Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Antioxidant Capacity of Resveratrol
2.3.1. DPPH Free Radical Scavenging Activity
2.3.2. Ferric-Reducing Antioxidant Power (FRAP)
2.3.3. Oxygen Radical Absorbance Capacity (ORAC)
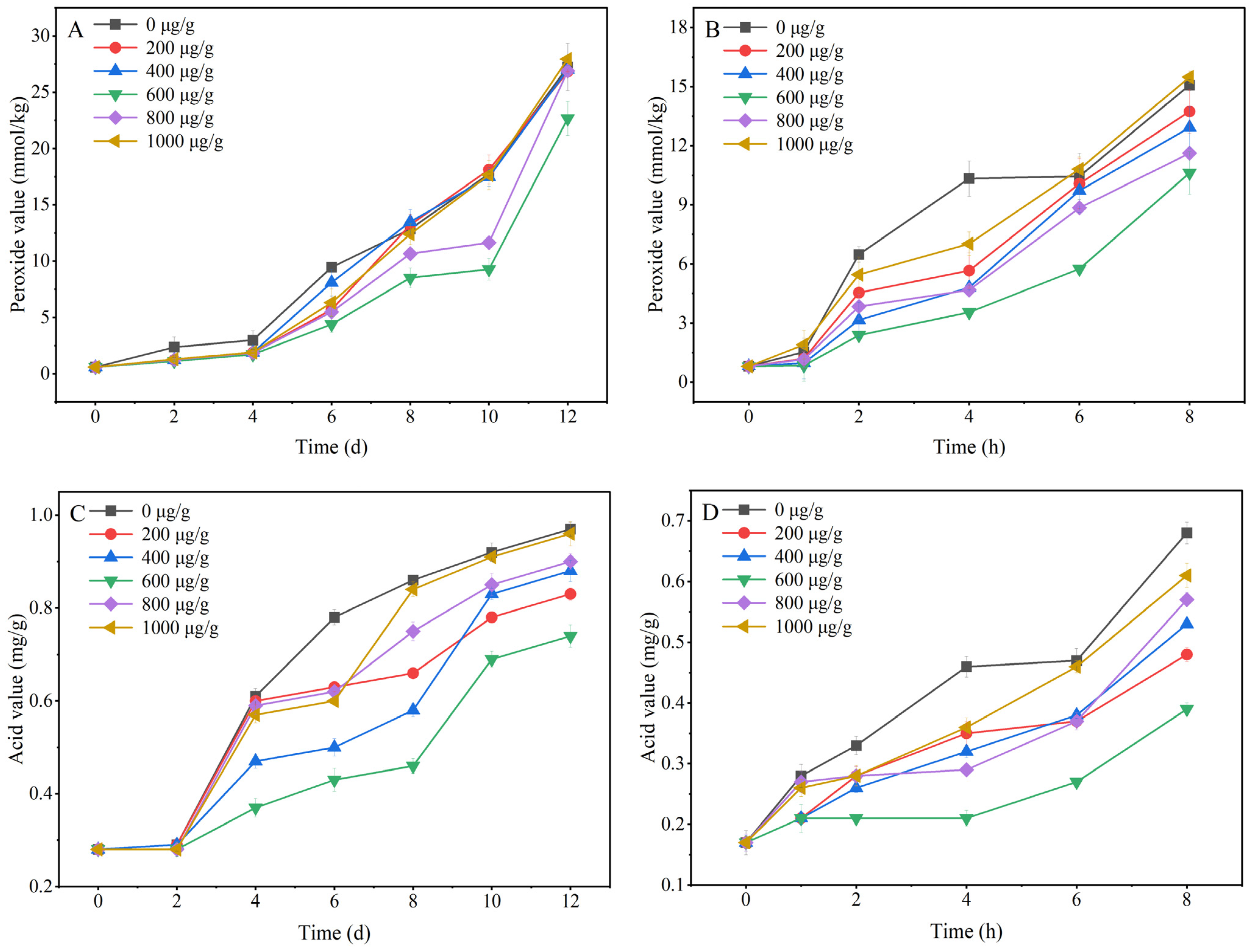
2.3.4. Analysis of Peroxide Value and Acid Value
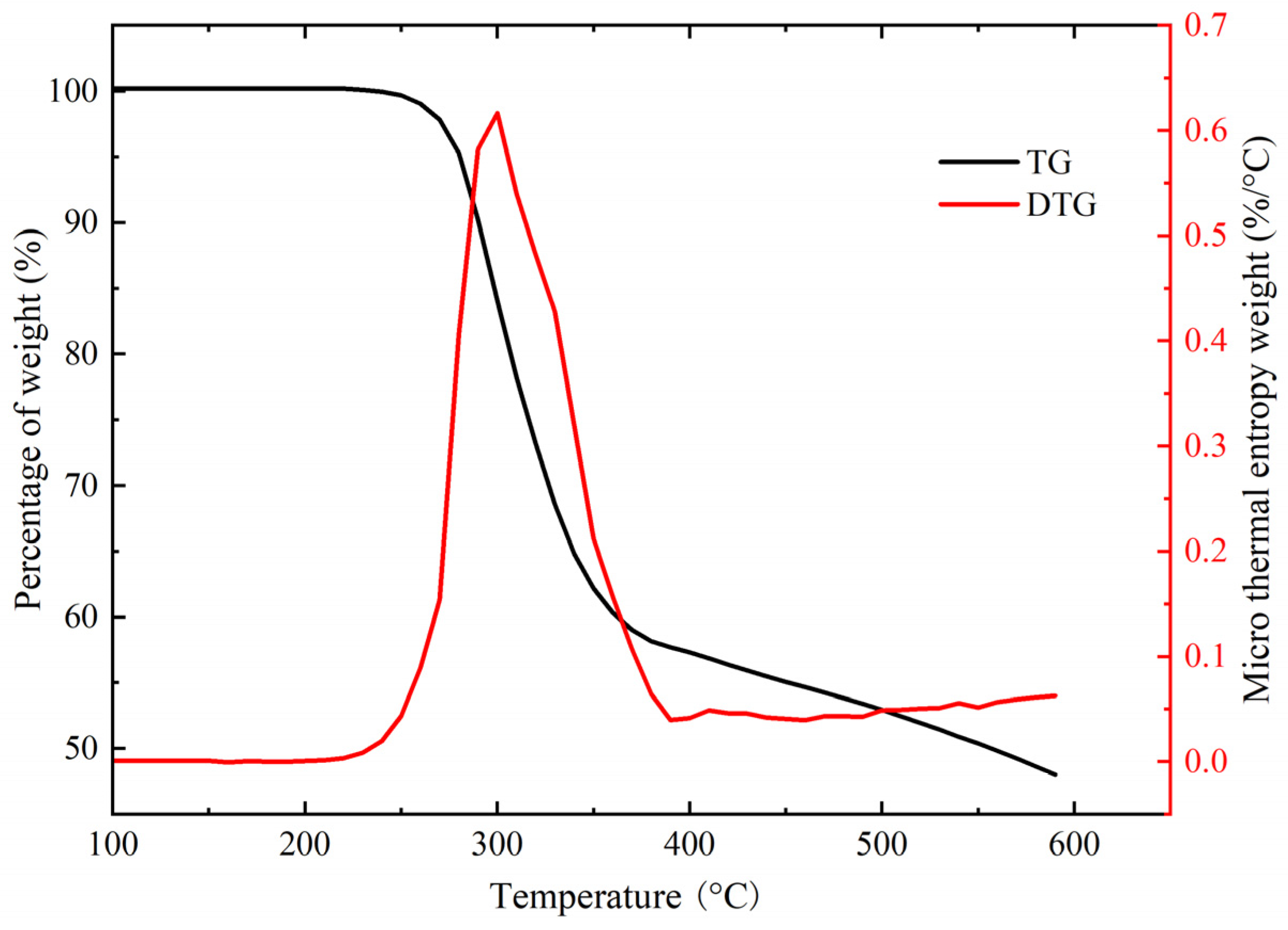
2.4. Thermogravimetric Analysis
2.5. Determination of Resveratrol in Oil
2.6. Analysis of Resveratrol Oxidation Products
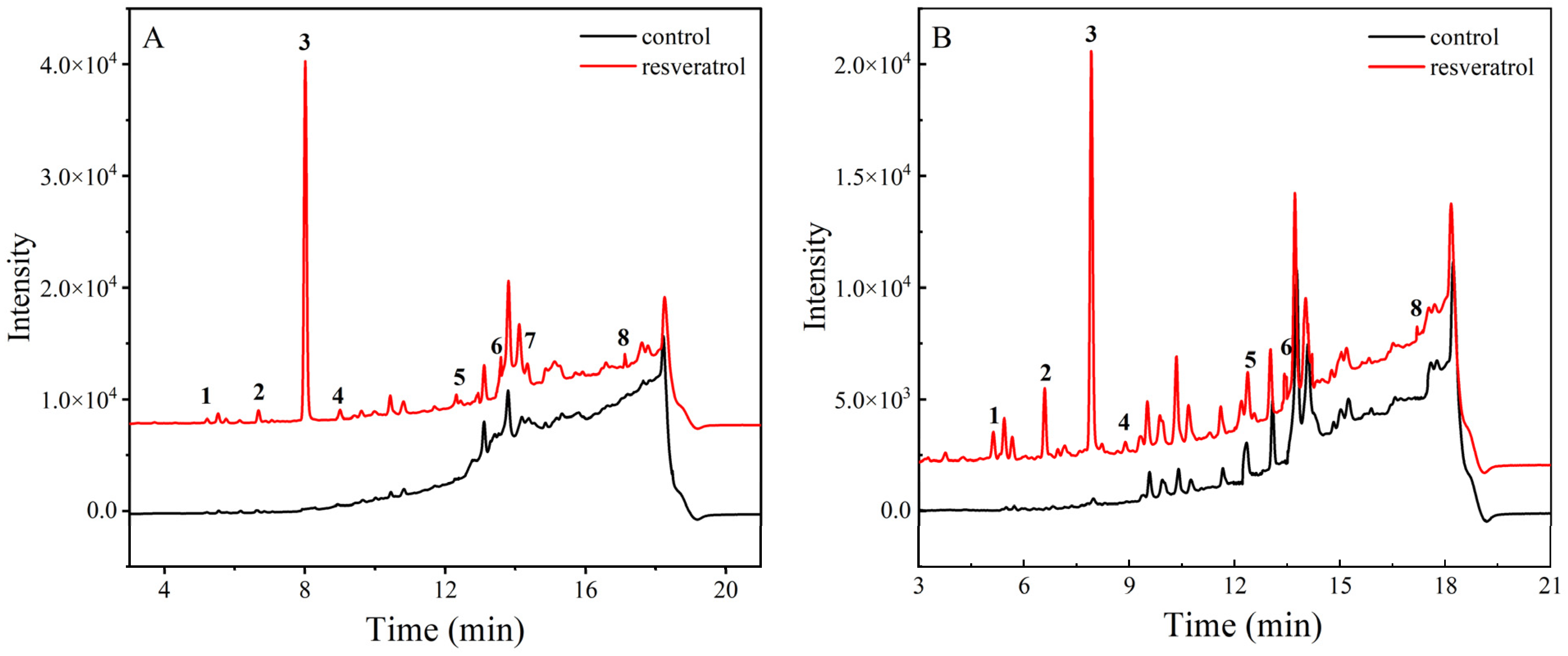
2.6.1. Identification of Non-Volatile Thermal Oxidation Products by LC-MS
2.6.2. Identification of Volatile Thermal Oxidation Products by GC-MS
2.7. Statistical Analysis
3. Results
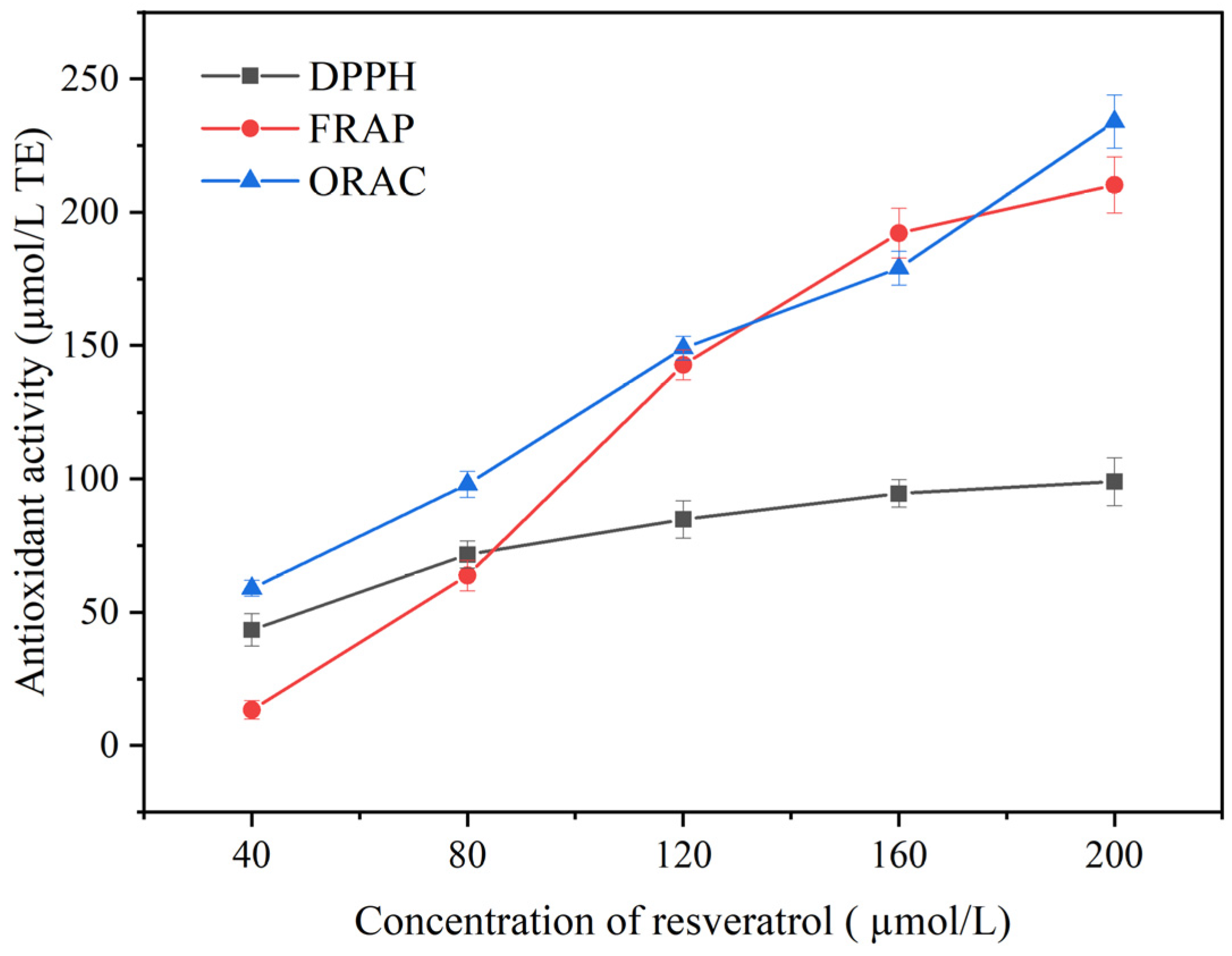
3.1. In Vitro Antioxidant Capacity of Resveratrol
3.2. Antioxidant Capacity of Resveratrol in Oil
3.3. Thermal Stability of Resveratrol
3.3.1. Thermogravimetric Analysis
3.3.2. Degradation of Resveratrol during Heating in Oils
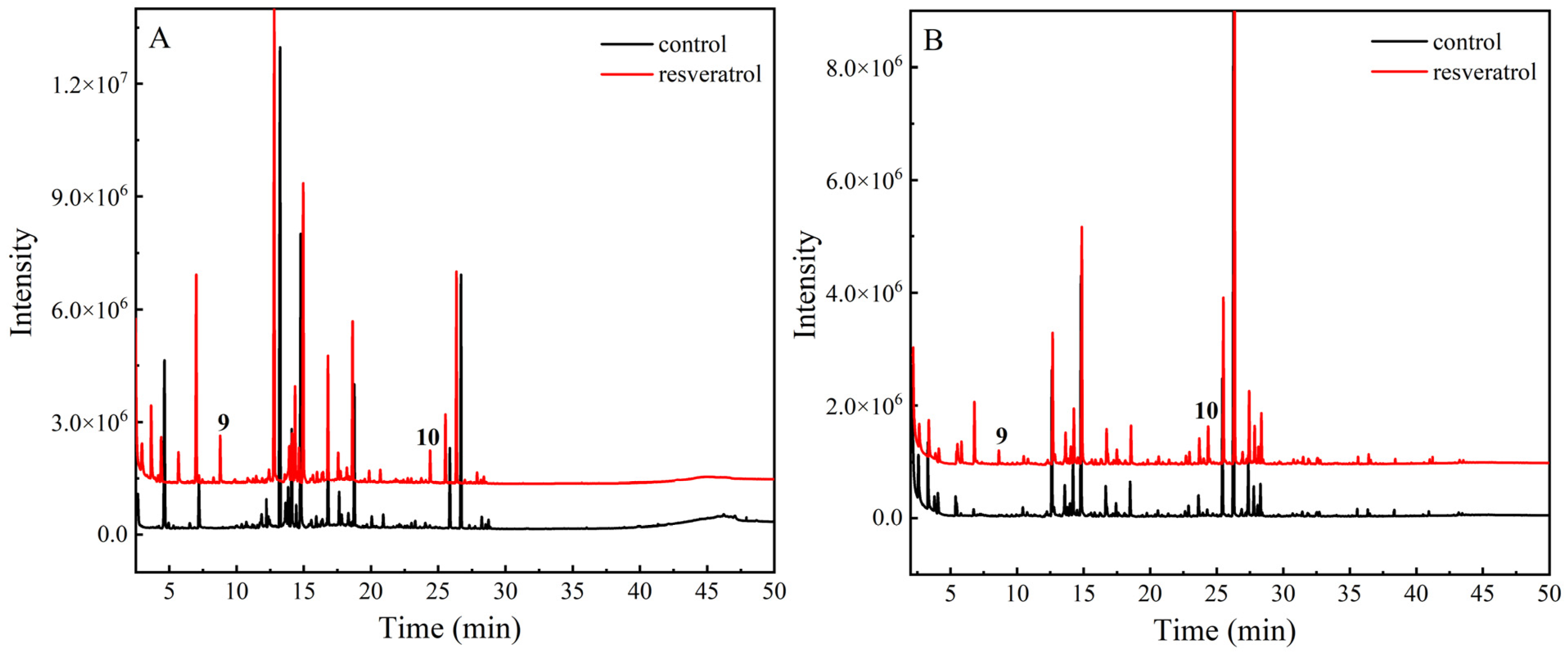
3.4. Identification of Oxidation Products
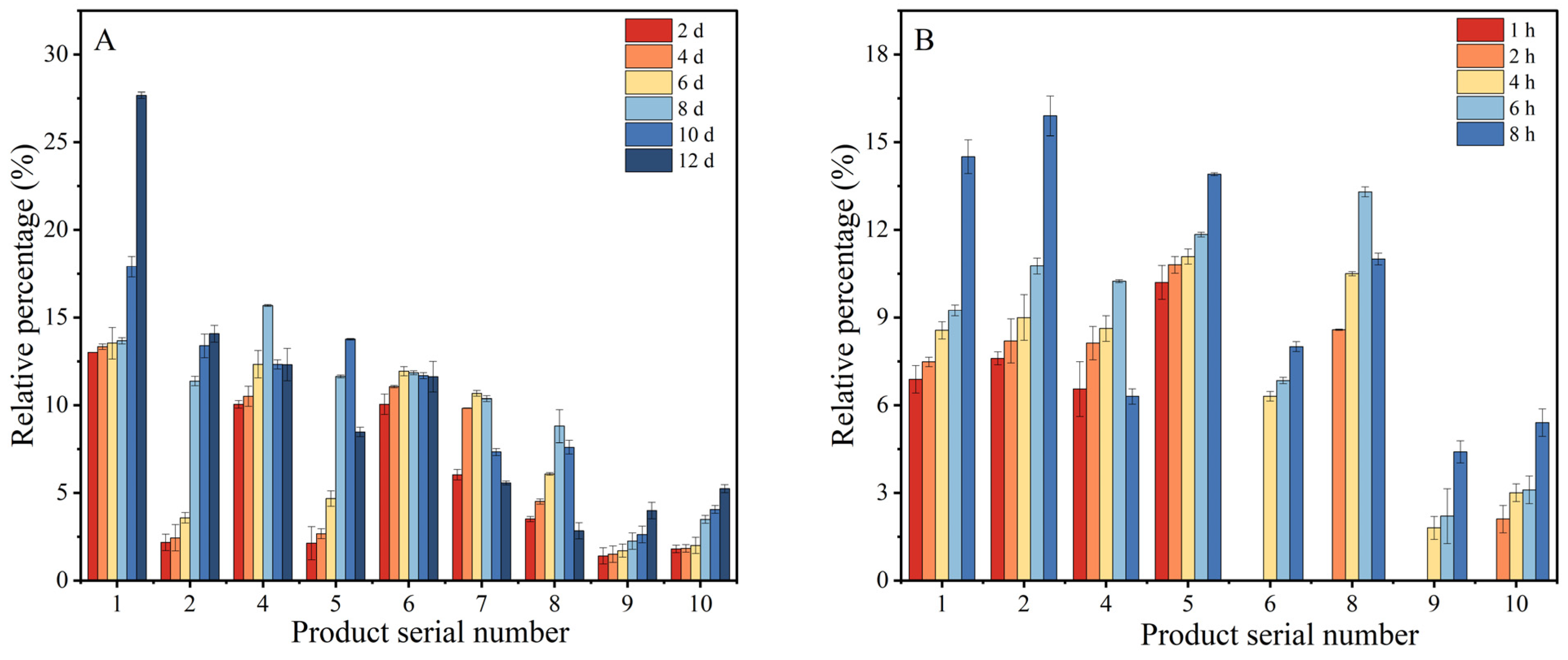
