Characterization of Second-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours
Abstract
:1. Introduction
| Moisture | Protein | Fat | Ash | Carbohydrates | Fiber | Starch | ||
|---|---|---|---|---|---|---|---|---|
| Ax | Root a | 76 (2) | 4.76 (0.86) | 2.2 (0.3) | 2.3 (0.3) | 15.1 (2.3) | 3 (1) | 14 (2) |
| Flour f | 6.2 (0.2) | 2.1 (0.2) | 0.69 (0.08) | 4.5 (0.2) | 77.3 (0.5) | 9.25 (0.02) | 42.8 (0.6) | |
| Ci | Root b | 73.04 (0.33) | 3.93 (0.04) | 3.40 (0.04) | 2.70 (0.02) | 14.15 (0.02) | 2.78 (0.03) | 13.63 (0.52) |
| Flour f | 5.9 (0.2) | 4.8 (0.2) | 0.63 (0.29) | 8.04 (0.04) | 69.09 (0.04) | 11.51 (0.05) | 52.18 (0.72) | |
| Ce | Root b | 72.7 (0.2) | 5.7 (0.2) | 2.2 (0.2) | 2.96 (0.26) | 13.2 (0.2) | 3.4 (0.2) | 12.2 (0.2) |
| Flour f | 6.22 (0.26) | 8.4 (0.2) | 0.73 (0.08) | 5.55 (0.02) | 64.2 (0.2) | 14.90 (0.02) | 59.98 (0.82) | |
| Ib | Root c | 74.2 (0.2) | 5.6 (0.2) | 1.1 (0.2) | 1.9 (0.2) | 13.7 (0.2) | 3.4 (0.2) | 12.7 (0.2) |
| Flour f | 6.20 (0.27) | 4.65 (0.24) | 0.37 (0.06) | 3.24 (0.49) | 74.35 (0.36) | 11.2 (0.2) | 40.05 (0.55) | |
| Ot | Root d | 79.22 (0.62) | 3.11 (0.02) | 1.71 (0.03) | 1.29 (0.03) | 13.01 (0.02) | 1.66 (0.08) | 9.6 (0.2) |
| Flour f | 16.4 (0.2) | 1.63 (0.07) | 1.06 (0.06) | 3.04 (0.24) | 72.5 (0.2) | 5.33 (0.05) | 28.12 (0.39) | |
| Tt | Root e | 82.8 (0.2) | 5.5 (0.2) | 1.7 (0.2) | 0.6 (0.2) | 8.5 (0.2) | 0.9 (0.2) | 7.1 (0.2) |
| Flour f | 18.87 (0.06) | 9.12 (0.13) | 0.59 (0.07) | 4.94 (0.05) | 56.9 (0.3) | 9.60 (0.05) | 22.2 (0.3) |
- First-generation snacks (1G) are minimally processed by roasting and frying. They are used for snacking (i.e., eating small amounts of food between meals) and include, for example, nuts, potato chips, and popped popcorn [15].
- Second-generation snacks (2G) are the most popular and commonly called “ready-to-eat” or “three-dimensional” snacks. These snacks are directly expanded through the extrusion exit [16]. Examples of snacks in this category include puffed corn, onion rings, and flavored loops.
- Third-generation snacks (3G), called “pellets” or “glassy half-products”, are not expanded directly through the extrusion exit and must be expanded before being consumed using additional processes, such as frying or microwave heating. These pellets are shelf-stable without refrigeration due to their low moisture (around 10% wb) as long as their packaging prevents moisture from increasing [17]. These snacks are made for export due to their high bulk density and stability.
2. Materials and Methods
2.1. Raw Materials
2.2. Flour Manufacturing
2.3. Production of 2G Snacks
2.4. Characterization of 2G Snacks
2.4.1. Water Content (xw) and Water Activity (aw)
2.4.2. Sectional Expansion Index (SEI)
2.4.3. Hygroscopicity (Hy)
2.4.4. Bulk Density (ρb) and Porosity (ε)
2.4.5. Water Absorption Index (WAI), Water Solubility Index (WSI), and Swelling Index (SWE)
2.4.6. Texture Properties
2.4.7. Optical Properties
2.4.8. Bioactive compounds
- Total carotenoids (TC)
- Lycopene (LP)
- Total phenols (TP)
- Antioxidant capacity (AC)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of 2G Snacks
3.1.1. Water Content (xw) and Water Activity (aw)
3.1.2. Sectional Expansion Index (SEI)
3.1.3. Hygroscopicity (Hy)
3.1.4. Bulk Density (ρb) and Porosity (ε)
3.1.5. Water Absorption Index (WAI), Water Solubility Index (WSI), and Swelling Index (SWE)
3.1.6. Texture Properties
3.1.7. Optical Properties
3.1.8. Bioactive Compounds
4. Advantages and Limits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, S.; Kaur, H. Determinants of food security in Sub-Saharan Africa, South Asia and Latin America. In Global Economic Cooperation: Views from G20 Countries; Springer: Berlin/Heidelberg, Germany, 2016; pp. 81–102. [Google Scholar]
- Kärenlampi, S.O.; White, P.J. Potato proteins, lipids, and minerals. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 99–125. [Google Scholar]
- Quiroga Ledezma, C.C. Native food crops for present and future generations: Their role in nutrition and health. In Sustainability of the Food System. Sovereignty, Waste, and Nutrients Biovailability; Betoret, N., Betoret, E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 3–23. [Google Scholar]
- Petropoulos, S.A.; Sampaio, S.L.; Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Ferreira, I. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part I: Root vegetables. Antioxidants 2019, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Matsuguma, L.S.; Lacerda, L.G.; Schnitzler, E.; da Silva Carvalho Filho, M.A.; Landi Franco, C.M.; Mottin Demiate, I. Characterization of native and oxidized starches of two varieties of Peruvian carrot (Arracacia xanthorrhiza, B.) from two production areas of Paraná state, Brazil. Braz. Arch. Biol. Technol. 2009, 52, 701–713. [Google Scholar] [CrossRef]
- Tresina, S.; Doss, A.; Mohan, V.R. Nutritional and antinutritional assessment of some underutilized corms, rhizomes and tubers. Trop. Subtrop. Agroecosystems 2020, 23, 2907. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)-A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.E.; Rossi, A.; Sammán, N. Health properties of oca (Oxalis tuberosa) and yacon (Smallanthus sonchifolius). Food Funct. 2015, 6, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Apaza Ticona, L.N.; Tena Pérez, V.; Bermejo Benito, P. Local/traditional uses, secondary metabolites and biological activities of Mashua (Tropaeolum tuberosum Ruíz & Pavón). J. Ethnopharmacol. 2020, 247, 112152. [Google Scholar] [PubMed]
- Salazar, D.; Arancibia, M.; Ocaña, I.; Rodríguez-Maecker, R.; Bedón, M.; López-Caballero, M.E.; Montero, M.P. Characterization and technological potential of underutilized ancestral andean crop flours from Ecuador. Agronomy 2021, 11, 1693. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Statistics Database. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 24 May 2023).
