Effects of High-Pressure Homogenization Treatment on the Development of Antioxidant Zanthoxylum bungeanum Leaf Powder Films for Preservation of Fresh-Cut Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zanthoxylum bungeanum Leaf-Based Films
2.3. Characterization of the Films
2.3.1. Film Thickness
2.3.2. Mechanical Property Measurement
2.3.3. Color Measurement
2.3.4. Transparency Measurement
2.3.5. Water Vapor Permeability
2.3.6. Infrared Spectral Analysis
2.3.7. Scanning Electron Microscopy
2.3.8. Water Contact Angle
2.3.9. DPPH Free Radical Scavenging Ability Measurement
2.3.10. ABTS Cation Radical Scavenging Capacity Determination
2.3.11. Ferrous Ion Reducing Power Determination
2.4. Application of ZBL Film in Freshly Cut Apple Packaging
2.4.1. Sample Preparation
2.4.2. Weight Loss Rate
2.4.3. Determination of Browning Index, Hardness, Total Soluble Solids, Titratable Acid Content, and pH
Browning Index (BI) Determination
Hardness Determination
Total Soluble Solid Content Determination
Titratable Acid Content Determination
pH Determination
2.5. Statistical Analysis
3. Results and Analysis
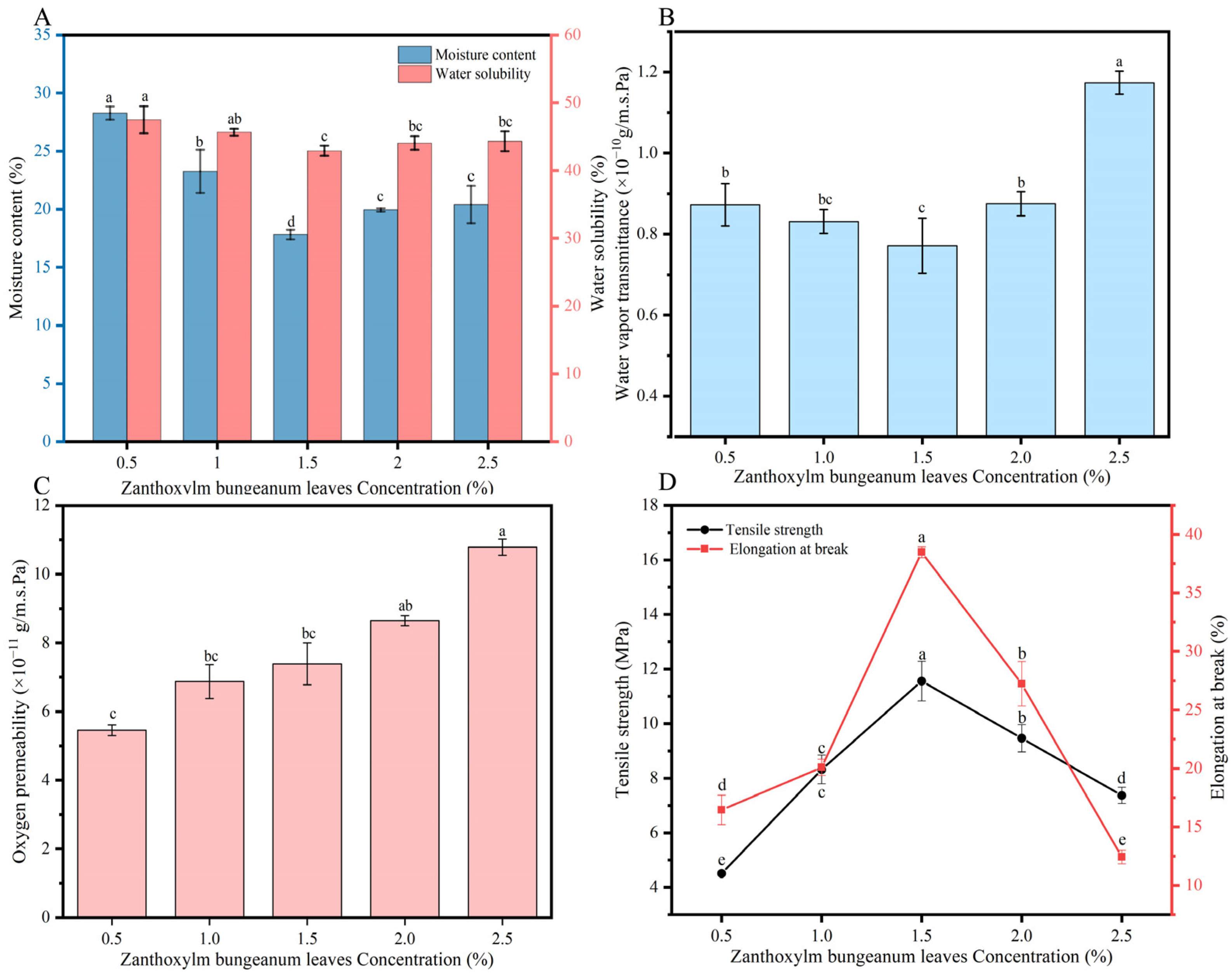
3.1. Screening of the Amount of ZBL Powder Added
3.1.1. Influence of the Amount of ZBL Powder Added on the Thickness and Optical Properties of ZBL Film
3.1.2. Influence of the Amount of ZBL Powder Added on the Moisture Content (MC) and Water Solubility (WS)
3.1.3. Influence of the Amount of ZBL Powder Added on the Water Vapor Permeability (WVP)
3.1.4. Influence of the Amount of ZBL Powder Addition on the Oxygen Permeability
3.1.5. Influence of the Amount of Zanthoxylum bungeanum Leaf Powder Added on the Mechanical Properties of ZBL Film
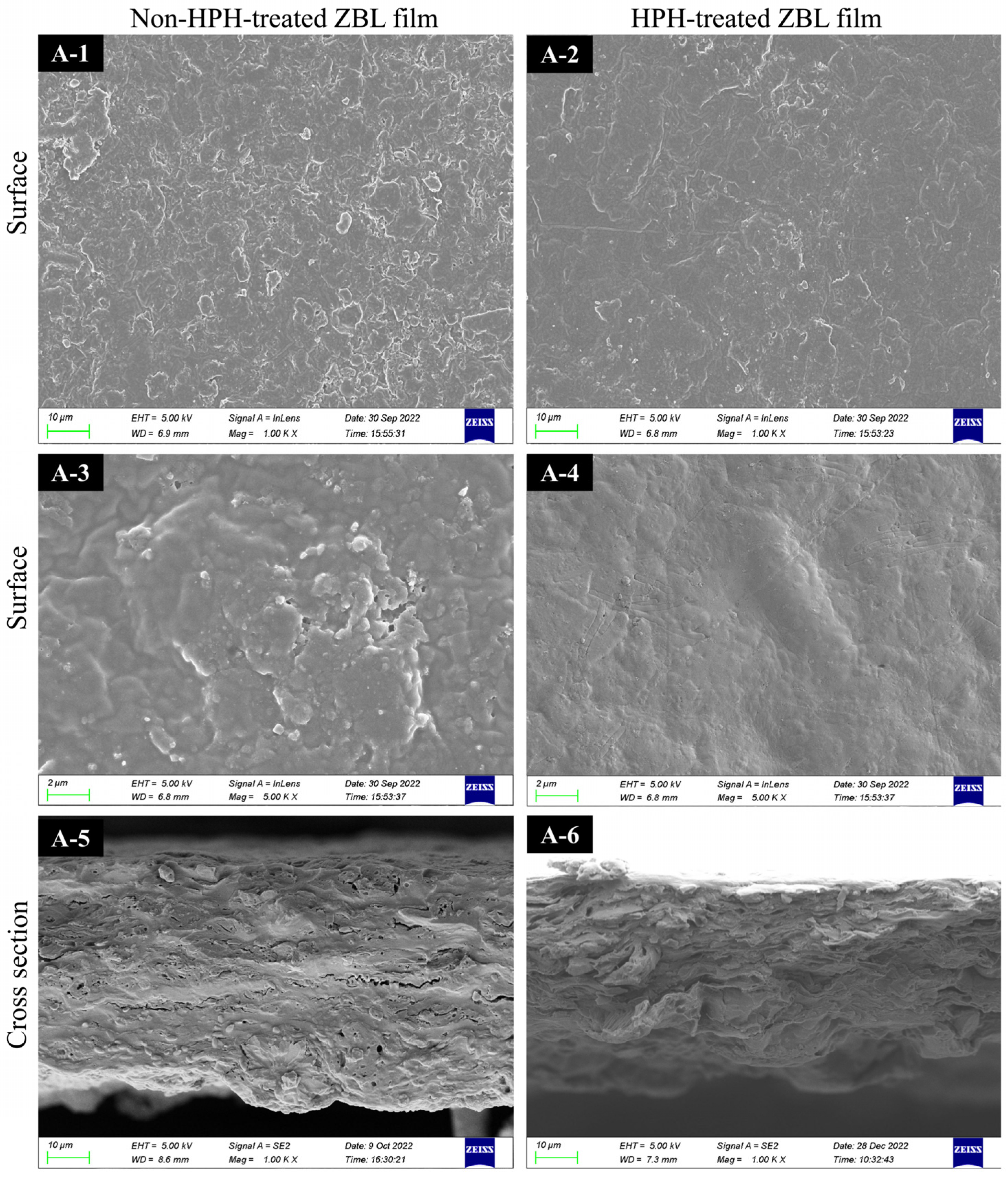
3.2. Influence of HPH on the Characteristics of Zanthoxylum bungeanum Leaf-Based Films
3.2.1. Influence of HPH Treatment on Morphology, Thickness, and Optical Characteristics
3.2.2. Enhancement of Mechanical and Water Barrier Properties of ZBL Film with HPH Treatment
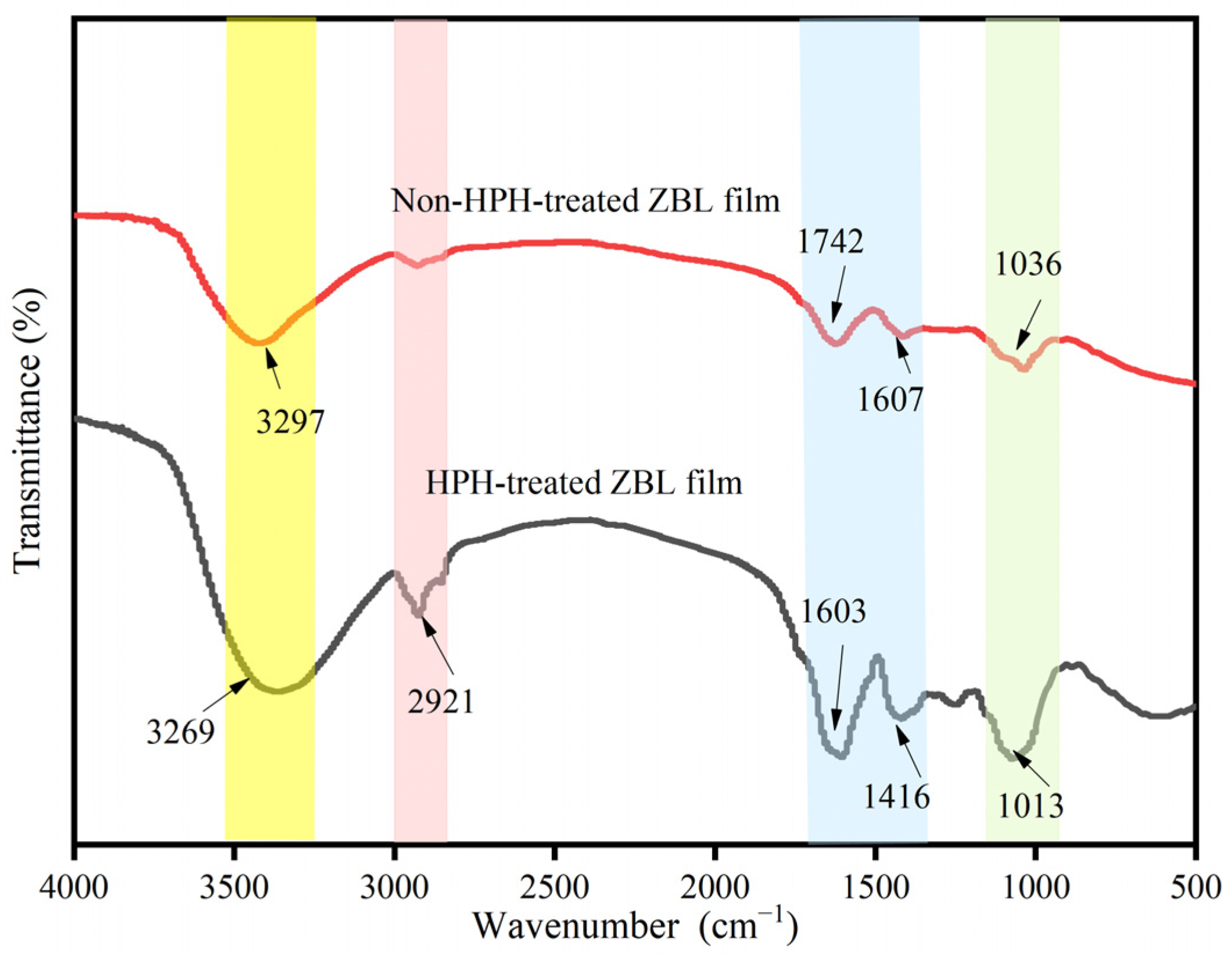
3.2.3. Infrared Spectral Analysis of HPH-Treated ZBL Active Film
3.2.4. Thermogravimetric Analysis of ZBL Active Film Treated by High Pressure Homogenization
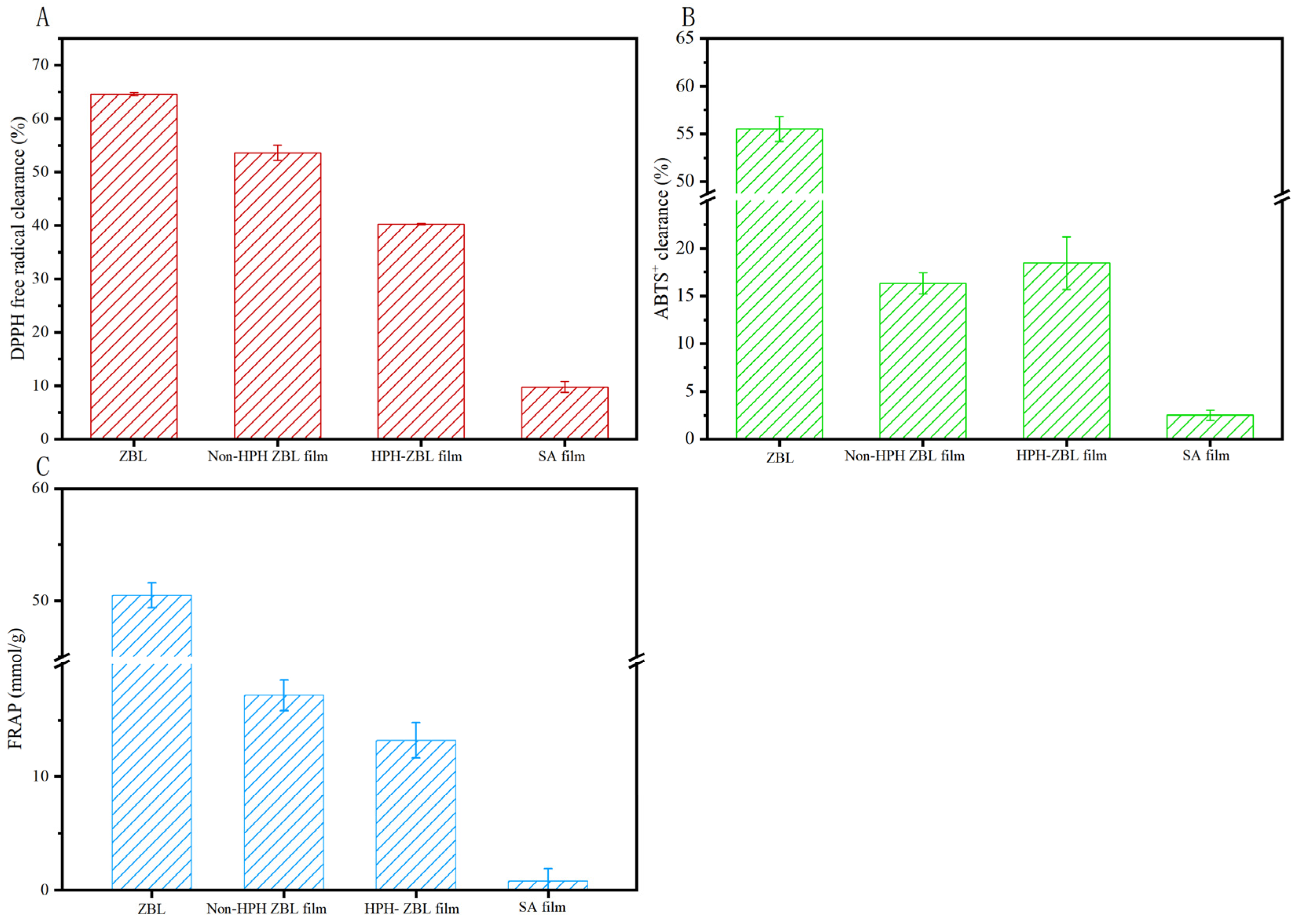