3.5. Variation in Relative Content of Resveratrol Oxidation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Yang, H.; Xie, Y.; Ding, Y.; Kong, D.; Yu, H. Research Progress on Alzheimer’s Disease and Resveratrol. Neurochem. Res. 2020, 45, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Milanovic, M.; Milic, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- de Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]
- López-Hernández, J.; Paseiro-Losada, P.; Sanches-Silva, A.T.; Lage-Yusty, M.A. Study of the changes of trans-resveratrol caused by ultraviolet light and determination of trans- and cis-resveratrol in Spanish white wines. Eur. Food Res. Technol. 2006, 225, 789–796. [Google Scholar] [CrossRef]
- Jensen, J.S.; Wertz, C.F.; O’Neill, V.A. Preformulation Stability of trans-Resveratrol and trans-Resveratrol Glucoside (Piceid). J. Agric. Food Chem. 2010, 58, 1685–1690. [Google Scholar] [CrossRef]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol 2020, 64, 276–280. [Google Scholar] [CrossRef]
- Li, T.; Guo, Q.; Qu, Y.; Liu, H.; Liu, L.; Zhang, Y.; Wang, Q. Inhibition mechanism of trans-resveratrol on thermally induced trans fatty acids in peanut oil. Food Chem. 2023, 406, 134863. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-C.; Shi, N.; Wang, X.-Y.; Wang, Z.-H.; Wang, K.-L.; Gao, M.; Yu, L.; Chen, D.; Xu, X. Application of natural cotton fibers as an extraction sorbent for the detection of trans-resveratrol in adulterated peanut oils. Food Chem. 2021, 339, 127885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Liu, R.; Zhong, S.Y.; Peng, X.T.; Ye, T.T.; Yu, Q.W.; Feng, Y.Q. Characterization of Trans-Resveratrol in Peanut Oils Based on Solid-Phase Extraction with Loofah Sponge Combined with High-Performance Liquid Chromatography-Ultraviolet (HPLC-UV). Food Anal. Methods 2022, 15, 3153–3161. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Zhang, L.; Gao, Y.; Li, Q.; Yu, X. Antioxidant properties of lipid concomitants in edible oils: A review. Food Chem. 2023, 422, 136219. [Google Scholar] [CrossRef] [PubMed]
- Softić, M.; Karić, E.; Zukić, A.; Ibišević, M.; Kozarević, E.C.; Seferović, S.; Bjelić, E.; Alibašić, H.; Horozić, E.; Begić, S. Antioxidant Capacity and Total Phenolic