- Lugo-Morin, D.R. Innovate or perish: Food policy design in an indigenous context in a post-pandemic and climate adaptation era. J. Open Innov. Technol. Mark. Complex. 2022, 8, 34. [Google Scholar] [CrossRef]
- McGinnis, J.M.; Kraak, V.I.; Appleton Gootman, J. Food Marketing to Children and Youth: Threat or Opportunity? National Academies Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Riaz, M.N. Extruded snacks. In Handbook of Food Science, Technology, and Engineering; Hui, Y.H., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 4, p. 168. [Google Scholar]
- Serna-Saldivar, S.O. Snack Foods: Processing, Innovation, and Nutritional Aspects; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Seker, M. Extrusion of snacks, breakfast cereals, and confectioneries. In Advances in Food Extrusion Technology; Aylin Altan, M.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 169–208. [Google Scholar]
- Singh, B.; Sharma, C.; Sharma, S. Extrusion Processing. In Novel Food Processing Technologies; Nanda, V., Sharma, S., Eds.; New India Publishing Agency: New Delhi, India, 2017; pp. 1–46. [Google Scholar]
- Ortiz, S.; Guevara, G. Education and poverty: A socio-demographic analysis on the education of the Ecuadorian population between 5–75 years old segmented by poverty condition. In Proceedings of the ICERI2017 Proceedings, Seville, Spain, 16–18 November 2017; pp. 764–770. [Google Scholar]
- Patil, R.T.; Berrios, J.d.J.; Tang, J.; Swanson, B.G. Evaluation of methods for expansion properties of legume extrudates. Appl. Eng. Agric. 2007, 23, 777–783. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Akram, W.; Karim, A. Heat and Mass Transfer Modelling during Drying: Empirical to Multiscale Approaches; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Cai, Y.-Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Qiu, J.; Khalloufi, S.; Martynenko, A.; Van Dalen, G.; Schutyser, M.; Almeida-Rivera, C. Porosity, bulk density, and volume reduction during drying: Review of measurement methods and coefficient determinations. Dry. Technol. 2015, 33, 1681–1699. [Google Scholar] [CrossRef]
- Gujska, E.; Khan, K. Functional properties of extrudates from high starch fractions of navy and pinto beans and corn meal blended with legume high protein fractions. J. Food Sci. 1991, 56, 431–435. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of insects and pea powder as alternative protein and mineral sources in extruded snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Singh, N.; Smith, A.C. A comparison of wheat starch, whole wheat meal and oat flour in the extrusion cooking process. J. Food Eng. 1997, 34, 15–32. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Influence of microalgae addition in formulation on colour, texture, and extrusion parameters of corn snacks. Food Sci. Technol. Int. 2020, 26, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration properties of dietary fibre and resistant starch: A European collaborative study. LWT-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Bouvier, J.M. Melt expansion in extrusion-cooking. Feed. Technol. 1997, 1, 13–15. [Google Scholar]
