Isolation and Characterization of n-3 Polyunsaturated Fatty Acids in Enteromorpha prolifera Lipids and Their Preventive Effects on Ulcerative Colitis in C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Isolation of Enteromorpha prolifera Oil by SFE
2.3. Fatty Acid Methyl Esters and GC-MS Analysis
2.4. Animals and Treatment
2.5. Histopathological Examination
2.6. Serum Inflammatory Cytokines
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
2.8. Western Blotting
2.9. Hepatic Lipidomics Analysis
2.10. Statistical Analysis
3. Results and Discussion
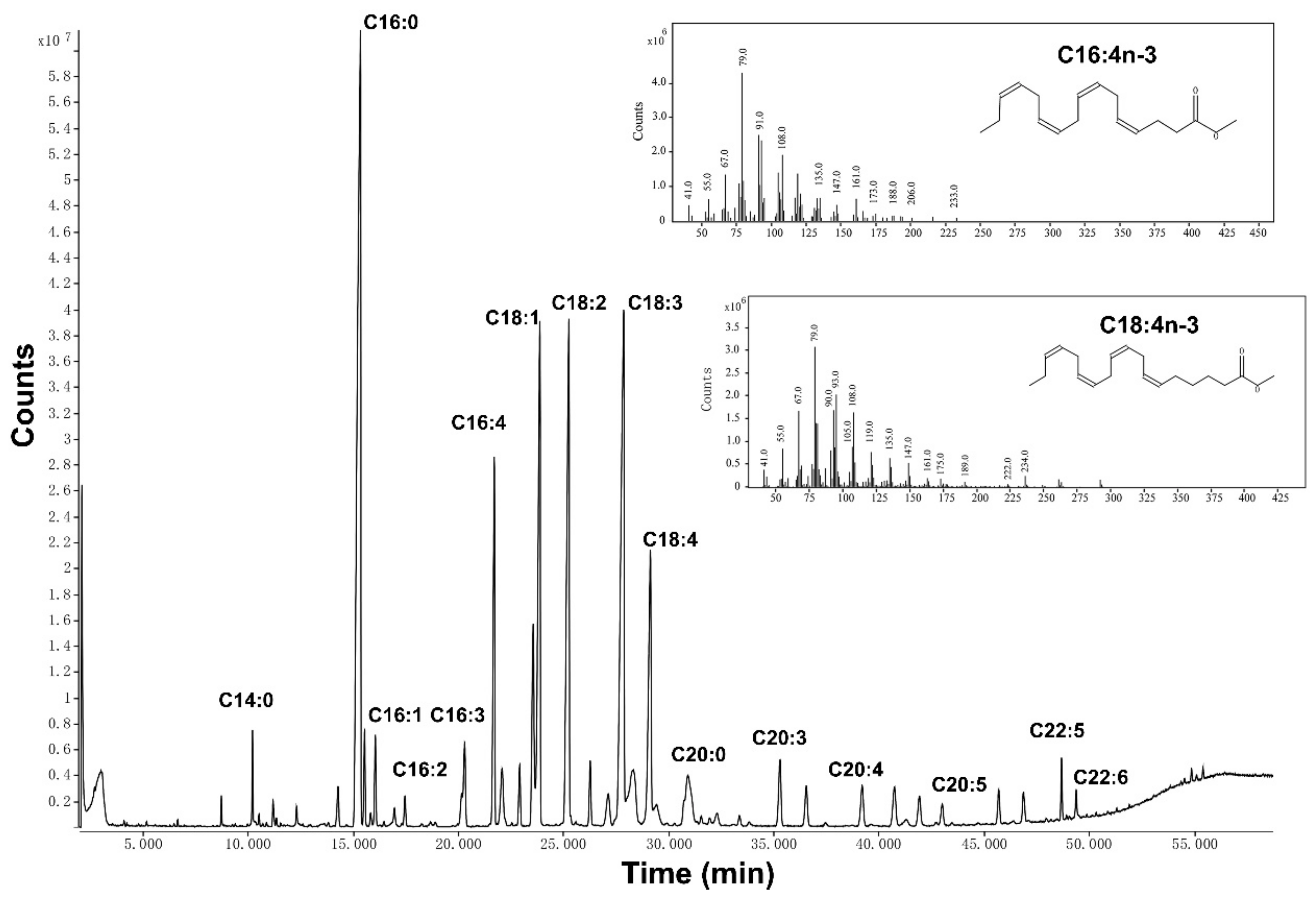
3.1. Identification of Fatty Acids of Enteromorpha prolifera Oil Extracted by SFE
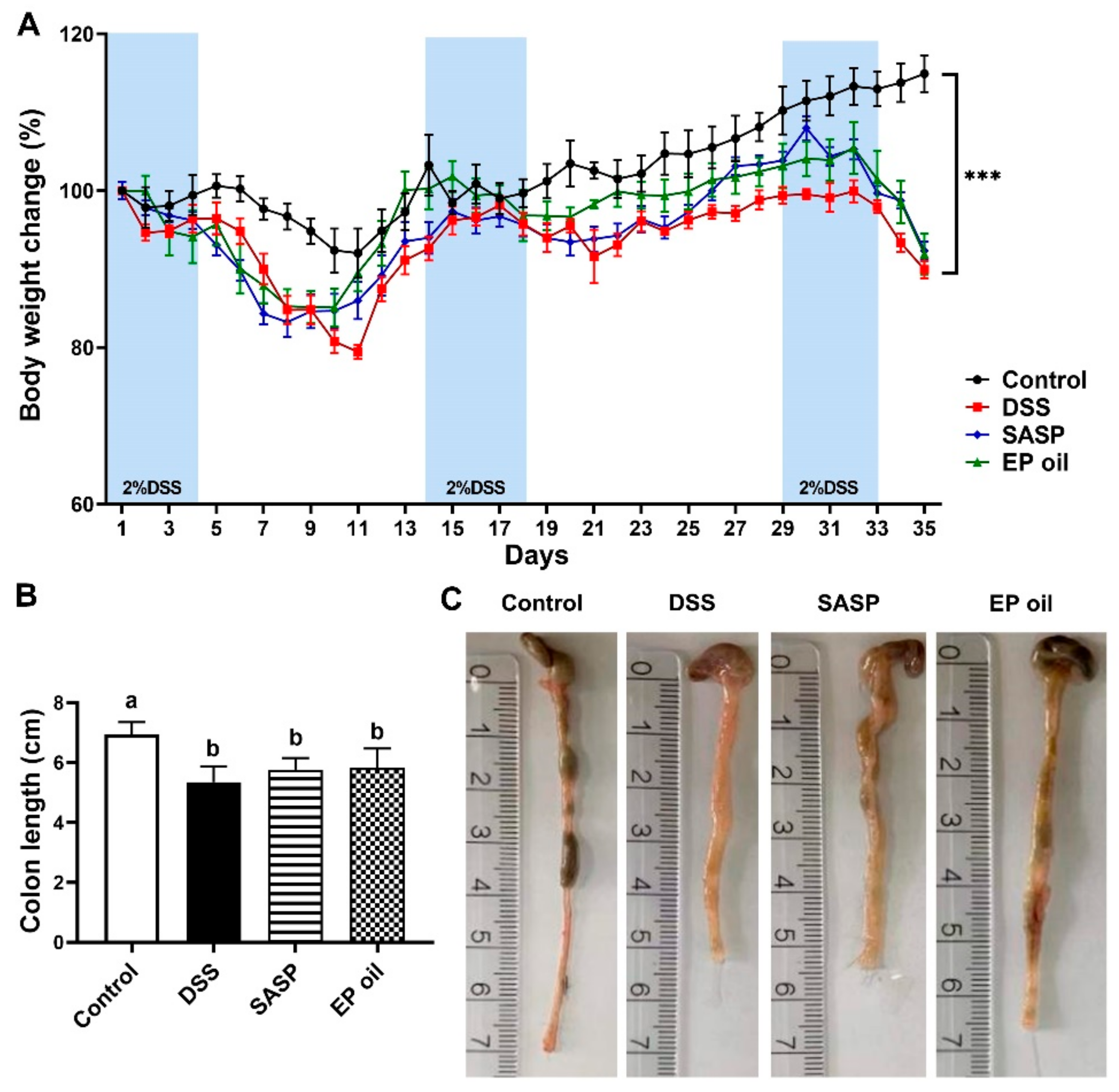
3.2. Impact of Enteromorpha prolifera Oil on DSS-Induced Mice
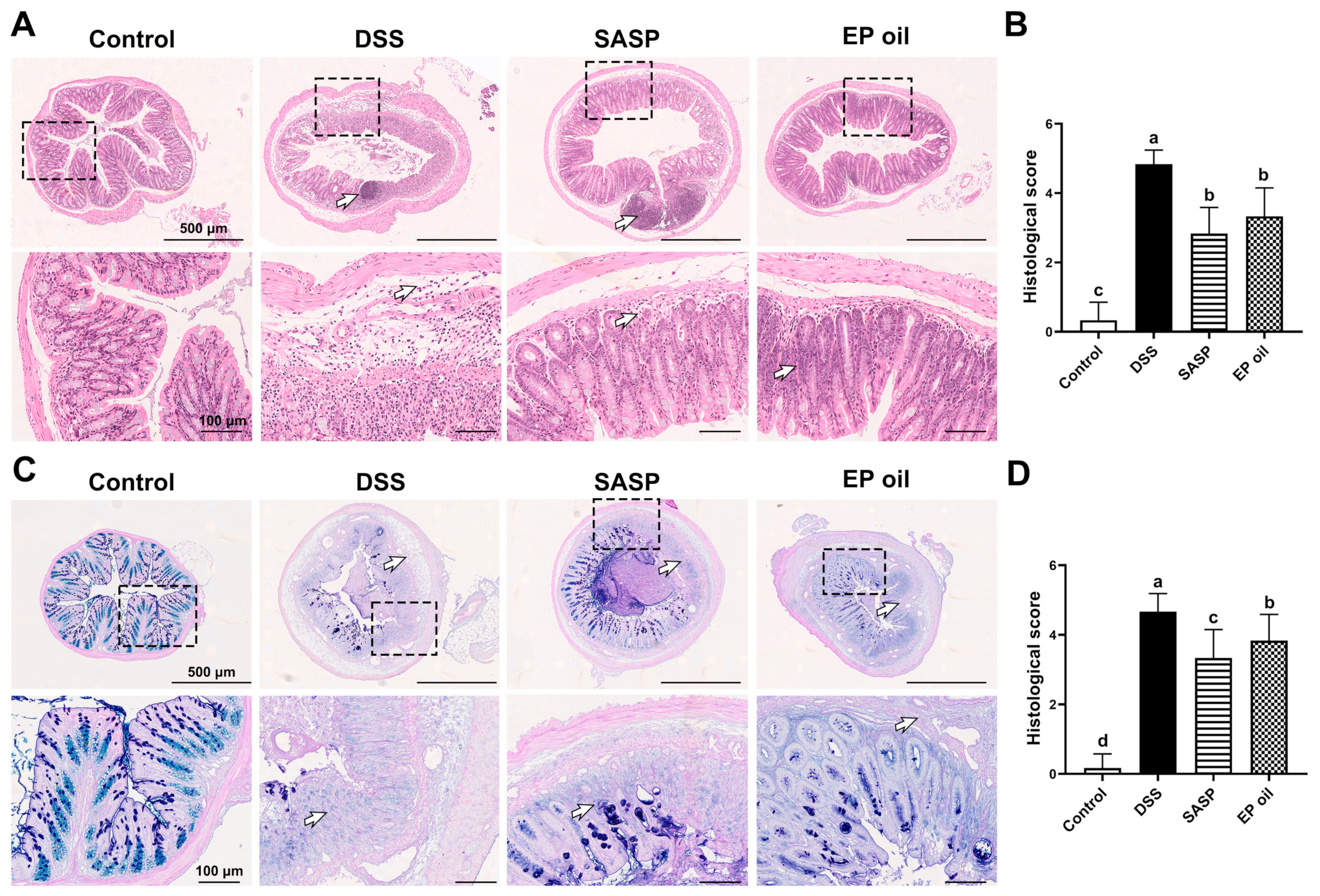
3.3. Effect of Enteromorpha prolifera Oil on Colon Histomorphology
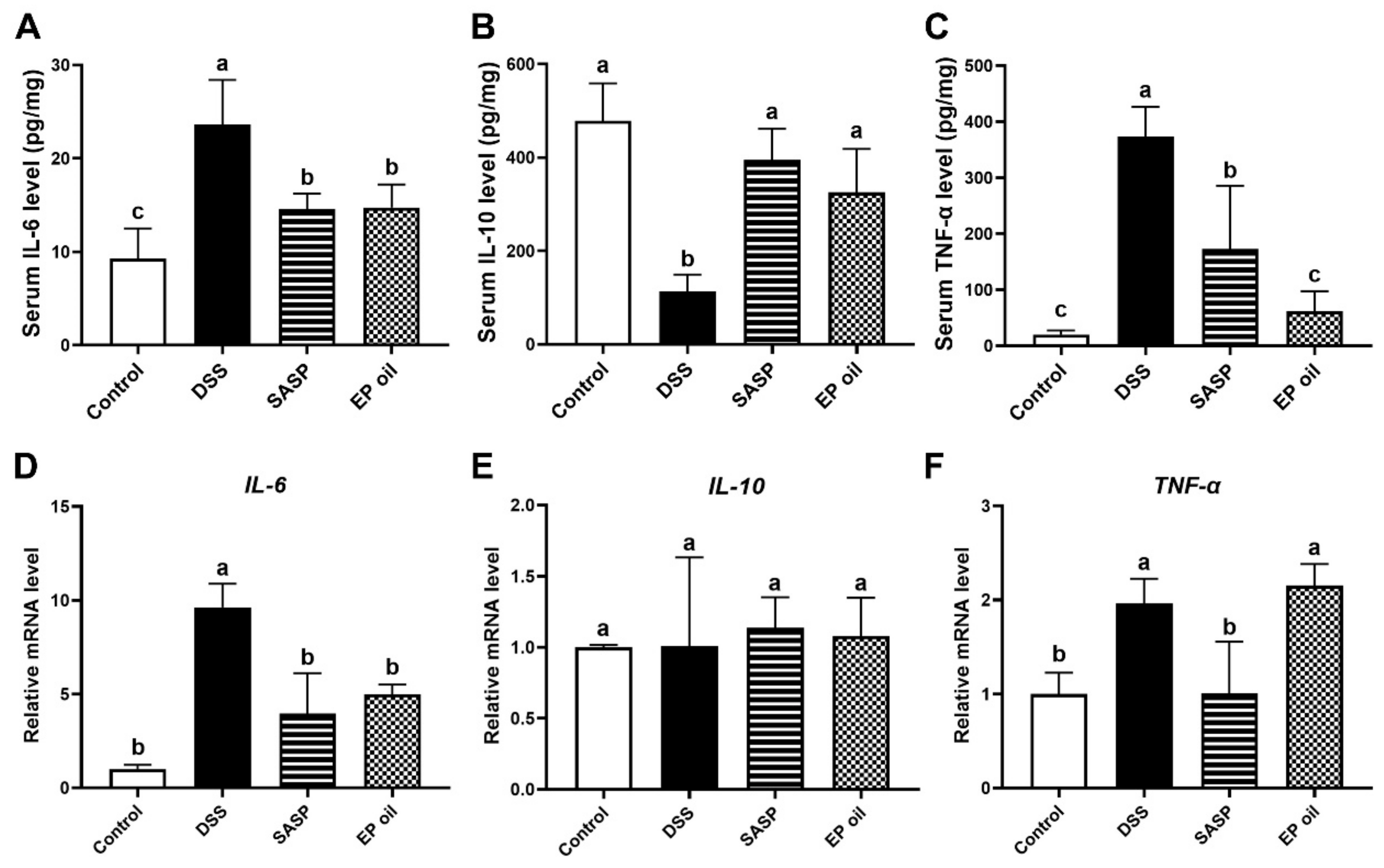
3.4. Anti-Inflammatory Effects of Enteromorpha prolifera Oil on DSS-Induced Mice
3.5. Enteromorpha prolifera Oil Inhibited Inflammation by JAK/STAT Signaling Pathway
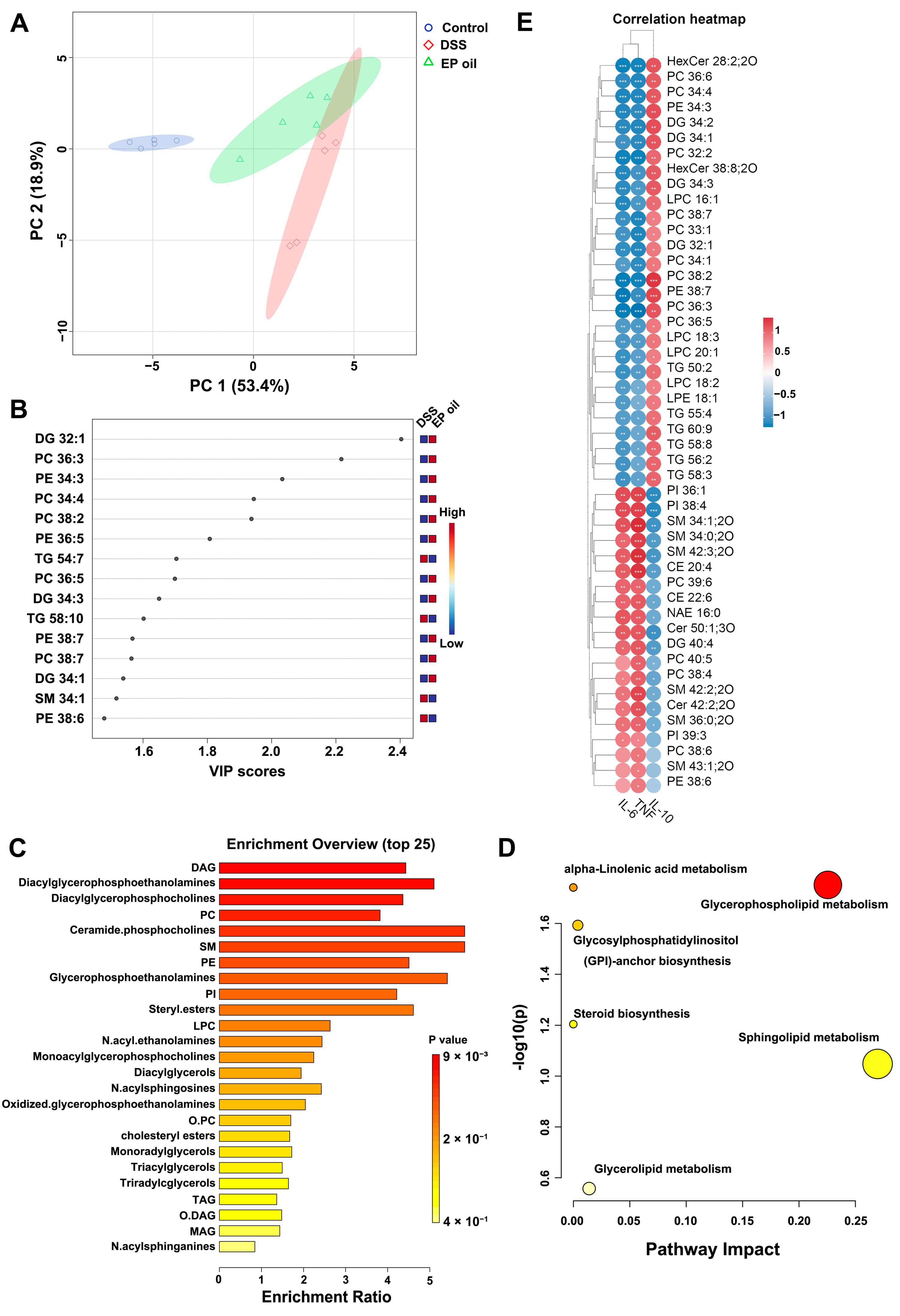
3.6. Effect of Enteromorpha prolifera Oil on Hepatic Lipid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Panah, F.M.; Nielsen, K.D.; Simpson, G.L.; Schönherz, A.; Schramm, A.; Lauridsen, C.; Nielsen, T.S.; Højberg, O.; Fredborg, M.; Purup, S.; et al. A westernized diet changed the colonic bacterial composition and metabolite concentration in a dextran sulfate sodium pig model for ulcerative colitis. Front. Microbiol. 2023, 14, 1018242. [Google Scholar] [CrossRef]