3.2.5. Antioxidant Analysis of HPH-ZBL Film
3.3. The Effects of Zanthoxylum bungeanum Leaf-Based Films on the Preservation of Freshly Cut Apples
3.3.1. Weight Loss Rate
3.3.2. Hardness
3.3.3. Browning
3.3.4. Soluble Solids Content
3.3.5. Titratable Acidity
3.3.6. pH Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamed, A.; Solmaz, P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 7, 108–117. [Google Scholar]
- Caio, G.; Roberto, J.; Henriette, M.; Marcos, V.; Márcia, R.; Moura, L.; Tara, H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar]
- Mathiazhagan, S.; Periasamy, V.; Vadivel, A. Ecofriendly antimicrobial Acalypha indica leaf extract immobilized polycaprolactone nanofibrous mat for food package applications. J. Food Process. Preserv. 2021, 45, 122–128. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.Y.J.; Mirhosseini, H.; Abas, F.; Hussain, N.; Hedayatnia, S.; Chong, H.L.F. Antioxidant activity enhancement of biodegradable film as active packaging utilizing crude extract from durian leaf waste. Food Control 2018, 8, 56–69. [Google Scholar]
- Novita, I.; Miete, C.; Jelle, V.; Raymond, P.; Ann, V.; Marc, H. Functionalization of pectin-depleted residue from different citrus by-products by high pressure homogenization. Food Hydrocoll. 2022, 3, 34–47. [Google Scholar]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crops Prod. 2020, 56, 182–197. [Google Scholar] [CrossRef]
- Bin, W.; Jie, S.; Bin, Y. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2020, 44, 57–69. [Google Scholar]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film withefficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Mariah, A.; Amauri, R. High pressure homogenization applied to fruit juices: Effects on microbial inactivation and on maintenance of bioactive components. Food Sci. Technol. Int. 2022, 4, 121–132. [Google Scholar]
- Zhou, L.; Guan, Y.; Bi, J.; Liu, X.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Change of the rheological properties of mango juice by high pressurehomogenization. LWT—Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Shi, X.; Xia, X.; He, Y.; Zhu, Y.; Xie, T.; Liu, T.; Xu, X.; Luo, X. Comparison of chemical constituents in diverse zanthoxylum herbs, and evaluation of their relative antibacterial and nematicidal activity. Food Biosci. 2021, 42, 55–67. [Google Scholar] [CrossRef]
- Lei, Y.; Zhu, R.; Zhao, M.; Wu, H.; Zhang, Z.; Shen, G.; Li, S. Preparation and characteiization of the edible films based on pomelo peel. Acta Agric. Nucleatae Sin. 2019, 33, 290–296. [Google Scholar]
- GB/T 6672-2001; National Standard of the People Republic of China—Mechanical Method for Determination of Thickness of Plastic Films and Sheets. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2001; pp. 1–5.
- GB/T 1040.3-2006; National Standard of the People Republic of China—Testing of Tensile Properties of Plastics Part 3: Testing of Films and Sheets. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2006; pp. 2–7.
- Mehran, G.; Nahal, A.; Ronak, F.; Saeedeh, S.; Behnam, K.; Marlene, J.; Ramn, K. Physical mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar]
- GB/T 2410-2008; National Standard of the People Republic of China for Determination of Transmittance and Haze of Transparent Plastics. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008; pp. 1–6.