- Igual, M.; Chiş, M.S.; Păucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of rose hip (Rosa canina) puree co-product in enriched corn extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef]
- Olives Barba, A.I.; Cámara Hurtado, M.; Sánchez Mata, M.C.; Fernández Ruiz, V.; López Sáenz De Tejada, M. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Khamis, H.; Lusweti, K.; Mwevura, H.; Nyanzi, S.; Kiremire, B.T. Quantification of lycopene from tomatoes and watermelons by using Beer-Lambert principle. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 38, 119–133. [Google Scholar]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.-J.; Kowalski, R.J.; Ganjyal, G.M. Food Extrusion Processing: An Overview; WSU Extension: Pullman, WA, USA, 2017. [Google Scholar]
- Adem, M.; JA, S.; Worku, A.; Neela, S. Optimization of lupine (Lupinus albus L.) composition, feed moisture content and barrel temperatures for best quality maize based extruded snack food. Nutr. Food Sci. 2020, 50, 853–869. [Google Scholar] [CrossRef]
- Sharifi, S.; Majzoobi, M.; Farahnaky, A. Effects of particle size and moisture content of maize grits on physical properties of expanded snacks. J. Texture Stud. 2021, 52, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Jiménez, X.; Caro-Corrales, J.; Gómez-Aldapa, C.A.; Zazueta-Morales, J.; Limón-Valenzuela, V.; Castro-Rosas, J.; Hernández-Ávila, J.; Aguilar-Palazuelos, E. Third generation snacks manufactured from orange by-products: Physicochemical and nutritional characterization. J. Food Sci. Technol. 2015, 52, 6607–6614. [Google Scholar] [CrossRef] [PubMed]
- Acurio, L.; Salazar, D.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Third-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours: Microwave Expansion Kinetics and Characterization. Foods 2023, 12, 2168. [Google Scholar] [CrossRef]
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana Rao, M.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.; Singh, R.R.B.; Arora, S. Evaluation of quality changes in nutritionally enriched extruded snacks during storage. J. Food Sci. Technol. 2018, 55, 3939–3948. [Google Scholar] [CrossRef]
- Jothi, J.S.; Le, T.N.D.; Kawai, K. Effects of trehalose and corn starch on the mechanical glass transition temperature and texture properties of deep-fried food with varying water and oil contents. J. Food Eng. 2020, 267, 109731. [Google Scholar] [CrossRef]
- Min, S.C.; Kim, Y.T.; Han, J.H. 18 Packaging and the Shelf Life of Cereals and Snack Foods. In Food Packaging Shelf Life. A practical Guide; Robertson, G.L., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 339–352. [Google Scholar]
- Syamaladevi, R.M.; Tang, J.; Villa-Rojas, R.; Sablani, S.; Carter, B.; Campbell, G. Influence of water activity on thermal resistance of microorganisms in low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 353–370. [Google Scholar] [CrossRef]