- Saadh, M.J.; Pal, R.S.; Arias-Gonzáles, J.L.; Orosco Gavilán, J.C.; Jc, D.; Mohany, M.; Al-Rejaie, S.S.; Bahrami, A.; Kadham, M.J.; Amin, A.H.; et al. A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Durkin, L.A.; Childs, C.E.; Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium—A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Nusrat, A. Saving Problematic Mucosae: SPMs in Intestinal Mucosal Inflammation and Repair. Trends Mol. Med. 2019, 25, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dong, L.; Yan, Q.; Dong, Y.; Wang, L.; Wang, F. Preparation and Characterization of an Anticancer Peptide from Oriental Tonic Food Enteromorpha prolifera. Foods 2022, 11, 3507. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; He, P.; Wang, Z.; Shi, Y.; Wang, Y. The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications. Estuar. Coast. Shelf Sci. 2013, 129, 2–10. [Google Scholar] [CrossRef]
- Zhao, S.; He, Y.; Wang, C.; Assani, I.; Hou, P.; Feng, Y.; Yang, J.; Wang, Y.; Liao, Z.; Shen, S. Isolation, Characterization and Bioactive Properties of Alkali-Extracted Polysaccharides from Enteromorpha prolifera. Mar. Drugs 2020, 18, 552. [Google Scholar] [CrossRef]
- Song, W.; Wang, Z.; Zhang, X.; Li, Y. Ethanol Extract from Ulva prolifera Prevents High-Fat Diet-Induced Insulin Resistance, Oxidative Stress, and Inflammation Response in Mice. Biomed Res. Int. 2018, 2018, 1374565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Chen, W.; Huang, F.; Chen, S.; Wang, X.; Yang, C. Algal oil alleviates antibiotic-induced intestinal inflammation by regulating gut microbiota and repairing intestinal barrier. Front. Nutr. 2022, 9, 1081717. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO₂ Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Siriamornpun, S.; Li, D. Polyunsaturated Fatty Acid Content of Edible Insects in Thailand. J. Food Lipids 2006, 13, 277–285. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Li, X.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Zeng, M.; Zhao, Y. Effects of microbial diversity and phospholipids on flavor profile of caviar from hybrid sturgeon (Huso dauricus × Acipenser schrencki). Food Chem. 2022, 377, 131969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Fan, Z.-K.; Gao, X.; Zhou, F.; Guo, X.-F.; Sinclair, A.J.; Li, D. n-3 polyunsaturated fatty acids in phospholipid or triacylglycerol form attenuate nonalcoholic fatty liver disease via mediating cannabinoid receptor 1/adiponectin/ceramide pathway. J. Nutr. Biochem. 2024, 123, 109484. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Wang, X.; Lan, H.; Sun, T.; Cong, P.; Xue, C.; Xu, J. Serum metabolomics analysis reveals amelioration effects of sea cucumber ether phospholipids on oxidative stress and inflammation in high-fat diet-fed mice. Food Funct. 2022, 13, 10134–10146. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-K.; Ma, W.-J.; Zhang, W.; Li, H.; Zhai, J.; Zhao, T.; Guo, X.-F.; Sinclair, A.J.; Li, D. Elevated serum phosphatidylcholine (16:1/22:6) levels promoted by fish oil and vitamin D(3) are highly correlated with biomarkers of non-alcoholic fatty liver disease in Chinese subjects. Food Funct. 2022, 13, 11705–11714. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Tian, H.; Liu, C.; Zhang, X.; Peng, Y.; Yang, X.; Chen, F.; Li, J. Metformin and cyanidin 3-O-galactoside from Aronia melanocarpa synergistically alleviate cognitive impairment in SAMP8 mice. Food Funct. 2021, 12, 10994–11008. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yamazaki, Y.; Koike, S.; Hakozaki, M.; Nagahora, N.; Yuki, S.; Yano, A.; Tsurumi, K.; Okumura, T. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica. Sci. Rep. 2017, 7, 9944. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.G.; Lasiter, A.D.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. III. Four cold-adapted, peridinin-containing taxa and the presence of trigalactosyldiacylglycerol as an additional glycolipid. Eur. J. Phycol. 2009, 44, 439–445. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 1, 30–40. [Google Scholar] [CrossRef]
- De Paula do Nascimento, R.; Lima, A.V.; Oyama, L.M.; Paiotti, A.P.R.; Cardili, L.; Martinez, C.A.R.; Pereira, J.A.; Silva, M.F.; Garofolo, I.C.; Silveira, V.L.F.; et al. Extra virgin olive oil and flaxseed oil have no preventive effects on DSS-induced acute ulcerative colitis. Nutrition 2020, 74, 110731. [Google Scholar] [CrossRef]