and Flavonoid Contents of Methanolic Extracts of Urtica dioica L. by Different Extraction Techniques. Int. Res. J. Pure Appl. Chem. 2020, 21, 207–214. [Google Scholar] [CrossRef]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of polyphenols, tocopherols, and antioxidant capacity in virgin argan oil (Argania spinosa, Skeels). Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of Extraction Conditions for the Antioxidant Potential of Different Pumpkin Varieties (Cucurbita maxima). Sustainability 2020, 12, 1305. [Google Scholar] [CrossRef]
- Silva, R.d.C.d.; Teixeira, J.A.; Nunes, W.D.G.; Zangaro, G.A.C.; Pivatto, M.; Caires, F.J.; Ionashiro, M. Resveratrol: A thermoanalytical study. Food Chem. 2017, 237, 561–565. [Google Scholar] [CrossRef]
- Polunina, I.A.; Polunin, K.E.; Voytova, V.M.; Senchikhin, I.N.; Ul’yanov, A.V.; Buryak, A.K.; Roldughin, V.I. Thermal transitions of resveratrol on a carbon black surface. Prot. Met. Phys. Chem. Surf. 2013, 49, 649–654. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, D.-Q.; Tao, M.; Ning, W.-J.; Yang, Y.-J.; Meng, K.-Y.; Mei, Y.; Feng, Y.