- Rahman, M.S.; Labuza, T.P. Water activity and food preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 465–494. [Google Scholar]
- Ramos Diaz, J.M.; Kirjoranta, S.; Tenitz, S.; Penttilä, P.A.; Serimaa, R.; Lampi, A.-M.; Jouppila, K. Use of amaranth, quinoa and kañiwa in extruded corn-based snacks. J. Cereal Sci. 2013, 58, 59–67. [Google Scholar] [CrossRef]
- Singh, D.; Chauhan, G.S.; Suresh, I.; Tyagi, S.M. Nutritional quality of extruded snacks developed from composite of rice brokens and wheat bran. Int. J. Food Prop. 2000, 3, 421–431. [Google Scholar] [CrossRef]
- Han, Y.J.; Tra Tran, T.T.; Man Le, V.V. Corn snack with high fiber content: Effects of different fiber types on the product quality. LWT 2018, 96, 1–6. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C. Interaction between starch and dietary compounds: New findings and perspectives to produce functional foods. Food Res. Int. 2023, 172, 113182. [Google Scholar] [CrossRef] [PubMed]
- Gat, Y.; Ananthanarayan, L. Effect of extrusion process parameters and pregelatinized rice flour on physicochemical properties of ready-to-eat expanded snacks. J. Food Sci. Technol. 2015, 52, 2634–2645. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.; Fatima, N.T.; Singh, M.; Singh, V.; Kaur, S.; Puranik, V.; Kumar, R.; Kaur, D. Effect of cultivar and maturity on functional properties, low molecular weight carbohydrate, and antioxidant activity of Jackfruit seed flour. J. Food Process. Preserv. 2021, 45, e15146. [Google Scholar] [CrossRef]
- Roudaut, G. Water activity and physical stability. In Water Activity in Foods: Fundamentals Applications; Barbosa-Cánovas, G.V., Fontana, A.J., Schmidt, S.J., Labuza, T.P., Eds.; Wiley: New York, NY, USA, 2020; pp. 255–269. [Google Scholar]
- Pardhi, S.D.; Singh, B.; Nayik, G.A.; Dar, B.N. Evaluation of functional properties of extruded snacks developed from brown rice grits by using response surface methodology. J. Saudi Soc. Agric. Sci. 2019, 18, 7–16. [Google Scholar] [CrossRef]
- Franco Lucas, B.; Greque de Morais, M.; Duarte Santos, T.; Vieira Costa, J.A. Effect of spirulina addition on the physicochemical and structural properties of extruded snacks. Food Sci. Technol. 2017, 37, 16–23. [Google Scholar] [CrossRef]
- Chanvrier, H.; Desbois, F.; Perotti, F.; Salzmann, C.; Chassagne, S.; Gumy, J.C.; Blank, I. Starch-based extruded cereals enriched in fibers: A behavior of composite solid foams. Carbohydr. Polym. 2013, 98, 842–853. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT-Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Bisharat, G.I.; Oikonomopoulou, V.P.; Panagiotou, N.M.; Krokida, M.K.; Maroulis, Z.B. Effect of extrusion conditions on the structural properties of corn extrudates enriched with dehydrated vegetables. Food Res. Int. 2013, 53, 1–14. [Google Scholar] [CrossRef]
