Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Extract from Bergamot Juice Powder
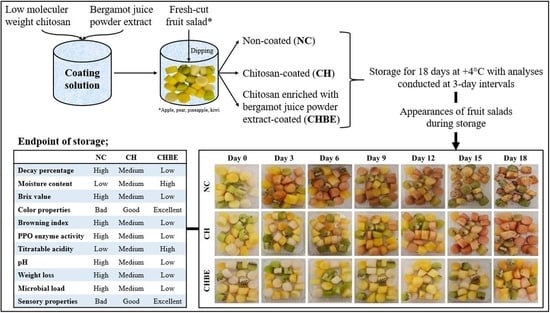
2.3. Edible Coating Application to Fresh-Cut Fruit Salad and Storage Analysis
2.3.1. Decay Percentage
2.3.2. Moisture Content
2.3.3. Total Soluble Solid Content
2.3.4. Color
2.3.5. Titratable Acidity and pH
2.3.6. Browning Index
2.3.7. Extraction and Analysis of Polyphenol Oxidase Enzyme Activity
2.3.8. Determination of Microbial Quality
2.3.9. Weight Loss
2.3.10. Sensory Evaluation
2.4. Statistical Analysis
3. Results
3.1. Decay Percentage
3.2. Moisture Content
3.3. Total Soluble Solid Content
3.4. Color
3.5. Browning Index
3.6. Polyphenol Oxidase Enzyme Activity
3.7. Titratable Acidity and pH
3.8. Microbial Quality
3.9. Weight Loss
3.10. Sensory Evaluation
4. Discussion
- Decay percentage and shelf life: The observed decay percentage provides valuable insights into the effectiveness of the chitosan coating enriched with bergamot juice powder in extending the shelf life of fresh-cut fruit salads. The lower decay percentage in the coated groups, particularly the CHBE group, highlights the preservative capabilities of the coating. This aligns with previous studies that have demonstrated the ability of chitosan-based coatings to inhibit microbial growth and delay decay in various fruits [17,38,39];
- Moisture content and weight loss: Moisture content and weight loss are crucial parameters affecting the overall quality of fresh-cut fruit. The reduced weight loss in the CHBE-coated group suggests that the bergamot juice powder extract contributes to microbial control but also aids in moisture retention. This dual functionality emphasizes the multifaceted benefits of incorporating natural extracts into edible coatings;
- Total soluble solids content, titratable acidity, and pH: Changes in total soluble solids content, titratable acidity, and pH indicate the impact of coatings on fruit maturation and flavor. The gradual increase in total soluble solids and the moderate reduction in titratable acidity in coated groups suggest a controlled and balanced maturation process. The preservation of optimal moisture levels, pH regulation, and the antimicrobial properties of the coatings likely contribute to maintaining desirable flavor profiles;
- Color and browning index: The significant reduction in the browning index and the preservation of color attributes in coated groups underscore the efficacy of chitosan coatings enriched with bergamot juice powder in mitigating enzymatic browning. This aligns with studies emphasizing the role of edible coatings in controlling color changes in various fruits [28,33];
- Polyphenol oxidase enzyme activity: The decline in polyphenol oxidase enzyme activity in the coated groups reflects the effectiveness of the coatings in inhibiting enzymatic browning reactions. This outcome is consistent with previous research showcasing the ability of chitosan-based coatings to reduce PPO activity and extend the shelf life of fresh-cut produce [40,41];
