9 ± 1.9 | 3.6 ± 0.4 | 1 ± 0.1 | 7.6 ± 1.2 | |
| JpT | 13.8 ± 2.2 | 1.1 ± 1.1 | 3.3 ± 2.1 | 3.8 ± 0.6 | 2 ± 0.6 | 9.8 ± 2.9 | |
| PpT | 17.5 ± 3.5 | 1.6 ± 0.2 | 5.3 ± 0.1 | 4.1 ± 0.4 | 1.4 ± 0.2 | 12.9 ± 3.9 | |
| JpsT | 18.4 ± 1.2 | 1.8 ± 0.2 | 3.3 ± 1.3 | 5.2 ± 0.6 | 2.2 ± 0.2 | 10.1 ± 6.3 | |
| E | Wt | 1.9 ± 1.9 | 0.7 ± 0.7 | 5.2 ± 5.2 | 13.5 ± 4.4 | 1.9 ± 0.7 | 36.6 ± 12.7 |
| Jp | 8.6 ± 2.2 | 3.3 ± 0.5 | 8.2 ± 0.9 | 15 ± 7.1 | 2.7 ± 1.4 | 24.1 ± 6.2 | |
| Jpt | 6.2 ± 2.1 | 3.4 ± 0.4 | 7.8 ± 1.4 | 17.9 ± 2.5 | 2.6 ± 1.3 | 35 ± 0.6 | |
| P | 14.2 ± 0.8 | 1.7 ± 0.8 | 8.8 ± 1.5 | 21.2 ± 2.6 | 8.6 ± 1.6 | 27.9 ± 9.6 | |
| Pp | 7 ± 1.2 | 2.9 ± 0.0 | 8.1 ± 1.4 | 19.8 ± 0.1 | 4.8 ± 0.1 | 40.3 ± 5.8 |
| Location | Variation | Well-Fermented | Slightly Fermented | Violet | Slaty | Moldy |
|---|---|---|---|---|---|---|
| A | Wt | 62.7 ± 27 | 30.5 ± 27 | 6.2 ± 7 | 0.7 ± 1.1 | 0 |
| J | 62.7 ± 32.4 | 24.3 ± 22.9 | 12 ± 9.8 | 1 ± 1.4 | 0 | |
| Jp | 55.3 ± 29 | 27.7 ± 17.1 | 10 ± 13.4 | 3.7 ± 2.6 | 0 | |
| Jpt | 72.3 ± 20.2 | 20 ± 13.6 | 7.3 ± 7.1 | 0.3 ± 0.5 | 0 | |
| JpT | 56 ± 23.3 | 37.3 ± 16.4 | 6 ± 7.1 | 0.7 ± 0.9 | 0 | |
| PpT | 61 ± 28.6 | 33 ± 25.2 | 3 ± 4.2 | 0.3 ± 0.5 | 0 | |
| JpsT | 39 ± 29.7 | 53.3 ± 25 | 7.3 ± 9 | 0.3 ± 0.5 | 0 | |
| E | Wt | 34.4 ± 17.7 | 48.4 ± 19.4 | 16 ± 12.1 | 0.3 ± 0.5 | 0.1 ± 0.3 |
| J | 28 ± 9.1 | 51.8 ± 12.8 | 18.8 ± 14.5 | 1 ± 1.7 | 0.3 ± 0.4 | |
| Jp | 31.5 ± 12.6 | 53 ± 15.8 | 15.3 ± 13.1 | 0.2 ± 0.4 | 0 | |
| Jpt | 49.4 ± 12.7 | 39.8 ± 16.3 | 10 ± 5.8 | 0.4 ± 0.5 | 0.2 ± 0.4 | |
| P | 42.5 ± 24.5 | 41 ± 12 | 15.5 ± 11.5 | 0.5 ± 0.5 | 0.5 ± 0.5 | |
| Pp | 30.3 ± 5.4 | 57.3 ± 11.4 | 12.3 ± 8.8 | 0.5 ± 0.9 | 0 |
| Location | Variations | Acidity | Bitterness | Fruity, Citrus | Fruity, Banana | Floral | Nutty | Woody | Spicy | Cocoa | Roasted-Burnt | Earthy | Astringency |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.001 | <0.001 | <0.001 | 0.007 | 0.013 | 0.272 | <0.001 | 0.015 | 0.001 | <0.001 | <0.001 | <0.001 | ||
| A | Wt | 7.3 ab | 4.6 cde | 4.3 a | 1.1 a | 2.4 a | 3.2 | 2.1 cd | 3.1 a | 2.4 ab | 4.0 ab | 1.2 c | 4.4 bcde |