- GB/T 1038-2000; National Standard of the People Republic of China Gas Permeability Test Method for Plastic Films and Sheets. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2000; pp. 3–6.
- GB/T 1037-1988; National Standard of the People Republic of China Test Method for Water Vapor Permeability of Plastic Films and Sheets. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008; pp. 1–4.
- Wu, H.; Xiao, D.; Lu, J.; Jiao, C.; Li, S.; Liu, D.; Wang, J.; Zhang, Z.; Liu, Y.; Shen, G.; et al. Effect of high-pressure homogenization on microstructure and properties of pomelo peel flour film-forming dispersions and their resultant films. Food Hydrocoll. 2020, 102, 105–118. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties ofchitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Hossein, H.; Syméon, B.; Francesco, B.; Riccardo, D.; Elisa, B.; Frank, P.; Heinz, W.; Fabio, L.; Andrea, P. Comprehensive characterization of activechitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar]
- Sara, B. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Hadi, F.; Nooshin, N.; Mahdi, H.; Mohammad, F.; Hossein, S.; Véronique, C. Antioxidant and antimicrobial properties of carbohydrate-based films enriched with cinnamon essential oil by Pickering emulsion method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar]
- Mehmet, M.; Derya, A. Antioxidant effect of essential oils of rosemary, clove and cinnamonon hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar]
- Matthew, R.; Danielle, M.; Mark, A.; Mitchell, P.; Bradley, W. Browning Index of Anthocyanin-Rich Fruit Juice Depends on pH and Anthocyanin Loss More Than the Gain of Soluble Polymeric Pigments. J. Food Sci. 2018, 83, 113–121. [Google Scholar]
- Huang, X.; Song, S.; Li, Y. Effect of hardness on the mechanical properties of kiwifruit peel and flesh. Int. J. Food Prop. 2022, 25, 21–34. [Google Scholar] [CrossRef]
- Tomala, K.; Guzek, D.; Głąbska, D.; Małachowska, M.; Widłak, Ł.; Krupa, T.; Gutkowska, K. Maintaining the Quality of ‘Red Jonaprince’ Apples during Storage by 1-Methylcyclopropene Preharvest and Postharvest Treatment. Agriculture 2022, 12, 1189. [Google Scholar] [CrossRef]
- Du, T.; Li, X.; Wang, S.; Su, Z.; Sun, H.; Wang, J.; Zhang, W. Phytochemicals-based edible coating for photodynamic preservation of fresh-cut apples. Food Res. Int. 2023, 163, 112293. [Google Scholar] [CrossRef] [PubMed]
- Lambertus, A.; Rutger, J.; Frans, H.; Carmen, G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar]
- Chang, X.; Hou, Y.; Liu, Q.; Hu, Z.; Xie, Q.; Shan, Y.; Li, G.; Ding, S. Physicochemical and antimicrobial properties of chitosancomposite films incorporated with glycerol monolaurate and nano-TiO2. Food Hydrocoll. 2021, 119, 106846. [Google Scholar] [CrossRef]
- Mahdiyar, S.; Mahsa, M.; Asgar, F. Physical modification of starch by high-pressure homogenization for improving functional properties of κ-carrageenan/starch blend film. Food Hydrocoll. 2018, 85, 204–214. [Google Scholar]
- Purba, P.; Pulak, D.; Laxmikant, S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochemistry 2017, 36, 11–19. [Google Scholar]
- Moufida, C.; Khaoula, E.; Sarra, A.; Bouthina, B.; Mariam, F.; Ahlen, C.; Monia, E.; Tanmay, S.; Monhammad, A.; Maksim, R.; et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants 2022, 11, 2095–2096. [Google Scholar]
- Chen, S.; Zhang, Z.; Wei, X.; Sui, Z.; Geng, J.; Xiao, J.; Huang, D. Antibacterial and antioxidant water-degradable food packaging chitosan film prepared from American cockroach. Food Biosci. 2022, 49, 559–567. [Google Scholar] [CrossRef]