- Microbial quality: The antimicrobial properties of bergamot juice powder extract in the CHBE-coated group contributed to superior microbial inhibition throughout storage. This aligns with a growing body of evidence supporting using natural antimicrobial compounds in edible coatings to enhance the microbiological safety of fresh-cut products [1,5,32];
- Sensory evaluation: The sustained high scores in sensory evaluation, particularly in taste and overall preference, highlight the positive impact of chitosan coatings enriched with bergamot juice powder on consumer satisfaction. The absence of undesirable taste or aroma interference from bergamot’s typical odor further accentuates the bioactive potential of such coatings.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruit and vegetables by application of different edible coatings: A review. LWT-Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruit quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. Aagric. Eng. 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruit and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Rojas-Grau, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef] [PubMed]
- Demircan, B.; Ozdestan Ocak, O. The effects of ethyl lauroyl arginate and lemon essential oil added edible chitosan film coating on biogenic amines formation during storage in mackerel fillets. J. Food Process. Preserv. 2021, 45, e15454. [Google Scholar] [CrossRef]
- Demircan, B.; Velioglu, Y.S.; Giuffrè, A.M. Bergamot juice powder with high bioactive properties: Spray-drying for the preservation of antioxidant activity and ultrasound-assisted extraction for enhanced phenolic compound extraction. J. Food Sci. 2023, 88, 3694–3713. [Google Scholar] [CrossRef]
- Santana Moreira, M.; de Almeida Paula, D.; Maurício Furtado Martins, E.; Nascif Rufino Vieira, É.; Mota Ramos, A.; Stringheta, P.C. Vacuum impregnation of β-carotene and lutein in minimally processed fruit salad. J. Food Process. Preserv. 2018, 42, e13545. [Google Scholar] [CrossRef]
- Demircan, B.; Ozdestan-Ocak, O. Effects of lemon essential oil and ethyl lauroyl arginate on the physico-chemical and mechanical properties of chitosan films for mackerel fillet coating application. J. Food Meas. Charact. 2021, 15, 1499–1508. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Fai, A.E.C.; de Souza, M.R.A.; de Barros, S.T.; Bruno, N.V.; Ferreira, M.S.L.; de Andrade Gonçalves, É.C.B. Development and evaluation of biodegradable films and coatings obtained from fruit and vegetable residues applied to fresh-cut carrot (Daucus carota L.). Postharvest Biol. Technol. 2016, 112, 194–204. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Nunes, C.; Maia, A.; Ferreira, P.; Coimbra, M.A. Conditions for producing long shelf life fruit salads processed using mild pasteurization. LWT-Food Sci. Technol. 2017, 85, 316–323. [Google Scholar] [CrossRef]
- Karagoz, S.; Demirdoven, A. Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT-Food Sci. Technol. 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Lacivita, V.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Pomegranate peel powder as a food preservative in fruit salad: A sustainable approach. Foods 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Valenzuela-Soto, E.; Lizardi-Mendoza, J.; Goycoolea, F.; Martínez-Téllez, M.A.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 2009, 89, 15–23. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int. J. Biol. Macromol. 2017, 104, 821–826. [Google Scholar] [CrossRef]