- Hudert, C.A.; Weylandt, K.H.; Lu, Y.; Wang, J.; Hong, S.; Dignass, A.; Serhan, C.N.; Kang, J.X. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11276–11281. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Murata, M.; Kaneniwa, M.; Saito, H.; Komatsu, W.; Shinohara, K. Purification of stearidonic acid (18:4(n-3)) and hexadecatetraenoic acid (16:4(n-3)) from algal fatty acid with lipase and medium pressure liquid chromatography. Biosci. Biotechnol. Biochem. 2000, 64, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Nunes, L.V.; Duarte, M.T.S.; Ferreira, L.F.R.; Soriano, R.N.; Iqbal, H.M.N. Exploitation of Marine-Derived Robust Biological Molecules to Manage Inflammatory Bowel Disease. Mar. Drugs 2021, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-N.; Choi, Y.-S.; Kim, S.H.; Zhong, X.; Kim, W.; Park, J.S.; Saeidi, S.; Han, B.W.; Kim, N.; Lee, H.S.; et al. Resolvin D1 suppresses inflammation-associated tumorigenesis in the colon by inhibiting IL-6-induced mitotic spindle abnormality. FASEB J. 2021, 35, e21432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xue, Z.; Yang, H.; Zhao, F.; Liu, C.; Chen, J.; Lu, S.; Zou, Z.; Zhou, Y.; Zhang, X. Differential effects of EPA and DHA on DSS-induced colitis in mice and possible mechanisms involved. Food Funct. 2021, 12, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Hansen, T.; Goll, R.; Stenlund, H.; Ahnlund, M.; Jensen, E.; Moritz, T.; Florholmen, J.; Forsdahl, G. Lipidomics in Ulcerative Colitis Reveal Alteration in Mucosal Lipid Composition Associated With the Disease State. Inflamm. Bowel Dis. 2019, 25, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in Lipid, Amino Acid, and Energy Metabolism Distinguish Crohn’s Disease from Ulcerative Colitis and Control Subjects by Serum Metabolomic Profiling. Metabolomics 2018, 14, 17. [Google Scholar] [CrossRef]