- Udenigwe, C.C. Food Proteins and Peptides: Emerging Biofunctions, Food and Biomaterial Applications; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Choi, O.-J.; Zhao, C.-C.; Ameer, K.; Eun, J.-B. Effects of soy flour types and extrusion-cooking conditions on physicochemical, microstructural and sensory characteristics of puffed rice snack base. Int. J. Food Eng. 2021, 17, 473–483. [Google Scholar] [CrossRef]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Ek, P.; Kowalski, R.J.; Ganjyal, G.M. Chapter 4-Raw material behaviors in extrusion processing I (Carbohydrates). In Extrusion Cooking; Ganjyal, G.M., Ed.; Woodhead Publishing: Thorston, UK, 2020; pp. 119–152. [Google Scholar]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Natabirwa, H.; Muyonga, J.H.; Nakimbugwe, D.; Lungaho, M. Physico-chemical properties and extrusion behaviour of selected common bean varieties. J. Sci. Food Agric. 2018, 98, 1492–1501. [Google Scholar] [CrossRef]
- Mu, T.-H.; Tan, S.-S.; Xue, Y.-L. The amino acid composition, solubility and emulsifying properties of sweet potato protein. Food Chem. 2009, 112, 1002–1005. [Google Scholar] [CrossRef]
- Coloma, A.; Flores-Mamani, E.; Quille-Calizaya, G.; Zaira-Churata, A.; Apaza-Ticona, J.; Calsina-Ponce, W.C.; Huata-Panca, P.; Inquilla-Mamani, J.; Huanca-Rojas, F. Characterization of nutritional and bioactive compounds in three genotypes of mashua (Tropaeolum tuberosum Ruiz and Pavón) from different agroecological areas in Puno. Int. J. Food Sci. 2022, 2022, 7550987. [Google Scholar] [CrossRef]
- Chiang, A. Protein-Protein Interaction of Soy Protein Isolate from Extrusion Processing; University of Missouri-Columbia: Columbia, MO, USA, 2007. [Google Scholar]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Tabibloghmany, F.S.; Mazaheri Tehrani, M.; Koocheki, A. Optimization of the extrusion process through response surface methodology for improvement in functional and nutritional properties of soybean hull. J. Food Sci. Technol. 2020, 57, 4054–4064. [Google Scholar] [CrossRef]
- Chaunier, L.; Chanvrier, H.; Courcoux, P.; Della Valle, G.; Lourdin, D. Structural basis and process requirements for corn-based products crispness. In Bubbles in Food 2. Novelty, Health and Luxury; Campbell, G.M., Scanlon, M.G., Oyle, D.L., Eds.; Elsevier: St. Paul, MN, USA, 2008; pp. 369–380. [Google Scholar]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Effect of Brewer’s spent grain and temperature on physical properties of expanded extrudates from rice. LWT-Food Sci. Technol. 2017, 79, 145–151. [Google Scholar] [CrossRef]
- Duizer, L. A review of acoustic research for studying the sensory perception of crisp, crunchy and crackly textures. Trends Food Sci. Technol. 2001, 12, 17–24. [Google Scholar] [CrossRef]
- Njintang, N.Y.; Mbofung, C.M.F.; Balaam, F.; Kitissou, P.; Scher, J. Effect of taro (Colocasia esculenta) flour addition on the functional and alveographic properties of wheat flour and dough. J. Sci. Food Agric. 2008, 88, 273–279. [Google Scholar] [CrossRef]