- Zahra, A.; Mohammad, M.; Vahid, B.; Zahra, G.; Maryam, M.; Ali, E. Physico-chemical and antimicrobial characteristics of novel biodegradable films based on gellan and carboxymethyl cellulose containing rosemary essential oil. Int. J. Biol. Macromol. 2022, 234, 101–112. [Google Scholar]
- Liu, J.; Song, F.; Chen, R.; Deng, G.; Chao, Y.; Yang, Z.; Wu, H.; Bei, M.; Zhang, P.; Hu, Y. Effect of cellulose nanocrystal-stabilized cinnamon essential oil Pickering emulsions on structure and properties of chitosan composite films. Carbohydr. Polym. 2022, 275, 118704. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wu, H.; Jiao, C.; Yao, J.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidantand antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated withtea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Furkan, T.; Serpil, T.; Osman, G.; Sadettin, T. High pressure homogenization of mechanically deboned chicken meat protein suspensions to improve mechanical and barrier properties of edible films. Food Hydrocoll. 2018, 84, 135–145. [Google Scholar]
- Roberta, M.; Mariana, S.; Édira, A. Development and Characterization of Edible Films Based on Fruit and Vegetable Residues. J. Food Sci. 2016, 81, E412–E418. [Google Scholar]
- Liu, Y.; Liu, A.; Ibrahim, S.; Yang, H.; Huang, W. Isolation and characterization of microcrystalline cellulose frompomelo peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, S.; Chen, S.; Wang, C.; Liu, D.; Li, X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020, 159, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.; Ian, T.; Hannah, K.; Konstantinos, G.; Fotios, S.; Pauio, S. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar]
- Zhong, T.; Huang, R.; Sui, S.; Lian, Z.; Sun, X.; Wan, A.; Li, H. Effects of ultrasound treatment on lipid self-association and properties of methylcellulose/stearic acid blending films. Carbohydr. Polym. 2015, 131, 415–423. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive review of polysaccharide-based materials in edible packaging: A sustainable approach. Foods. 2021, 10, 1845. [Google Scholar] [CrossRef]
- Wang, C.; Han, F.; Chen, X.; Zhao, A.; Wang, D. Time-series based metabolomics reveals the characteristics of the color-related metabolites during the different coloration stages of Zanthoxylum bungeanum peel. Food Res. Int. 2022, 155, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Hu, Z.; Li, G.; Hu, L.; Chen, X.; Hu, Y. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. Int. J. Biol. 2021, 189, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, W.; Qi, J.; Qi, J.; Huang, Q.; Xioa, J. Oregano essential oil loaded soybean polysaccharide films: Effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll. 2019, 87, 165–172. [Google Scholar] [CrossRef]
- Moreira, M.; Álvarez, M.; Martin-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments and pectin edible coatings on the quality of fresh-cut apples: A hurdle technology approach. J. Sci. Food Agr. 2017, 97, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qing, Y.; Qiang, L.; Du, G.; Shi, K.; Tang, J.; Yan, X.; Chen, H.; Yue, Y.; He, Y.; et al. Improving microbiological and physicochemical properties of fresh-cut apples using carvacrol emulsions. Food Biosci. 2023, 52, 45–55. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Liu, Y.; Song, Y.; Wu, T.; Zhang, M. Using soy protein SiOx nanocomposite film coating to extend the shelf life of apple fruit. Int. J. Food Sci. Technol. 2017, 52, 119–128. [Google Scholar] [CrossRef]