-Q. Rapid magnetic solid-phase extraction based on alendronate sodium grafted mesoporous magnetic nanoparticle for the determination of trans-resveratrol in peanut oils. Food Chem. 2019, 279, 187–193. [Google Scholar] [CrossRef]
- Wang, S.-T.; Le, J.; Peng, R.; Li, Y. Efficient extraction and sensitive LC-MS quantification of hydroxytyrosol in wine, oil and plasma. Food Chem. 2020, 323, 126803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, D.; Zheng, W.; Jiao, L.; Lang, Y. Discrimination of Adulterated Sesame Oil Using Mid-infrared Spectroscopy and Chemometrics. Food Anal. Methods 2015, 8, 2308–2314. [Google Scholar] [CrossRef]
- Emberger, M.E.; Lin, J.; Pika, J.; Christ, I.; Eigenbrodt, B. Automated Solid-Phase Microextraction GC-MS/MS Method for Quantification of Volatile Limonene Oxidation Products in Encapsulated Orange Oil. Flavour Fragr. J. 2019, 34, 52–62. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2013, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.J.; Zhao, S.R.; Li, J.M.; Xue, B. Stilbene profiles in different tissues ofVitis viniferaL. cv. Cabernet Sauvignon and a comparison of their antioxidant activity. Aust. J. Grape Wine Res. 2016, 22, 226–231. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef]
