Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Chemicals, and Reagents
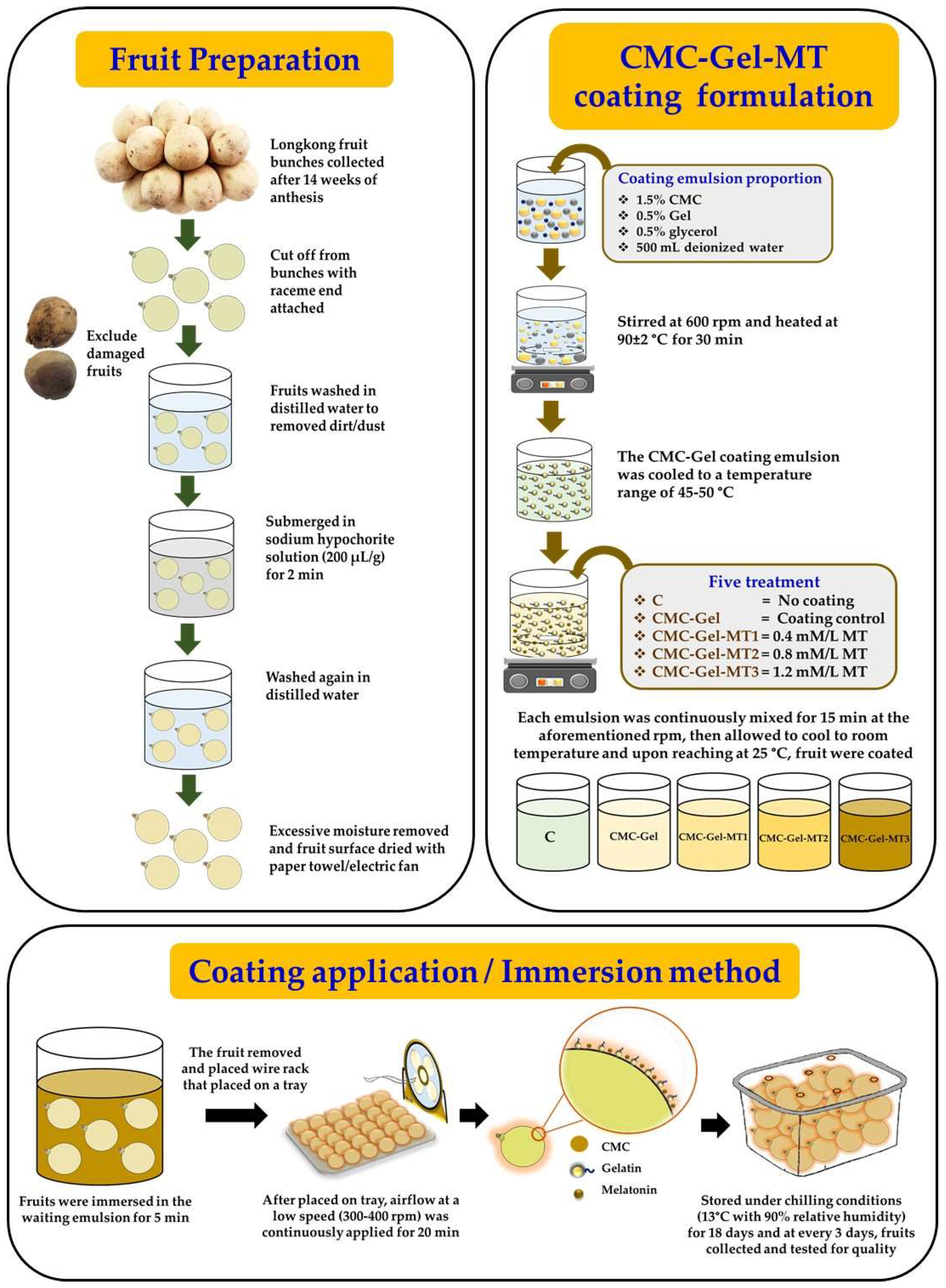
2.2. Formulation of Coating and Treatment
2.3. Quality Analysis
2.3.1. Pericarp Color and Observation of Fruit Surface Appearance
2.3.2. Chilling Injury Index (CI)
2.3.3. Electrolytic Leakage (EL)
2.3.4. Malondialdehyde (MDA) Content
2.3.5. Fruit Weight Loss
2.3.6. Respiration Rate
2.3.7. Determination of Hydrogen Peroxide (H2O2)
2.3.8. Determination of Hydroxyl Radical (OH−)
2.3.9. Determination of Superoxide Anion (O2−) Radical
2.3.10. Total Phenolic Content (TPC)
2.3.11. Antioxidant Activities
2.3.12. Pericarp Enzyme Activities
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pericarp Color and Appearance
3.2. CI Index, EL, and MDA
3.3. Fruit Weight Loss and Respiration Rate
3.4. Production of Reactive Oxygen Species
3.5. Pericarp PAL Enzyme Activity, Phenolic Content, and Antioxidant Activities
3.6. Lipid Metabolism-Based Enzyme Activities
3.7. Browning Related Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keawpeng, I.; Paulraj, B.; Venkatachalam, K. Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication. Membranes 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Sangkasanya, S. Flavor and Its Related Quality in Longkong (Aglaia dookkoo Griff.) during On-Tree Maturation and Storage. Ph.D. Thesis, Prince of Songkla University, Songkhla, Thailand, 2014. [Google Scholar]
- Venkatachalam, K. Postharvest Physiology and Handling of Longkong Fruit: A Review. Fruits 2016, 71, 289–298. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M. Lansium Domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients 2022, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Sathya, R.; Rasane, P.; Singh, J.; Kaur, S.; Bakshi, M.; Gunjal, M.; Kaur, J.; Sharma, K.; Sachan, S.; Singh, A.; et al. Strategic Advances in the Management of Browning in Fruits and Vegetables. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Charoenphun, N.; Ali, A.M.M.; Paulraj, B.; Venkatachalam, K. Effect of Aqueous N-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage. Horticulturae 2023, 9, 938. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Meenune, M. Physiological Changes of Longkong Fruit during Different Storage Conditions. Adv. Environ. Biol. 2014, 8, 362–369. [Google Scholar]
- Techavuthiporn, C.; Kanlayanarat, S. Quality changes of different form of minimally processed longkong at low temperature storage. Acta Hortic. 2010, 877, 635–640. [Google Scholar] [CrossRef]
- Wongs-Aree, C.; Noichinda, S. Chapter 10—Postharvest Physiology and Quality Maintenance of Tropical Fruits. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 275–312. ISBN 978-0-12-408137-6. [Google Scholar]
- Noichinda, C.W.-A. Sompoch Rambutan and Longkong. In Postharvest Physiological Disorders in Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-315-26747-0. [Google Scholar]
- Venkatachalam, K. Changes in Quality and Enzymes of Longkong (Aglaia dookkoo Griff.) Fruit during Storages as Affected by Maturation, Package and Methyl Jasmonate Treatment. Ph.D. Thesis, Prince of Songkla University, Yai, Thailand, 2013. [Google Scholar]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible Coatings and Antimicrobial Nanoemulsions for Enhancing Shelf Life and Reducing Foodborne Pathogens of Fruits and Vegetables: A Review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Cerqueira, M.A. Active Carboxymethyl Cellulose-Based Edible Coatings for the Extension of Fresh Goldenberries Shelf-Life. Horticulturae 2022, 8, 936. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Samsi, M.S.; Kamari, A.; Din, S.M.; Lazar, G. Synthesis, Characterization and Application of Gelatin–Carboxymethyl Cellulose Blend Films for Preservation of Cherry Tomatoes and Grapes. J. Food Sci. Technol. 2019, 56, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Esteghlal, S.; Niakousari, M.; Hosseini, S.M.H. Physical and Mechanical Properties of Gelatin-CMC Composite Films under the Influence of Electrostatic Interactions. Int. J. Biol. Macromol. 2018, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent Advances in the Improvement of Carboxymethyl Cellulose-Based Edible Films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae 2023, 9, 1099. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.-X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Hernandez, M.; Blanch, M.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. High CO2 Alleviates Cell Ultrastructure Damage in Autumn Royal Table Grapes by Modulating Fatty Acid Composition and Membrane and Cell Oxidative Status during Long-Term Cold Storage. Postharvest Biol. Technol. 2020, 160, 111037. [Google Scholar] [CrossRef]
- Njie, A.; Zhang, W.; Dong, X.; Lu, C.; Pan, X.; Liu, Q. Effect of Melatonin on Fruit Quality via Decay Inhibition and Enhancement of Antioxidative Enzyme Activities and Genes Expression of Two Mango Cultivars during Cold Storage. Foods 2022, 11, 3209. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; Von Borries-Medrano, E.; Valle-Guadarrama, S.; Aguilar-Méndez, M.A. Development of Gelatin-Carboxymethylcellulose Coatings Incorporated with Avocado Epicarp and Coconut Endocarp Extracts to Control Fungal Growth in Strawberries for Shelf-Life Extension. CyTA J. Food 2022, 20, 27–38. [Google Scholar] [CrossRef]
- Venkatachalam, K. Exogenous Nitric Oxide Treatment Impacts Antioxidant Response and Alleviates Chilling Injuries in Longkong Pericarp. Sci. Hortic. 2018, 237, 311–317. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.G. Relationship between Browning and the Activities of Polyphenoloxidase and Phenylalanine Ammonia Lyase in Banana Peel during Low Temperature Storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Du, R.; Liu, Y.; Ying, T.; Mao, L. Effect of Nitric Oxide on Antioxidative Response and Proline Metabolism in Banana during Cold Storage. J. Agric. Food Chem. 2013, 61, 8880–8887. [Google Scholar] [CrossRef] [PubMed]
- Noonim, P.; Venkatachalam, K. Combination of Salicylic Acid and Ultrasonication for Alleviating Chilling Injury Symptoms of Longkong. Food Qual. Saf. 2022, 6, fyab032. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of Clove Essential Oil and Eugenol on Quality and Browning Control of Fresh-Cut Lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Caleb, O.; Mahajan, P.; Opara, U.; Witthuhn, C. Modeling the Respiration Rates of Pomegranate Fruit and Arils. Postharvest Biol. Technol. 2012, 64, 49–54. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Chung, S.-K.; Osawa, T.; Kawakishi, S. Hydroxyl Radical-Scavenging Effects of Spices and Scavengers from Brown Mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 1997, 61, 118–123. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative Relation between the Reaction of Hydroxylamine and Superoxide Anion Radicals in Plants. Zhi Wu Sheng Li Xue Tong Xun 1990, 26, 55–57. [Google Scholar]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the Extraction of Phenolic Compounds from Apples Using Response Surface Methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon baladi CV fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-Induced Changes in Energy Status and Membrane Integrity of Harvested Litchi Fruit and Its Relation to Pathogen Resistance. J. Phytopathol. 2008, 156, 365–371. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Shi, J. Inhibitory Effects of Propyl Gallate on Browning and Its Relationship to Active Oxygen Metabolism in Pericarp of Harvested Longan Fruit. LWT Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Jiang, Y. Role of Anthocyanins, Polyphenol Oxidase and Phenols in Lychee Pericarp Browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of Peroxidase in Anthocyanin Degradation in Litchi Fruit Pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Lichanporn, I.; Srilaong, V.; Wongs-Aree, C.; Kanlayanarat, S. Postharvest Physiology and Browning of Longkong (Aglaia dookkoo Griff.) Fruit under Ambient Conditions. Postharvest Biol. Technol. 2009, 52, 294–299. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between Browning and Related Enzymes (PAL, PPO and POD) in Rambutan Fruit (Nephelium Lappaceum Linn.) Cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The Roles of Metabolism of Membrane Lipids and Phenolics in Hydrogen Peroxide-Induced Pericarp Browning of Harvested Longan Fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Saowakon, K.; Deewatthanawong, R.; Khurnpoon, L. Effect of Carboxymethyl Cellulose as Edible Coating on Postharvest Quality of Rambutan Fruit under Ambient Temperature. Int. J. Agric. Technol. 2017, 13, 1449–1457. [Google Scholar]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of Melatonin Treatment on the Enzymatic Browning and Nutritional Quality of Fresh-Cut Pear Fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Dolhaji, N.H.; Muhammad, I.D.; Yaakob, H.; Mohd Marsin, A. Chilling Injury in Pineapple Fruits: Physical Quality Attributes and Antioxidant Enzyme Activity. Food Res. 2020, 4, 86–95. [Google Scholar] [CrossRef]
- Marcantonini, G.; Bartolini, D.; Zatini, L.; Costa, S.; Passerini, M.; Rende, M.; Luca, G.; Basta, G.; Murdolo, G.; Calafiore, R.; et al. Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin. Molecules 2022, 27, 3254. [Google Scholar] [CrossRef]
- Jové, M.; Mota-Martorell, N.; Pradas, I.; Martín-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning Structure and Properties of Gelatin Edible Films through Pullulan Dialdehyde Crosslinking. LWT Food Sci. Technol. 2021, 138, 110607. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A State-of-the-Art Review on the Elaboration of Fish Gelatin as Bioactive Packaging: Special Emphasis on Nanotechnology-Based Approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Meenune, M. Physical and Chemical Quality Changes of Longkong (Aglaia dookkoo Griff.) during Passive Modified Atmospheric Storage. Int. Food Res. J. 2012, 19, 795–800. [Google Scholar]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water Loss of Fresh Fruit: Influencing Pre-Harvest, Harvest and Postharvest Factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Xanthopoulos, G.T.; Templalexis, C.G.; Aleiferis, N.P.; Lentzou, D.I. The Contribution of Transpiration and Respiration in Water Loss of Perishable Agricultural Products: The Case of Pears. Biosyst. Eng. 2017, 158, 76–85. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Nair, S.; Singh, Z. Chilling Injury during Storage Affects Respiration Rate and Fruit Quality in Kensington Pride Mango Fruit. Acta Hortic. 2006, 820, 737–744. [Google Scholar] [CrossRef]