- Ferru-Clément, R.; Boucher, G.; Forest, A.; Bouchard, B.; Bitton, A.; Lesage, S.; Schumm, P.; Lazarev, M.; Brant, S.; Duerr, R.H.; et al. Serum Lipidomic Screen Identifies Key Metabolites, Pathways, and Disease Classifiers in Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 1024–1037. [Google Scholar] [CrossRef]
- Murgia, A.; Hinz, C.; Liggi, S.; Denes, J.; Hall, Z.; West, J.; Santoru, M.L.; Piras, C.; Manis, C.; Usai, P.; et al. Italian cohort of patients affected by inflammatory bowel disease is characterised by variation in glycerophospholipid, free fatty acids and amino acid levels. Metabolomics 2018, 14, 140. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Zhou, E.; Tao, Y.; Wang, M.; Qi, S.; Zhao, L.; Tan, Y.; Wu, L. Integrated microbiomic and metabolomic analyses reveal the mechanisms by which bee pollen and royal jelly lipid extracts ameliorate colitis in mice. Food Res. Int. 2023, 171, 113069. [Google Scholar] [CrossRef]
- Fan, F.; Mundra, P.A.; Fang, L.; Galvin, A.; Moore, X.L.; Weir, J.M.; Wong, G.; White, D.A.; Chin-Dusting, J.; Sparrow, M.P.; et al. Lipidomic Profiling in Inflammatory Bowel Disease: Comparison Between Ulcerative Colitis and Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1511–1518. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| IL-10 | GCTGGACAACATACTGCTAACC | ATTTCCGATAAGGCTTGGCAA |
| TNF-α | CTGAACTTCGGGGTGATCGG | GGCTTGTCACTCGAATTTTGAGA |
