The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures
Abstract
:1. Introduction
2. Materials and Methods
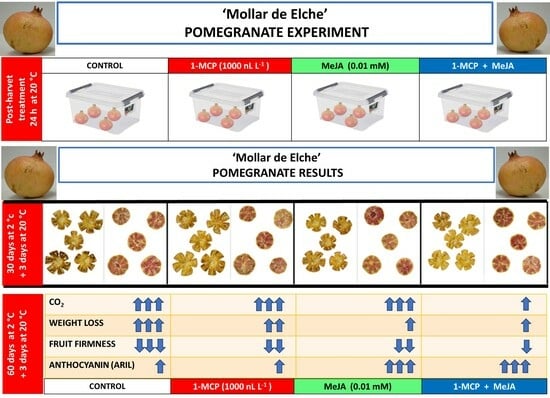
2.1. Fruit Material and Postharvest Treatments
2.2. Postharvest Quality Parameters
2.3. Bioactive Compounds
2.4. Membrane Permeability and Cold Damage
2.5. Statistical Analysis
3. Results and Discussion
3.1. Respiration Rate, Weight Loss, and Fruit Firmness Evolution
3.2. Pomegranate External and Internal Color
3.3. Total Soluble Solids (TSS) and Titratable Acidity (TA)
3.4. Effect of Postharvest Treatments on Total Bioactive Compounds
3.5. Membrane Permeability (MDA Content and EL) and Chilling Injury (CI)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fadavi, A.; Barzegar, M.; Azizi, M.H.; Bayat, M. Note. Physicochemical composition of ten pomegranate cultivars (Punica granatum L.) grown in Iran. Food Sci. Technol. Int. 2005, 11, 113–119. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer activity of Punica granatum (Pomegranate): A Review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol. Technol. 2007, 44, 26–33. [Google Scholar] [CrossRef]
- Maghoumi, M.; Amodio, M.L.; Fatchurrahman, D.; Cisneros-Zevallos, L.; Colelli, G. Pomegranate husk scald browning during Storage: A review on factors involved, their modes of action, and its association to postharvest treatments. Foods 2022, 11, 3665. [Google Scholar] [CrossRef] [PubMed]
- Lufu, R.; Ambaw, A.; Opara, U.L. The influence of internal packaging (Liners) on moisture dynamics and physical and physiological quality of pomegranate fruit during cold storage. Foods 2021, 10, 1388. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Valverde, J.M.; Valero, D.; Badiche, F.; Robles, A.; Díaz-Mula, H.M.; Serrano, M.; Martínez-Romero, D. Chilling injury control in pomegranate fruit with compostable stretchable skin film. Sci. Hortic. 2024, 323, 112480. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Chen, C.; Sun, C.; Wang, Y.; Gong, H.; Zhang, A.; Yang, Y.; Guo, F.; Cui, K.; Fan, X.; Li, X. The preharvest and postharvest application of salicylic acid and its derivatives on storage of fruit and vegetables: A review. Sci. Hortic. 2023, 312, 111858. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Habibi, F.; Valero, D.; Serrano, M.; Guillén, F. Exogenous application of glycine betaine maintains bioactive compounds, antioxidant activity, and physicochemical attributes of blood orange fruit during prolonged cold storage. Front. Nutr. 2022, 9, 873915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Jin, S.; Dong, W.; Zhang, Y.; Chen, W.; Shi, L.; Cao, S.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem. 2023, 404, 134661. [Google Scholar] [CrossRef] [PubMed]
- Maghoumi, M.; Amodio, M.L.; Cisneros-Zevallos, L.; Colelli, G. Prevention of chilling injury in pomegranates revisited: Pre- and post-harvest factors, mode of actions, and technologies involved. Foods 2023, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Faizy, A.H.; Ozturk, B.; Aglar, E.; Yıldız, K. Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J. Food Process. Preserv. 2021, 45, 15882. [Google Scholar] [CrossRef]
- Ozturk, A.; Yildiz, K.; Ozturk, B.; Karakaya, O.; Gun, S.; Uzun, S.; Gundogdu, M. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT 2019, 111, 117–124. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘Mollar de Elche’ at harvest and during postharvest storage. J. Sci. Food Agr. 2020, 100, 145–153. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Al-Otaibi, H.H.; Ali, M.R. A new approach for extending shelf-life of pomegranate arils with combined application of salicylic acid and methyl jasmonate. Horticulturae 2023, 9, 225. [Google Scholar] [CrossRef]
- Asghari, M.; Hasanlooe, A.R. Methyl jasmonate effectively enhanced some defense enzymes activity and total antioxidant content in harvested “Sabrosa” strawberry fruit. Food Sci. Nutr. 2016, 4, 377–383. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Liu, H.; Pei, H.; Jiao, J.; Jin, M.; Li, H.; Zhu, Q.; Ma, Y.; Rao, J. 1-Methylcyclopropene treatment followed with ethylene treatment alleviates postharvest chilling injury of ‘Xuxiang’ kiwifruit during low-temperature storage. Food Control 2021, 130, 108340. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Zhang, X.; Shu, P.; Cui, X.; Dong, L.; Ren, C.; Meng, D.; Li, J. Effect of methyl salicylate in combination with 1-methylcyclopropene on postharvest quality and decay caused by Botrytis cinerea in tomato fruit. J. Sci. Food Agric. 2018, 98, 3815–3822. [Google Scholar] [CrossRef]