- Sharma, M.; Saini, C.S. Postharvest shelf-life extension of fresh-cut guavas (Psidium guajava) using flaxseed protein-based composite coatings. Food Hydrocoll. Health 2021, 1, 100015. [Google Scholar] [CrossRef]
- Sharma, L.; Saini, C.S.; Sharma, H.K.; Sandhu, K.S. Biocomposite edible coatings based on cross linked-sesame protein and mango puree for the shelf life stability of fresh-cut mango fruit. J. Food Process. Eng. 2019, 42, e12938. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT-Food Sci. Technol. 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Donsì, F.; Marchese, E.; Maresca, P.; Pataro, G.; Vu, K.D.; Salmieri, S.; Ferrari, G. Green beans preservation by combination of a modified chitosan based-coating containing nanoemulsion of mandarin essential oil with high pressure or pulsed light processing. Postharvest Biol. Technol. 2015, 106, 21–32. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.S.; Rojas-Grau, M.A.; Carmona, A.; Rodríguez, F.J.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of alginate-and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008, 22, 1493–1503. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruit. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Nongtaodum, S.; Jangchud, A. Effects of edible chitosan coating on quality of fresh-cut mangoes (Fa-lun) during storage. Agric. Nat. Resour. 2009, 43, 282–289. [Google Scholar]
- Souza, M.P.; Vaz, A.F.; Cerqueira, M.A.; Texeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food Bioprocess Technol. 2015, 8, 647–654. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; Gonzalez-Martinez, C. Recent advances in edible coatings for fresh and minimally processed fruit. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Grau, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The use of packaging techniques to maintain freshness in fresh-cut fruit and vegetables: A review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Supapvanich, S.; Mitrsang, P.; Srinorkham, P.; Boonyaritthongchai, P.; Wongs-Aree, C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J. Food Sci. Technol. 2016, 53, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT-Food Sci. Technol. 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Jin, T.Z.; Chen, W.; Gurtler, J.B.; Fan, X. Effectiveness of edible coatings to inhibit browning and inactivate foodborne pathogens on fresh-cut apples. J Food Saf. 2020, 40, e12802. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Mahna, N. Pectin-based edible coating preserves antioxidative capacity of plum fruit during shelf life. Food Sci. Technol. 2020, 26, 583–592. [Google Scholar] [CrossRef]