| JAK1 | AGTGCAGTATCTCTCCTCTCTG | GATTCGGTTCGGAGCGTACC |
| JAK2 | GGAATGGCCTGCCTTACAATG | TGGCTCTATCTGCTTCACAGAAT |
| STAT2 | GTTACACCAGGTCTACTCACAGA | TGGTCTTCAATCCAGGTAGCC |
| STAT3 | AATATAGCCGATTCCTGCAAGAG | TGGCTTCTCAAGATACCTGCTC |
| TYK2 | TGCATCCACATCGCACACAA | CTCCTGGGGATTCATGCCA |
| SOCS3 | TGCGCCTCAAGACCTTCAG | GCTCCAGTAGAATCCGCTCTC |
| Fatty Acids | Fatty Acid | Formula | Methyl Ester (%) |
|---|---|---|---|
| C14:0 | Myristic acid | C14H28O2 | 0.78 ± 0.09 |
| C16:0 | Palmitic acid | C16H32O2 | 27.56 ± 1.41 |
| C18:0 | Stearic acid | C18H36O2 | 0.79 ± 0.27 |
| C20:0 | Eicosanoic acid | C20H40O2 | 0.48 ± 0.40 |
| SFA | 29.61 ± 2.16 | ||
| C16:1 | Palmitoleic acid | C16H30O2 | 1.62 ± 0.43 |
| C17:1 | cis-10-Heptadecenoic acid | C17H32O2 | 0.53 ± 0.02 |
| C18:1n-9 | Oleic acid | C18H34O2 | 7.11 ± 5.67 |
| C18:1n-7 | Oleic acid | C18H34O2 | 11.39 ± 0.38 |
| C22:1n-9 | 13-Docosenoic acid | C22H42O2 | 0.99 ± 0.17 |
| MUFA | 21.65 ± 6.68 | ||
| C16:2n-7 | 9,12-Hexadecadienoic acid | C16H28O2 | 0.46 ± 0.04 |
| C16:3n-3 | 7,10,13-Hexadecatrienoic acid | C16H26O2 | 1.80 ± 0.38 |
| C16:4n-3 | 6Z,9Z,12Z,15Z-hexadecatetraenoic acid | C16H24O2 | 5.96 ± 0.71 |
| C18:2n-6 | Linoleic acid | C18H32O2 | 13.14 ± 0.63 |
| C18:3n-6 | γ-Linolenic acid | C18H30O2 | 0.95 ± 0.07 |
| C18:3n-3 | α-Linolenic acid | C18H30O2 | 16.41 ± 1.11 |
| C18:4n-3 | Stearidonic acid, SDA | C18H28O2 | 6.23 ± 0.91 |
| C20:3n-6 | Eicosatrienoic acid | C20H34O2 | 1.45 ± 0.09 |
| C20:4n-6 | Eicosatetraenoic acid | C20H32O2 | 0.92 ± 0.10 |