- Liu, R.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Synergistic effect of 1-methylcyclopropene and carvacrol on preservation of red pitaya (Hylocereus polyrhizus). Food Chem. 2019, 283, 588–595. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, C.; Yi, P.; Li, L.; Wu, G.; Huang, F.; Huang, M.; Gan, T. The effects of combined 1-methylcyclopropene and melatonin treatment on the quality characteristics and active oxygen metabolism of mango fruit during storage. Foods 2023, 12, 1979. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Dai, B.; Wang, Y.; Du, X.; Huan, C.; Zheng, X. 1-methylcyclopropylene or methyl jasmonate-induced chilling tolerance in a stony hard peach cultivar. Sci. Hortic. 2022, 304, 111279. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. Food Chem. Toxicol. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Sharma, M.; Jacob, J.K.; Subramanian, J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of Abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Mitra, A.; Selvam, S.P.; Shehabudheen, S.; Anitha, P.M.; Kumar, M.M. Efficiency evaluation of cinnamon essential oil loaded nanoliposomal coating for the post-harvest management of apple (Malus domestica). Int. J. Emerg. Technol. 2020, 11, 554–559. [Google Scholar]
- Boonyaritthongchai, P.; Supapvanich, S. Effects of methyl jasmonate on physicochemical qualities and internal browning of ‘Queen’ pineapple fruit during cold storage. Hortic. Environ. Biotechnol. 2017, 58, 479–487. [Google Scholar] [CrossRef]
- Kader, A.A.; Chordas, A.; Elyatem, S.M. Responses of pomegranates to ethylene treatment and storage temperature. Calif. Agric. 1984, 38, 14–15. [Google Scholar]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence, and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Min, D.; Li, Z.; Ai, W.; Li, J.; Zhou, J.; Zhang, X.; Mu, D.; Li, F.; Li, X.; Guo, Y. The co-regulation of ethylene biosynthesis and ascorbate-glutathione cycle by methyl jasmonate contributes to aroma formation of tomato fruit during postharvest ripening. J. Agric. Food Chem. 2020, 68, 10822–10832. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Nunes, C.N.; Emond, J.P. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hortic. Soc. 2007, 120, 235–245. [Google Scholar]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Serrano, M.; Moral, J.; Castillo, S. Methyl salicylate treatments of sweet cherry trees improve fruit quality at harvest and during storage. Sci. Hortic. 2015, 197, 665–673. [Google Scholar] [CrossRef]
- Matityahu, I.; Glazer, I.; Holland, D.; Bar-Ya’akov, I.; Ben-Arie, R.; Amir, R. Total antioxidative capacity and total phenolic levels in pomegranate husks correlate to several postharvest fruit quality parameters. Food Bioproc. Technol. 2014, 7, 1938–1949. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Zeng, X.; Li, Z.; Qin, B.; He, N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: It’s interaction with malondiadehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef]
- Besada, C.; Llorca, E.; Novillo, P.; Hernando, I.; Salvador, A. Short-term high CO2 treatment alleviates chilling injury of persimmon cv. Fuyu by preserving the parenchyma structure. Food Control 2014, 51, 163–170. [Google Scholar] [CrossRef]
- Valdenegro, M.; Fuentes, L.; Bernales, M.; Huidobro, C.; Monsalve, L.; Hernández, I.; Schelle, M.; Simpson, R. Antioxidant and fatty acid changes in pomegranate peel with induced chilling injury and browning by ethylene during long storage times. Front. Plant Sci. 2022, 9, 771094. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, R.; Zhan, N.; Qin, L. Methyl jasmonate and selenium synergistically mitigative cadmium toxicity in hot pepper (Capsicum annuum L.) plants by improving antioxidase activities and reducing Cd accumulation. Environ. Sci. Pollut. Res. 2023, 30, 82458–82469. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, X.; Xie, Y.; Chen, C. Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. Food Chem. 2012, 131, 456–461. [Google Scholar] [CrossRef]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente-Mento, J.M.; Serrano, M.; Martínez-Romero, D.; Ruiz-Aracil, M.C.; Valero, D.; Guillén, F. The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures. Foods 2024, 13, 60. https://doi.org/10.3390/foods13010060
Lorente-Mento JM, Serrano M, Martínez-Romero D, Ruiz-Aracil MC, Valero D, Guillén F. The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures. Foods. 2024; 13(1):60. https://doi.org/10.3390/foods13010060
Chicago/Turabian StyleLorente-Mento, José Manuel, María Serrano, Domingo Martínez-Romero, María Celeste Ruiz-Aracil, Daniel Valero, and Fabián Guillén. 2024. "The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures" Foods 13, no. 1: 60. https://doi.org/10.3390/foods13010060
APA StyleLorente-Mento, J. M., Serrano, M., Martínez-Romero, D., Ruiz-Aracil, M. C., Valero, D., & Guillén, F. (2024). The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures. Foods, 13(1), 60. https://doi.org/10.3390/foods13010060