- Constantions, E.; Aris, E.; Dimitrios, M.; Eleni, K.; Stavros, G.; Christina, G.; Areti, L.; Apostolos, A.; Anna, K.; Learda, A.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 48–60. [Google Scholar]
- Predrag, P.; Shahin, R.; Ralf, G.; Daniel, G.; Alaa, E.; Danijela, B. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419. [Google Scholar]
- Meng, F.; Zhang, C.; Li, J.; Wang, L. Self-assembling crystals of an extract of Flos Sophorae Immaturus for improvingtheantioxidant, mechanical and barrier properties of a cassia gum film. Int. J. Biol. Macromol. Struct. Funct. Interact. 2021, 167, 207–216. [Google Scholar]
- Li, X.; Shen, D.; Mao, J.; Zhang, L.; Mao, J.; Chen, B.; Bi, Y. Increased weight loss and internal air space, degraded starch and pectin combined to cause pulp mealiness in ‘Oregon Spur Ⅱ’ apples during ambient storage. Sci. Hortic. 2024, 324, 325–336. [Google Scholar] [CrossRef]



| Zanthoxylum bungeanum Leaf | Content |
|---|---|
| Moisture content | 2.09 ± 0.13% |
| Lipid | 1.47 ± 0.05% |
| Protein | 19.98 ± 0.25% |
| Crude polysaccharide | 7.33 ± 0.77% |
| Ash content | 46.03 ± 1.11% |
| Total dietary fiber | 20.48 ± 0.06 mg/g |
| Total phenol | 26.82 ± 0.26 mg/g |
| Flavone | 12.28 ± 0.22% |
| ZBL Concentration (%) | Thickness (μm) | L | a | b | ΔE | T (%) |
|---|---|---|---|---|---|---|
| 0.5 | 75 ± 0.01 e | 48.14 ± 0.12 a | 13.47 ± 0.29 c | 66.66 ± 0.34 a | - | 65.22 ± 1.08 a |
| 1.0 | 84 ± 0.00 d | 40.61 ± 3.61 b | 16.96 ± 1.78 b | 65.10 ± 1.93 a | 35.71 ± 0.38 d | 53.18 ± 0.89 b |
| 1.5 | 89 ± 0.00 c | 31.16 ± 1.31 c | 19.89 ± 0.66 a | 52.83 ± 2.11 b | 260.4 ± 0.79 c | 39.48 ± 1.74 c |
| 2.0 | 95 ± 0.00 b | 28.28 ± 1.01 d | 18.79 ± 0.22 a | 48.09 ± 1.66 c | 383.8 ± 0.21 b | 29.94 ± 0.44 d |
| 2.5 | 112 ± 0.00 a | 17.10 ± 0.88 e | 13.78 ± 0.12 c | 29.42 ± 1.09 d | 1175 ± 0.73 a | 19.90 ± 0.56 e |
| Film Type | Non-HPH-Treated ZBL Film | HPH-Treated ZBL Film |
|---|---|---|
| TS (MPa) | 4.61 ± 0.13 | 12.13 ± 0.14 |
| EAB (%) | 21.25 ± 0.88 | 42.86 ± 0.66 |
| WVP (×10−10 g/m·s·Pa) | 0.99 ± 0.02 | 0.80 ± 0.01 |
| T (%) | 25.36 ± 0.48 | 38.50 ± 0.98 |
| MC (%) | 29.60 ± 0.65 | 23.32 ± 0.62 |
| WS (%) | 35.22 ± 0.15 | 41.27 ± 1.27 |
| L | 30.67 ± 1.09 | 31.10 ± 1.16 |
| a | 23.12 ± 0.12 | 24.19 ± 0.4 |
| b | 52.16 ± 1.76 | 52.92 ± 1.91 |
| ΔE | - | 0.96 ± 0.79 |
| Water contact Angle |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, F.; Chen, R.; Ma, Z.; Wu, H.; Zhang, Z.; Yin, S.; Zhou, M. Effects of High-Pressure Homogenization Treatment on the Development of Antioxidant Zanthoxylum bungeanum Leaf Powder Films for Preservation of Fresh-Cut Apple. Foods 2024, 13, 22. https://doi.org/10.3390/foods13010022
Li F, Zhang F, Chen R, Ma Z, Wu H, Zhang Z, Yin S, Zhou M. Effects of High-Pressure Homogenization Treatment on the Development of Antioxidant Zanthoxylum bungeanum Leaf Powder Films for Preservation of Fresh-Cut Apple. Foods. 2024; 13(1):22. https://doi.org/10.3390/foods13010022
Chicago/Turabian StyleLi, Fuli, Fan Zhang, Ruixian Chen, Zexiang Ma, Hejun Wu, Zhiqing Zhang, Shutao Yin, and Man Zhou. 2024. "Effects of High-Pressure Homogenization Treatment on the Development of Antioxidant Zanthoxylum bungeanum Leaf Powder Films for Preservation of Fresh-Cut Apple" Foods 13, no. 1: 22. https://doi.org/10.3390/foods13010022