| C20:5n-3 | Eicosapentaenoic acid, EPA | C20H30O2 | 0.98 ± 0.13 |
| C22:4n-6 | Docosatetraenoic acid | C22H36O2 | 0.48 ± 0.19 |
| C22:5n-3 | Docosapentaenoic acid, DPA | C22H34O2 | 0.63 ± 0.14 |
| C22:6n-3 | Docohexaenoic acid, DHA | C22H32O2 | 0.31 ± 0.10 |
| PUFA | 49.72 ± 4.61 | ||
| PUFAs n-6 | 17.09 ± 1.03 | ||
| PUFAs n-3 | 32.17 ± 3.53 | ||
| Ratio n-6/n-3 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Leong, P.M.; Wang, X.; Li, D. Isolation and Characterization of n-3 Polyunsaturated Fatty Acids in Enteromorpha prolifera Lipids and Their Preventive Effects on Ulcerative Colitis in C57BL/6J Mice. Foods 2024, 13, 46. https://doi.org/10.3390/foods13010046
Wen H, Leong PM, Wang X, Li D. Isolation and Characterization of n-3 Polyunsaturated Fatty Acids in Enteromorpha prolifera Lipids and Their Preventive Effects on Ulcerative Colitis in C57BL/6J Mice. Foods. 2024; 13(1):46. https://doi.org/10.3390/foods13010046
Chicago/Turabian StyleWen, Haichao, Pooi Mun Leong, Xincen Wang, and Duo Li. 2024. "Isolation and Characterization of n-3 Polyunsaturated Fatty Acids in Enteromorpha prolifera Lipids and Their Preventive Effects on Ulcerative Colitis in C57BL/6J Mice" Foods 13, no. 1: 46. https://doi.org/10.3390/foods13010046
APA StyleWen, H., Leong, P. M., Wang, X., & Li, D. (2024). Isolation and Characterization of n-3 Polyunsaturated Fatty Acids in Enteromorpha prolifera Lipids and Their Preventive Effects on Ulcerative Colitis in C57BL/6J Mice. Foods, 13(1), 46. https://doi.org/10.3390/foods13010046