- Bastos-Cardoso, I.; de, J.; Zazueta-Morales, J.; Martínez-Bustos, F.; Kil-Chang, Y. Development and characterization of extruded pellets of whole potato (Solanum tuberosum L.) flour expanded by microwave heating. Cereal Chem. 2007, 84, 137–144. [Google Scholar] [CrossRef]
- Kaur, G.J.; Rehal, J.; Singh, B.; Singh, A.K.; Kaur, A. Development of multigrain breakfast cereal using extrusion technology. Asian J. Dairy Food Res. 2015, 34, 219–224. [Google Scholar] [CrossRef]
- Velasco, C.; Barbosa Escobar, F.; Spence, C.; Olier, J.S. The taste of colours. Food Qual. Prefer. 2023, 112, 105009. [Google Scholar] [CrossRef]
- Minda Tedes, T.V.; Terán Rosero, G.J. Determinants of the price of potatoes and their profitability for the producer. Lat. Sci. Multidiscip. Sci. Mag. 2023, 7, 6214–6230. [Google Scholar]
- Viteri Viteri, G.I.; Zambrano, C.E. Sales prices of rice in Ecuador producer-consumer and their correlation links in the production chain. Agrar. Sci. 2016, 9, 11–17. [Google Scholar]
- Zambrano, C.E.; Andrade Arias, M.S. Productivity and prices of hard cron pre and post Covid-19 in Ecuador. Univ. Soc. J. 2021, 13, 143–150. [Google Scholar]
- Leidi, E.O.; Altamirano, A.M.; Mercado, G.; Rodriguez, J.P.; Ramos, A.; Alandia, G.; Sørensen, M.; Jacobsen, S.-E. Andean roots and tubers crops as sources of functional foods. J. Funct. Foods 2018, 51, 86–93. [Google Scholar] [CrossRef]
- Campos, D.; Noratto, G.; Chirinos, R.; Arbizu, C.; Roca, W.; Cisneros-Zevallos, L. Antioxidant capacity and secondary metabolites in four species of Andean tuber crops: Native potato (Solanum sp.), mashua (Tropaeolum tuberosum Ruiz & Pavón), oca (Oxalis tuberosa Molina) and ulluco (Ullucus tuberosus Caldas). J. Sci. Food Agric. 2006, 86, 1481–1488. [Google Scholar]
- Praseptiangga, D.; Tryas, A.A.; Affandi, D.R.; Atmaka, W.; Ariyantoro, A.R.; Minardi, S. Physical and chemical characterization of composite flour from canna flour (Canna edulis) and lima bean flour (Phaseolus lunatus). In Proceedings of the AIP Conference Proceedings, Jatinangor, Indonesia, 8–9 August 2018. [Google Scholar]
- Ligarda-Samanez, C.A.; Palomino-Rincón, H.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Arévalo-Quijano, J.C.; Huamán-Carrión, M.L.; Quispe-Quezada, U.R.; Muñoz-Saenz, J.C.; Gutiérrez-Gómez, E.; Cabel-Moscoso, D. Bioactive compounds and sensory quality in chips of native potato clones (Solanum tuberosum spp. andigena) grown in the high Andean region of Perú. Foods 2023, 12, 2511. [Google Scholar] [CrossRef]
- Catunta Quispe, D.L. Effect of Osmotic Dehydration on Antioxidant Compounds in Two Mashua Accessions (Tropaeolum Tuberosum R&P); National University of the Altiplano: Puno-Perú, Peru, 2016. [Google Scholar]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Hernández-Hernández, O.; Moreno, F.J.; Villamiel, M. Andean tubers grown in Ecuador: New sources of functional ingredients. Food Biosci. 2020, 35, 100601. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef]
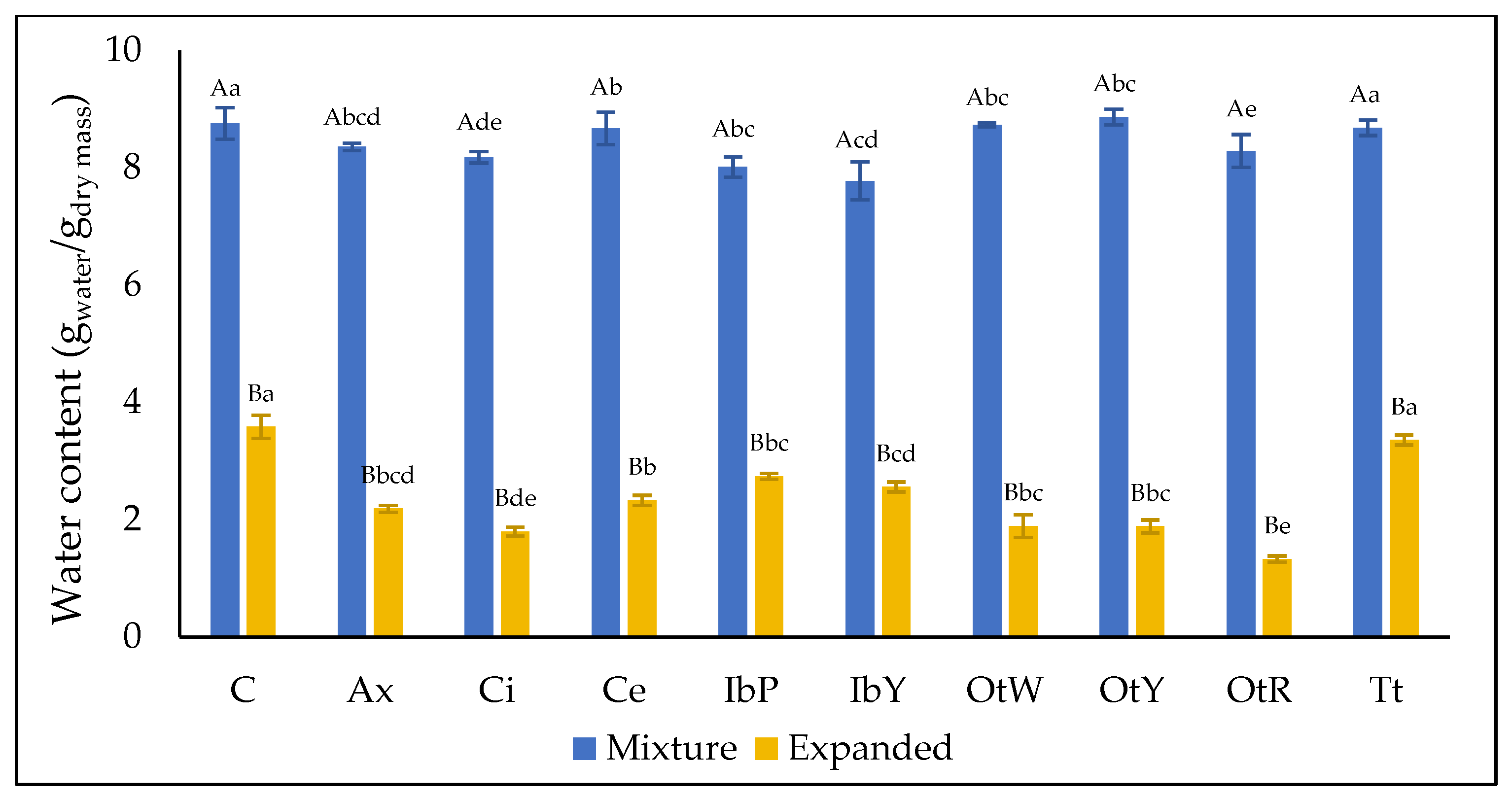
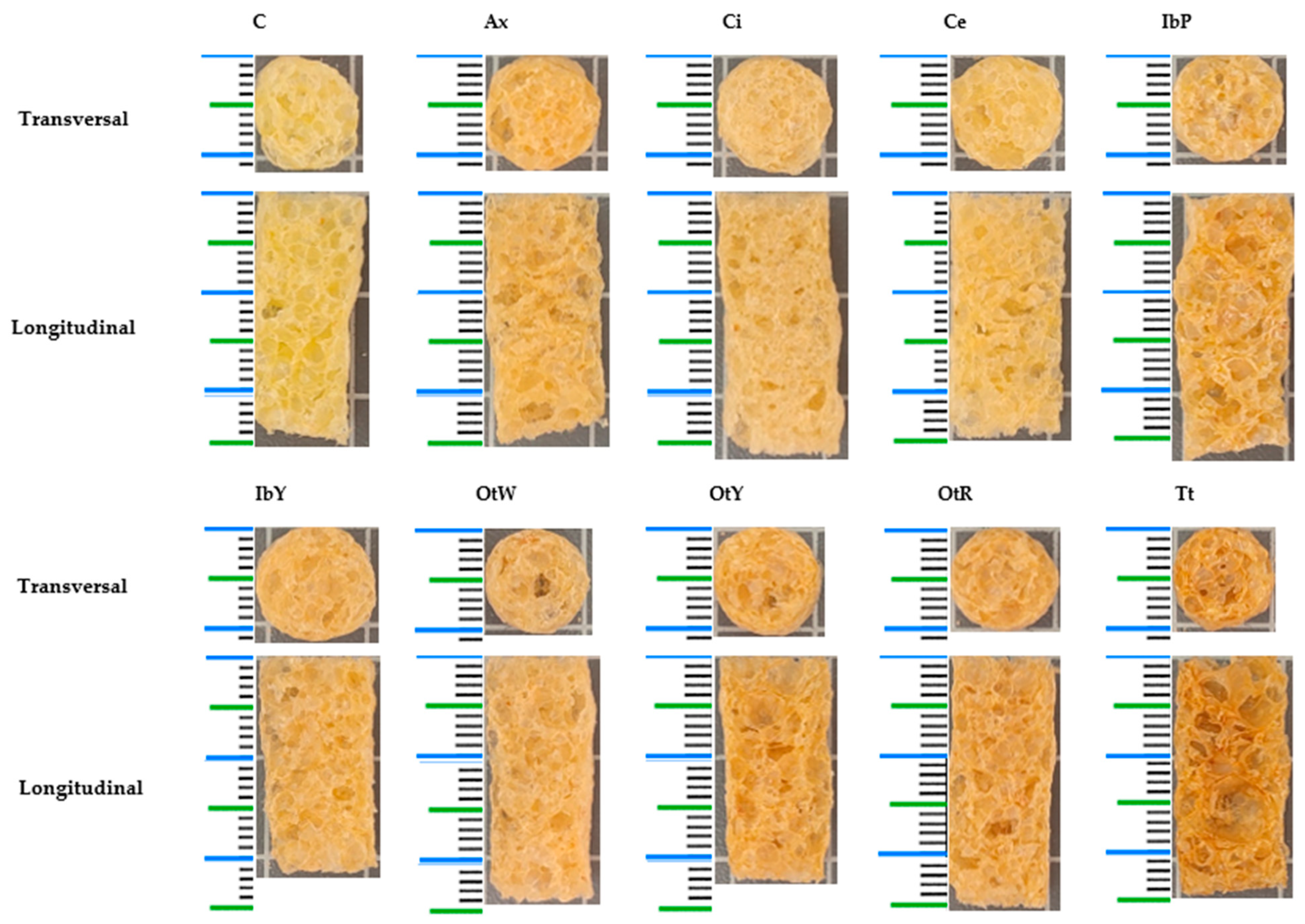
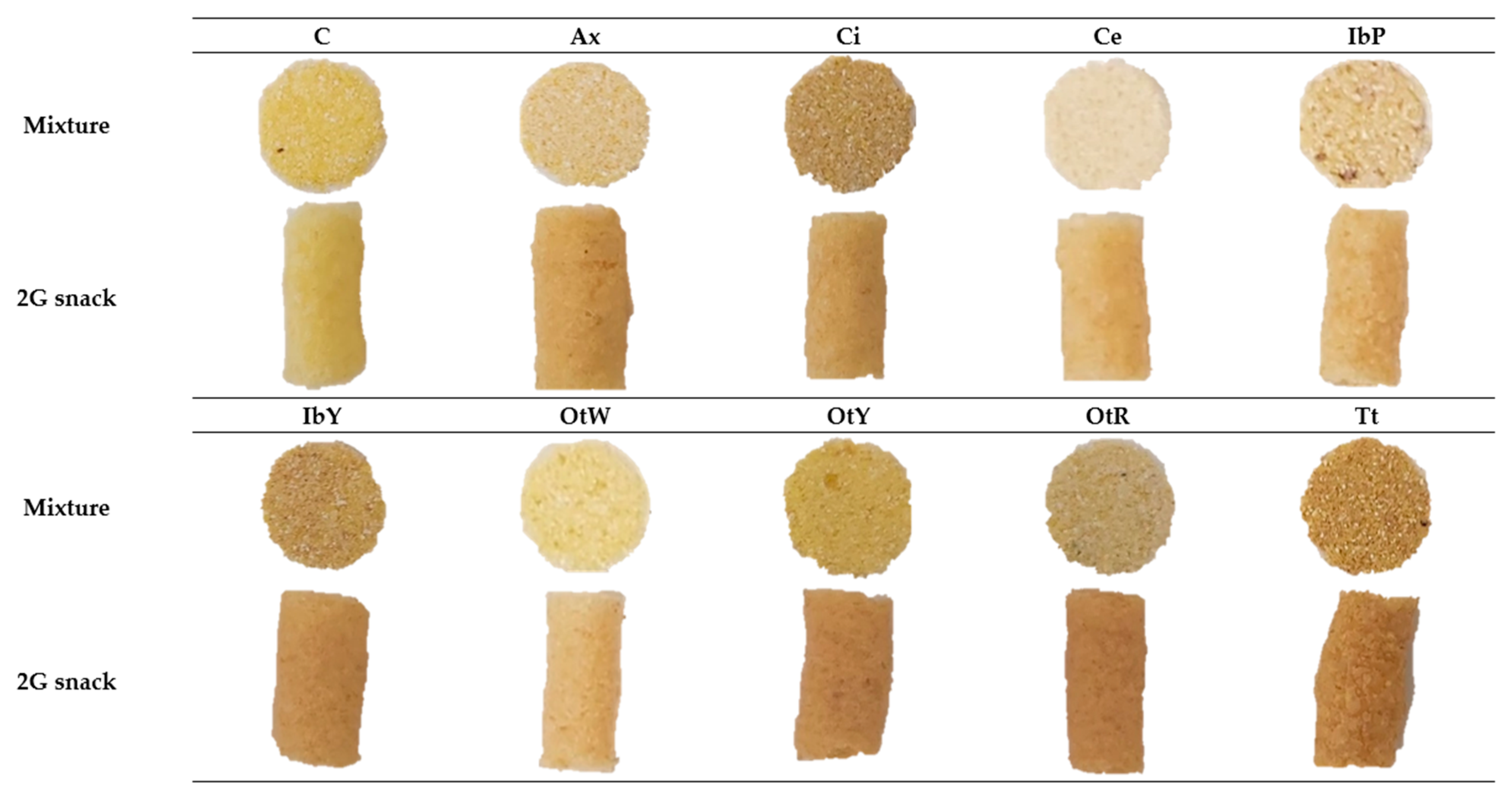