- Babazadeh, A.; Taghvimi, A.; Hamishehkar, H.; Tabibiazar, M. Development of new ultrasonic–solvent assisted method for determination of trans-resveratrol from red grapes: Optimization, characterization, and antioxidant activity (ORAC assay). Food Biosci. 2017, 20, 36–42. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Fang, J.-G.; Chen, W.-F.; Zhou, B.; Yang, L.; Liu, Z.-L. Structure–activity relationship studies of resveratrol and its analogues by the reaction kinetics of low density lipoprotein peroxidation. Bioorganic Chem. 2006, 34, 142–157. [Google Scholar] [CrossRef]
- He, X.; Andersson, G.; Lindgren, U.; Li, Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem. Biophys. Res. Commun. 2010, 401, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Antus, C.; Radnai, B.; Dombovari, P.; Fonai, F.; Avar, P.; Matyus, P.; Racz, B.; Sumegi, B.; Veres, B. Anti-inflammatory effects of a triple-bond resveratrol analog: Structure and function relationship. Eur. J. Pharmacol. 2015, 748, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Bulut, U.; Crivello, J.V. Investigation of the Reactivity of Epoxide Monomers in Photoinitiated Cationic Polymerization. Macromolecules 2005, 38, 3584–3595. [Google Scholar] [CrossRef]
- Li, T.; Guo, Q.; Qu, Y.; Li, Y.; Liu, H.; Liu, L.; Zhang, Y.; Jiang, Y.; Wang, Q. Solubility and physicochemical properties of resveratrol in peanut oil. Food Chem. 2022, 368, 130687. [Google Scholar] [CrossRef]
- Camont, L.; Collin, F.; Couturier, M.; Thérond, P.; Jore, D.; Gardès-Albert, M.; Bonnefont-Rousselot, D. Radical-induced oxidation of trans-resveratrol. Biochimie 2012, 94, 741–747. [Google Scholar] [CrossRef]
- Li, W.; Ran, L.; Li, H.; Chao, G.; Kang, X.; Lei, T. Regioselective Biomimetic Synthesis of Dimeric Oxyresveratrol Derivatives. Synlett 2020, 31, 1809–1812. [Google Scholar] [CrossRef]


| Peak | T (min) | Major Fragments ESI Negative/Positive | ||
|---|---|---|---|---|
| [M + H]+ | [M − H]− | Production Scan Experiments | ||
| 1 (3,5-dihydroxybenzaldehyde) | 5.2 | 138.03 | 227→138 | |
| 2 (7-hydroxy-1-naphthaldehyde) | 6.7 | 171.04 | 227→185→171 | |