- Vishwasrao, C.; Ananthanarayan, L. Delayed post-harvest ripening-associated changes in Manilkara zapota L. var. Kalipatti with composite edible coating. J. Sci. Food Agric. 2017, 97, 536–542. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut ‘Royal delicious’ apple with edible coatings and anti-browning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Del Real, A.; González-Reza, R.M.; Galindo-Pérez, M.J.; Quintanar-Guerrero, D. Fresh-cut Red Delicious apples coating using tocopherol/mucilage nanoemulsion: Effect of coating on polyphenol oxidase and pectin methylesterase activities. Food Res. Int. 2014, 62, 974–983. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Nguyen, D.H. Effects of nano-chitosan and chitosan coating on the postharvest quality, polyphenol oxidase activity and malondialdehyde content of strawberry (Fragaria ananassa Duch.). J. Hortic. Postharvest Res. 2020, 3, 11–24. [Google Scholar]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Rojas-Grau, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Emamifar, A.; Ghaderi, Z.; Ghaderi, N. Effect of salep-based edible coating enriched with grape seed extract on postharvest shelf life of fresh strawberries. J. Food Saf. 2019, 39, e12710. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT-Food Sci. Technol. 2017, 75, 210–219. [Google Scholar]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruit: A case of study with kiwifruit slices. LWT-Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Ozdemir, K.S.; Gokmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT-Food Sci. Technol. 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Motamedi, E.; Nasiri, J.; Malidarreh, T.R.; Kalantari, S.; Naghavi, M.R.; Safari, M. Performance of carnauba wax-nanoclay emulsion coatings on postharvest quality of ‘Valencia’ orange fruit. Sci. Hortic. 2018, 240, 170–178. [Google Scholar] [CrossRef]
- Gautam, S.; Lapcik, L.; Lapcikova, B.; Repka, D.; Szyk-Warszyńska, L. Physicochemical characterisation of polysaccharide films with embedded bioactive substances. Foods 2023, 12, 4454. [Google Scholar] [CrossRef] [PubMed]



| Fresh-Cut Fruit | Sample Group | Storage Time (day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | |||
| Decay percentage (%) | Apple | NC | 0 ± 0.00 | 73.33 ± 10.00 a | 86.67 ± 6.67 a | 93.33 ± 3.33 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| CH | 0 ± 0.00 | 6.67 ± 3.33 d | 20.00 ± 8.67 d | 33.33 ± 6.67 d | 40.00 ± 3.33 e | 46.67 ± 3.33 d | 53.33 ± 6.67 b | ||
| CHBE | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 6.67 ± 3.33 e | 20.00 ± 6.67 h | 26.67 ± 3.33 g | 33.33 ± 3.33 e | ||
| Pear | NC | 0 ± 0.00 | 26.67 ± 3.33 b | 73.33 ± 10.00 b | 86.67 ± 5.77 a | 93.33 ± 4.71 b | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| CH | 0 ± 0.00 | 6.67 ± 0.10 d | 13.33 ± 2.00 d | 26.67 ± 1.50 d | 33.33 ± 3.00 f | 40.00 ± 1.00 e | 46.67 ± 1.75 c | ||
| CHBE | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 6.67 ± 0.33 | 13.33 ± 4.71 h | 20.00 ± 3.06 h | 26.67 ± 3.06 ef | ||
| Pineapple | NC | 0 ± 0.00 | 0 ± 0.00 e | 40 ± 4.00 c | 46.67 ± 9.08 c | 60.00 ± 5.56 d | 73.33 ± 4.84 c | 100.00 ± 0.00 a | |
| CH | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 0 ± 0.00 e | 20.00 ± 3.33 h | 26.67 ± 0.00 g | 26.67 ± 0.00 ef | ||