| Sample | aw | SEI | Hy | ρb | ε | WAI | WSI | SWE |
|---|---|---|---|---|---|---|---|---|
| (gw/100 g dry solid) | (g/cm3) | (%) | (%) | (mL swollen/g dry solid) | ||||
| C | 0.567 (0.003) b | 11.1 (0.8) bcd | 11.5 (0.4) d | 0.085 (0.005) c | 92.4 (0.2) a | 4.73 (0.06) c | 11.57 (0.14) a | 4.03 (0.12) bcd |
| Ax | 0.665 (0.003) a | 12.1 (0.6) a | 17.2 (0.6) a | 0.084 (0.005) c | 91.9 (0.2) ab | 4.65 (0.07) c | 12.1 (0.3) a | 4.1 (0.3) bcd |
| Ci | 0.525 (0.003) b | 10.4 (0.8) d | 14.563 (0.107) bc | 0.08 (0.007) c | 90.3 (0.6) bc | 4.72 (0.05) c | 11.6 (0.2) a | 4.32 (0.12) bcd |
| Ce | 0.631 (0.003) a | 11.6 (0.9) ab | 13.6 (0.7) c | 0.11 (0.002) b | 89.3 (0.8) c | 4.72 (0.04) c | 11.960 (0.002) a | 3.67 (0.08) d |
| IbP | 0.606 (0.003) a | 10.6 (0.7) cd | 17.0 (0.4) a | 0.156 (0.003) a | 84.9 (0.7) e | 5.27 (0.02) a | 5.68 (0.17) d | 6.1 (0.4) a |
| IbY | 0.585 (0.003) b | 11.14 (0.8) bc | 17.0 (0.6) a | 0.095 (0.006) bc | 91.5 (0.5) ab | 5.03 (0.07) b | 9.59 (0.12) b | 4.3 (0.2) bcd |
| OtW | 0.645 (0.003) a | 11.1 (0.7) bcd | 15.0 (0.3) b | 0.098 (0.004) bc | 90.82 (0.05) abc | 5.00 (0.07) b | 9.6 (0.3) b | 4.59 (0.19) bc |
| OtY | 0.674 (0.003) a | 11.6 (0.8) ab | 15.6 (0.6) b | 0.097 (0.002) bc | 90.59 (0.13) bc | 5.26 (0.03) a | 8.0 (0.6) c | 3.94 (0.15) cd |
| OtR | 0.593 (0.003) b | 11.1 (0.7) bcd | 15.4 (0.3) b | 0.09 (0.006) c | 91.4 (0.3) ab | 4.55 (0.04) c | 12.4 (0.5) a | 3.98 (0.12) cd |
| Tt | 0.546 (0.003) b | 11.2 (0.4) bc | 15.691 (0.159) b | 0.16 (0.003) a | 87.54 (0.05) d | 5.29 (0.02) a | 5.9 (0.5) d | 4.76 (0.03) b |
| Sample | Fs (N) | Fp (N) | Nsr (mm−1) | N0 | Wc (N.mm) |
|---|---|---|---|---|---|
| C | 3.1 (0.4) b | 2.2 (0.3) b | 6.1 (0.5) d | 61 (5) c | 0.50 (0.03) b |
| Ax | 1.6 (0.2) f | 1.2 (0.2) c | 6.7 (0.4) bcd | 71 (5) ab | 0.23 (0.03) e |
| Ci | 2.5 (0.12) cd | 2.01 (0.08) b | 6.2 (0.5) d | 70 (5) ab | 0.40 (0.02) cd |
| Ce | 2.2 (0.2) de | 1.8 (0.2) b | 6.6 (0.5) cd | 66 (6) b | 0.33 (0.05) d |
| IbP | 5.1 (0.9) a | 4.2 (0.6) a | 6.5 (0.7) cd | 62 (6) c | 0.82 (0.13) a |
| IbY | 1.2 (0.3) g | 0.9 (0.3) d | 7.4 (0.6) ab | 77 (5) a | 0.16 (0.04) f |
| OtW | 1.74 (0.14) ef | 1.34 (0.08) c | 7.2 (0.4) ab | 72 (3) ab | 0.23 (0.03) e |
| OtY | 1.6 (0.2) fg | 1.3 (0.2) cd | 7.4 (0.3) a | 72 (3) ab | 0.22 (0.03) ef |
| OtR | 1.55 (0.06) fg | 1.17 (0.06) cd | 7.5 (0.5) abc | 70 (5) ab | 0.22 (0.02) ef |
| Tt | 2.9 (0.5) bc | 2.3 (0.5) b | 7.3 (0.5) abc | 74 (7) ab | 0.39 (0.08) bc |
| Sample | L* | a* | b* | C* | h* | ΔE |
|---|---|---|---|---|---|---|
| Mixtures before extrusion | ||||||
| Control | 81.53 (0.04) Aa | 7.5 (0.2) Bfg | 42.7 (0.3) Ba | 43.4 (0.3) Ba | 80.1 (0.04) Aa | - |
| Ax | 79.5 (0.3) Ad | 5.3 (0.2) Bd | 27.8 (0.2) Bf | 28.3 (0.3) Be | 79.2 (0.3) Ae | - |
| Ci | 72.93 (0.02) Af | 6 (0.03) Be | 30.6 (0.2) Be | 31.2 (0.2) Bd | 79.01 (0.04) Acd | - |
| Ce | 83.67 (0.02) Ab | 4.13 (0.02) Bg | 23.87 (0.02) Bh | 24.22 (0.02) Bg | 80.18 (0.02) Ab | - |