| 3 (resveratrol) | 7.9 | 227.08 | ||
| 4 (4-((1E,4E)-3-methylenehexa-1,4-dien-1-yl)phenol) | 9.0 | 185.10 | 227→185 | |
| 5 (2-naphthalenemethanol) | 12.7 | 157.07 | 227→185→157 | |
| 6 (dimer1) | 13.4 | 436.13 | 453→436 | |
| 7 (3,4,3′,5′-tetrahydroxy-trans-stilbene) | 14.3 | 244.07 | 228→244 | |
| 8 (dimer2) | 17.2 | 306.09 | 453→306 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Yuan, H.; Chen, C.; Liang, J.; Li, C. Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil. Foods 2024, 13, 29. https://doi.org/10.3390/foods13010029
Yao Y, Yuan H, Chen C, Liang J, Li C. Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil. Foods. 2024; 13(1):29. https://doi.org/10.3390/foods13010029
Chicago/Turabian StyleYao, Yunping, Huiping Yuan, Chen Chen, Jia Liang, and Changmo Li. 2024. "Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil" Foods 13, no. 1: 29. https://doi.org/10.3390/foods13010029
APA StyleYao, Y., Yuan, H., Chen, C., Liang, J., & Li, C. (2024). Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil. Foods, 13(1), 29. https://doi.org/10.3390/foods13010029