| CHBE | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 0 ± 0.00 e | 6.67 ± 3.06 i | 13.33 ± 1.18 i | 13.33 ± 2.00 g | ||
| Kiwi | NC | 0 ± 0.00 | 13.33 ± 3.33 c | 46.67 ± 0.13 c | 73.33 ± 3.10 b | 80.00 ± 1.50 c | 86.67 ± 1.00 b | 100.00 ± 0.00 a | |
| CH | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 6.67 ± 0.00 e | 26.67 ± 0.33 g | 33.33 ± 1.00 f | 40.00 ± 10.16 d | ||
| CHBE | 0 ± 0.00 | 0 ± 0.00 e | 0 ± 0.00 e | 6.67 ± 0.00 e | 13.33 ± 0.67 h | 13.33 ± 4.17 i | 20.00 ± 0.50 f | ||
| Moisture content (%) | Apple | NC | 84.58 ± 1.20 f | 82.56 ± 0.90 e | 81.25 ± 0.20 f | 80.53 ± 0.74 i | 80.10 ± 0.84 g | 78.70 ± 0.10 f | 75.50 ± 1.05 g |
| CH | 88.75 ± 0.55 c | 87.67 ± 0.70 c | 87.20 ± 0.50 d | 87.13 ± 1.05 e | 87.12 ± 0.94 d | 87.11 ± 1.06 d | 86.15 ± 0.41 d | ||
| CHBE | 89.18 ± 0.85 bc | 88.69 ± 0.48 bc | 88.12 ± 0.68 cd | 87.69 ± 0.17 de | 87.50 ± 0.54 d | 87.12 ± 1.05 d | 87.05 ± 1.52 cd | ||
| Pear | NC | 86.72 ± 0.70 e | 84.39 ± 0.66 d | 83.10 ± 0.58 e | 82.52 ± 0.34 g | 81.06 ± 0.90 fg | 80.72 ± 0.78 e | 78.19 ± 0.80 f | |
| CH | 90.02 ± 0.35 ab | 89.60 ± 0.20 ab | 89.12 ± 1.05 bc | 88.59 ± 1.81 bcd | 88.10 ± 0.99 cd | 87.92 ± 0.76 cd | 87.81 ± 0.30 bc | ||
| CHBE | 90.05 ± 0.64 ab | 89.71 ± 0.48 ab | 89.52 ± 0.27 ab | 88.35 ± 0.11 cde | 88.29 ± 0.19 bcd | 88.17 ± 0.20 cd | 88.02 ± 0.58 bc | ||
| Pineapple | NC | 88.00 ± 0.41 cd | 87.62 ± 0.52 c | 86.63 ± 0.87 d | 84.58 ± 0.17 f | 83.06 ± 0.69 e | 81.92 ± 0.58 e | 80.63 ± 0.44 e | |
| CH | 90.93 ± 1.05 a | 90.78 ± 0.57 a | 90.12 ± 1.20 ab | 89.93 ± 0.65 ab | 89.59 ± 0.58 ab | 89.48 ± 0.48 ab | 89.10 ± 0.72 ab | ||
| CHBE | 91.26 ± 0.29 a | 90.82 ± 0.03 a | 90.54 ± 0.84 a | 90.17 ± 0.74 a | 90.08 ± 1.03 a | 89.99 ± 1.00 a | 89.64 ± 0.23 a | ||
| Kiwi | NC | 87.10 ± 0.58 de | 84.99 ± 0.47 d | 83.69 ± 0.10 e | 83.10 ± 0.36 g | 81.69 ± 0.90 f | 81.06 ± 0.45 e | 79.29 ± 0.25 f | |
| CH | 90.45 ± 0.59 a | 90.12 ± 1.05 a | 90.02 ± 0.80 ab | 89.64 ± 0.66 abc | 89.10 ± 0.67 abc | 88.73 ± 0.31 bc | 88.34 ± 0.74 abc | ||
| CHBE | 90.49 ± 0.57 a | 90.28 ± 0.95 a | 90.21 ± 0.58 ab | 89.91 ± 0.10 ab | 89.12 ± 0.18 abc | 89.02 ± 0.52 abc | 88.67 ± 0.70 ab | ||
| Total soluble solid content (%) | Apple | NC | 15.42 ± 0.01 a | 14.28 ± 0.03 a | 13.58 ± 0.05 a | 15.43 ± 0.01 b | 15.48 ± 0.01 c | 15.79 ± 0.02 b | 15.82 ± 0.03 b |
| CH | 12.54 ± 0.12 e | 12.57 ± 0.04 e | 13.15 ± 0.10 bc | 13.57 ± 0.08 d | 13.84 ± 0.07 e | 13.99 ± 0.03 c | 14.03 ± 0.01 c | ||
| CHBE | 11.87 ± 0.06 f | 12.03 ± 0.18 f | 12.39 ± 0.10 e | 12.81 ± 0.07 e | 12.94 ± 0.05 g | 13.02 ± 0.13 e | 13.26 ± 0.25 de | ||
| Pear | NC | 14.55 ± 0.09 b | 13.83 ± 0.10 b | 13.29 ± 0.06 b | 15.62 ± 0.14 b | 15.73 ± 0.05 b | 15.89 ± 0.02 b | 16.01 ± 0.02 b | |
| CH | 11.95 ± 0.01 f | 12.05 ± 0.01 f | 12.09 ± 0.01 f | 12.24 ± 0.06 f | 13.25 ± 0.24 f | 13.36 ± 0.08 d | 13.39 ± 0.17 d | ||
| CHBE | 11.25 ± 0.06 g | 11.49 ± 0.05 g | 11.54 ± 0.27 h | 11.89 ± 0.11 g | 12.45 ± 0.18 h | 12.60 ± 0.05 f | 13.05 ± 0.02 e | ||
| Pineapple | NC | 14.02 ± 0.01 d | 13.24 ± 0.06 d | 12.70 ± 0.07 d | 14.89 ± 0.37 c | 15.05 ± 0.18 d | 16.17 ± 0.20 a | 17.18 ± 0.14 a | |
| CH | 10.31 ± 0.05 h | 10.86 ± 0.08 i | 11.52 ± 0.17 h | 11.57 ± 0.19 h | 12.10 ± 0.20 i | 12.61 ± 0.08 f | 12.53 ± 0.03 fg | ||