| IbP | 73.5 (0.2) Ag | 7.17 (0.03) Bc | 27.2 (0.2) Bf | 28.14 (0.13) Be | 75.24 (0.02) Af | - |
| IbY | 74.0 (0.7) Af | 5.82 (0.14) Bde | 30.3 (0.2) Be | 30.8 (0.2) Bd | 79.1 (0.2) Ad | - |
| OtW | 80.89 (0.02) Ac | 6.3 (0.2) Bd | 37.9 (0.3) Bc | 38.4 (0.3) Bc | 80.58 (0.04) Ad | - |
| OtY | 79.46 (0.02) Ae | 6.53 (0.02) Bb | 39.64 (0.02) Bb | 40.17 (0.02) Bb | 80.64 (0.02) Ae | - |
| OtR | 79.24 (0.02) Ae | 3.4 (0.2) Bf | 26.7 (0.2) Bg | 26.9 (0.2) Bf | 82.7 (0.2) Abc | - |
| Tt | 67.21 (0.02) Ah | 8.86 (0.02) Ba | 32.8 (0.2) Bd | 34 (0.2) Bc | 74.89 (0.03) Ag | - |
| 2G Snacks | ||||||
| Control | 72.9 (0.2) Ba | 5.57 (0.03) Afg | 40.1 (0.2) Aa | 40.5 (0.2) Aa | 82.1 (0.2) Ba | 9.22 (0.06) f |
| Ax | 61.7 (0.2) Bd | 9.72 (0.14) Ad | 32.8 (0.8) Af | 34.2 (0.8) Ae | 73.47 (0.14) Be | 18.9 (0.2) c |
| Ci | 65.4 (0.2) Bf | 8.2 (0.2) Ae | 31.7 (0.02) Ae | 32.73 (0.03) Ad | 75.58 (0.13) Bcd | 7.97 (0.02) g |
| Ce | 66.7 (0.6) Bb | 8.3 (0.2) Ag | 32.3 (0.2) Ah | 33.31 (0.13) Ag | 75.7 (0.2) Bb | 19.35 (0.02) b |
| IbP | 63.7 (0.2) Bg | 9.3 (0.2) Ac | 32.9 (0.2) Af | 34.2 (0.2) Ae | 74.2 (0.2) Bf | 11.6 (0.2) d |
| IbY | 64.6 (0.3) Bf | 8.9 (0.4) Ade | 32.4 (0.3) Ae | 33.6 (0.4) Ad | 74.6 (0.6) Bd | 10.1 (0.6) e |
| OtW | 61.8 (0.4) Bc | 9.0 (0.9) Ad | 30 (0.4) Ac | 31.2 (0.5) Ac | 73.4 (0.9) Bd | 20.8 (0.2) a |
| OtY | 60.2 (0.2) Be | 10.69 (0.04) Ab | 32.47 (0.02) Ab | 34.19 (0.02) Ab | 71.8 (0.2) Be | 21 (0.02) a |
| OtR | 61 (0.3) Be | 9.7 (0.4) Af | 30.5 (0.7) Ag | 32 (0.7) Af | 72.4 (0.3) Bbc | 19.65 (0.02) b |
| Tt | 57.5 (0.4) Bh | 11.8 (0.2) Aa | 33.7 (0.2) Ad | 35.7 (0.2) Ac | 70.8 (0.2) Bg | 10.21 (0.02) e |
| Sample | TC (mgβcarotene/100 g) | TP (mgGAE/100 g) | AC (mgTrolox/100 g) |
|---|---|---|---|
| C | 1.75 (0.02) e | 111 (4) d | 0.27 (0.12) d |
| Ax | 1.75 (0.02) e | 123 (2) c | 4.6 (0.2) ab |
| Ci | 2.14 (0.06) c | 130.6 (1.2) a | 4.1 (0.4) b |
| Ce | 1.42 (0.03) f | 112 (3) d | 0.9 (0.2) d |
| IbP | 1.68 (0.02) e | 124.2 (1.3) c | 2.53 (0.14) c |
| IbY | 2.18 (0.02) c | 123.9 (1.3) c | 4.5 (0.3) ab |
| OtW | 1.96 (0.07) d | 131 (2) a | 4.58 (0.14) ab |
| OtY | 4.74 (0.04) a | 127.4 (0.8) b | 4.9 (0.2) a |
| OtR | 2.20 (0.04) c | 126 (2) bc | 1.9 (0.6) c |
| Tt | 3.76 (0.02) b | 124.2 (1.3) c | 4.8 (0.4) ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acurio, L.; Salazar, D.; Castillo, B.; Santiana, C.; Martínez-Monzó, J.; Igual, M. Characterization of Second-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours. Foods 2024, 13, 51. https://doi.org/10.3390/foods13010051
Acurio L, Salazar D, Castillo B, Santiana C, Martínez-Monzó J, Igual M. Characterization of Second-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours. Foods. 2024; 13(1):51. https://doi.org/10.3390/foods13010051
Chicago/Turabian StyleAcurio, Liliana, Diego Salazar, Bagner Castillo, Cristian Santiana, Javier Martínez-Monzó, and Marta Igual. 2024. "Characterization of Second-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours" Foods 13, no. 1: 51. https://doi.org/10.3390/foods13010051