| J | 5.7 bc | 4.3 cde | 3.6 ab | 1.4 a | 2.2 a | 3 | 2.2 cd | 2.6 a | 2.8 ab | 4.6 ab | 1.4 bc | 5.1 bcde | |
| Jp | 1.6 d | 5.9 abc | 0.6 d | 0.9 a | 2.2 a | 4.2 | 4.4 ab | 1.7 a | 3.3 ab | 4.5 ab | 2.5 abc | 5.8 abc | |
| Jpt | 2.5 d | 5.8 abcd | 1.0 cd | 0.9 a | 2.8 a | 3.9 | 3.4 bc | 1.6 a | 3.7 a | 4.0 ab | 2.2 abc | 5.5 abcd | |
| JpT | 2.1 d | 6.6 ab | 0.7 d | 1.4 a | 2.3 a | 4.8 | 5.1 ab | 1.8 a | 2.4 ab | 5.0 a | 3.1 ab | 6.1 ab | |
| JpsT | 1.9 d | 7.1 a | 0.6 d | 1.1 a | 2.6 a | 4.6 | 5.4 a | 1.6 a | 2.1 b | 4.9 a | 3.8 a | 7.1 a | |
| E | Wt | 7.4 a | 4.0 de | 4.6 a | 1.5 a | 3.5 a | 3.6 | 1.2 d | 2.9 a | 2.4 ab | 3.0 b | 1.2 c | 3.5 e |
| Jp | 6.1 ab | 3.6 e | 2.8 abc | 1.3 a | 2.7 a | 3.4 | 1.9 cd | 2.3 a | 2.9 ab | 3.4 ab | 1.4 bc | 3.8 de | |
| Jpt | 6.3 ab | 4.8 bcde | 3.7 ab | 2.0 a | 3.5 a | 3.6 | 1.6 cd | 2.9 a | 2.1 b | 2.7 b | 1.3 c | 4.3 cde | |
| P | 4.3 c | 5.3 abcde | 2.3 bd | 2.4 a | 4.0 a | 3.6 | 3.4 bc | 2.3 a | 3.3 ab | 3.7 ab | 1.9 bc | 5.0 bcde | |
| Pp | 7.0 ab | 3.8 e | 4.6 a | 2.4 a | 3.7 a | 3.9 | 1.9 cd | 2.3 a | 2.7 ab | 2.8 b | 1.0 c | 3.7 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Motzer, D.; Poulose-Züst, M.; Miescher Schwenninger, S. Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory. Foods 2024, 13, 137. https://doi.org/10.3390/foods13010137
Streule S, Freimüller Leischtfeld S, Galler M, Motzer D, Poulose-Züst M, Miescher Schwenninger S. Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory. Foods. 2024; 13(1):137. https://doi.org/10.3390/foods13010137
Chicago/Turabian StyleStreule, Stefanie, Susette Freimüller Leischtfeld, Martina Galler, Dominik Motzer, Monja Poulose-Züst, and Susanne Miescher Schwenninger. 2024. "Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory" Foods 13, no. 1: 137. https://doi.org/10.3390/foods13010137
APA StyleStreule, S., Freimüller Leischtfeld, S., Galler, M., Motzer, D., Poulose-Züst, M., & Miescher Schwenninger, S. (2024). Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory. Foods, 13(1), 137. https://doi.org/10.3390/foods13010137