| CHBE | 10.17 ± 0.01 i | 10.81 ± 0.05 i | 11.08 ± 0.06 i | 11.16 ± 0.04 i | 11.87 ± 0.14 j | 12.05 ± 0.14 g | 12.40 ± 0.05 g | ||
| Kiwi | NC | 14.36 ± 0.03 c | 13.62 ± 0.10 c | 13.03 ± 0.15 c | 16.04 ± 0.23 a | 16.09 ± 0.04 a | 16.13 ± 0.07 a | 17.05 ± 0.31 a | |
| CH | 10.36 ± 0.06 h | 11.42 ± 0.09 g | 11.87 ± 0.16 g | 12.01 ± 0.10 fg | 12.35 ± 0.02 h | 12.72 ± 0.03 f | 12.68 ± 0.05 f | ||
| CHBE | 10.28 ± 0.05 h | 11.03 ± 0.17 h | 11.29 ± 0.10 i | 11.45 ± 0.11 h | 12.01 ± 0.05 ij | 12.54 ± 0.22 f | 12.57 ± 0.04 fg | ||
| Analyte | Sample Group | Storage Time (day) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | ||
| Browning index (A420/g) | NC | 0.38 ± 0.02 a | 0.38 ± 0.03 a | 0.40 ± 0.05 a | 0.42 ± 0.01 a | 0.45 ± 0.06 a | 0.54 ± 0.02 a | 0.70 ± 0.05 a |
| CH | 0.22 ± 0.01 b | 0.25 ± 0.02 b | 0.28 ± 0.02 b | 0.30 ± 0.05 b | 0.31 ± 0.07 b | 0.35 ± 0.08 b | 0.35 ± 0.01 b | |
| CHBE | 0.19 ± 0.03 b | 0.20 ± 0.01 c | 0.23 ± 0.01 ab | 0.27 ± 0.01 b | 0.28 ± 0.02 b | 0.29 ± 0.04 b | 0.27 ± 0.01 c | |
| Polyphenol oxidase enzyme activity (U mL−1) | NC | 1.04 ± 0.10 b | 4.93 ± 0.13 a | 5.41 ± 0.04 a | 6.93 ± 0.15 a | 10.57 ± 0.10 a | 13.87 ± 0.05 a | 19.48 ± 0.04 a |
| CH | 3.47 ± 0.04 a | 2.56 ± 0.10 b | 1.89 ± 0.16 b | 1.77 ± 0.10 b | 1.03 ± 0.05 b | 0.94 ± 0.07 b | 0.85 ± 0.03 b | |
| CHBE | 3.46 ± 0.12 a | 2.12 ± 0.11 c | 1.07 ± 0.04 c | 1.00 ± 0.01 c | 0.82 ± 0.01 c | 0.69 ± 0.13 c | 0.57 ± 0.08 c | |
| Titratable acidity (%) | NC | 1.57 ± 0.02 a | 1.33 ± 0.30 b | 1.30 ± 0.10 a | 1.27 ± 0.04 b | 0.87 ± 0.07 b | 0.50 ± 0.09 c | 0.45 ± 0.10 c |
| CH | 1.50 ± 0.05 a | 1.47 ± 0.02 a | 1.46 ± 0.11 a | 1.45 ± 0.10 a | 1.43 ± 0.03 a | 1.20 ± 0.04 b | 1.11 ± 0.05 b | |
| CHBE | 1.48 ± 0.20 a | 1.50 ± 0.10 a | 1.46 ± 0.07 a | 1.40 ± 0.06 ab | 1.37 ± 0.13 a | 1.35 ± 0.04 a | 1.33 ± 0.07 a | |
| pH | NC | 3.63 ± 0.10 a | 3.65 ± 0.11 a | 3.66 ± 0.08 a | 3.85 ± 0.09 a | 3.86 ± 0.75 a | 3.95 ± 0.70 a | 4.25 ± 0.06 a |
| CH | 3.68 ± 0.10 a | 3.70 ± 0.08 a | 3.71 ± 0.05 a | 3.71 ± 0.33 a | 3.74 ± 0.42 a | 3.76 ± 0.61 a | 3.77 ± 0.50 a | |
| CHBE | 3.67 ± 0.15 a | 3.68 ± 0.50 a | 3.69 ± 0.40 a | 3.69 ± 0.22 a | 3.71 ± 0.11 a | 3.72 ± 0.07 a | 3.73 ± 0.05 a | |
| Weight loss (%) | NC | 0 ± 0.00 | 1.75 ± 0.01 a | 3.85 ± 0.03 a | 4.64 ± 0.05 a | 5.10 ± 0.05 a | 6.43 ± 0.04 a | 6.61 ± 0.06 a |
| CH | 0 ± 0.00 | 0.36 ± 0.02 b | 0.58 ± 0.01 b | 0.96 ± 0.05 b | 1.74 ± 0.01 b | 2.30 ± 0.08 b | 2.45 ± 0.07 b | |
| CHBE | 0 ± 0.00 | 0.31 ± 0.01 c | 0.43 ± 0.02 c | 0.80 ± 0.03 c | 1.54 ± 0.04 c | 1.78 ± 0.03 c | 2.10 ± 0.02 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demircan, B.; Velioglu, Y.S. Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder. Foods 2024, 13, 147. https://doi.org/10.3390/foods13010147
Demircan B, Velioglu YS. Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder. Foods. 2024; 13(1):147. https://doi.org/10.3390/foods13010147
Chicago/Turabian StyleDemircan, Bahar, and Yakup Sedat Velioglu. 2024. "Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder" Foods 13, no. 1: 147. https://doi.org/10.3390/foods13010147