- Biale, J.B.; Young, R.E.; Olmstead, A.J. Fruit Respiration and Ethylene Production. Plant Physiol. 1954, 29, 168–174. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Özden, Ç.; Bayindirli, L. Effects of Combinational Use of Controlled Atmosphere, Cold Storage and Edible Coating Applications on Shelf Life and Quality Attributes of Green Peppers. Eur. Food Res. Technol. 2002, 214, 320–326. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Yahia, E.M.; Golding, J.B.; Vand, S.H. Postharvest Melatonin Treatment Reduces Chilling Injury in Sapota Fruit. J. Sci. Food Agric. 2020, 100, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Jannatizadeh, A. Exogenous Melatonin Applying Confers Chilling Tolerance in Pomegranate Fruit during Cold Storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Saha, M.; Das, S.; Manna, K.; Saha, K.D. Melatonin Targets Ferroptosis through Bimodal Alteration of Redox Environment and Cellular Pathways in NAFLD Model. Biosci. Rep. 2023, 43, BSR20230128. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm (2020) 2022, 3, e161. [Google Scholar] [CrossRef]
- Venkatachalam, K. Bioactive Compounds of Longkong Fruit (Lansium Domesticum Corr.); Springer International Publishing: New York, NY, USA, 2020; pp. 107–122. ISBN 978-3-030-30181-1. [Google Scholar]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant Peptides, the Guardian of Life from Oxidative Stress. Med. Res. Rev. 2023, 25, 21986. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Esmaeili, S.; Sharifi, M.; Ghanati, F.; Soltani, B.M.; Samari, E.; Sagharyan, M. Exogenous Melatonin Induces Phenolic Compounds Production in Linum Album Cells by Altering Nitric Oxide and Salicylic Acid. Sci. Rep. 2023, 13, 4158. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballesta, M.; Zacarías, L.; Granell, A.; Lafuente, M.T. Accumulation of Pal Transcript and Pal Activity as Affected by Heat-Conditioning and Low-Temperature Storage and Its Relation to Chilling Sensitivity in Mandarin Fruits. J. Agric. Food Chem. 2000, 48, 2726–2731. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the Bulwark: Unravelling the Efficient Applications of Plant Phenolics and Tannins against Environmental Stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical Bases of Appearance and Texture Changes in Fresh-Cut Fruit and Vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Chomkitichai, W. Oxidative Damage and Total and Antioxidant Capacity Involved in Pericarp Browning during Storage of Longkong Fruit. Burapha Sci. J. 2020, 25, 102–116. [Google Scholar]
- Nagarajan, M.; Rajasekaran, B.; Benjakul, S.; Venkatachalam, K. Influence of Chitosan-Gelatin Edible Coating Incorporated with Longkong Pericarp Extract on Refrigerated Black Tiger Shrimp (Penaeus monodon). Curr. Res. Food Sci. 2021, 4, 345–353. [Google Scholar] [CrossRef]
- Li, L.; Yi, P.; Huang, F.; Tang, J.; Sun, J.; Duan, X.; Li, J.; Su, Z.; Ling, D.; Tang, Y.; et al. Effects of Phospholipase D Inhibitors Treatment on Membrane Lipid Metabolism of Postharvest Banana Fruit in Response to Mechanical Wounding Stress. Horticulturae 2022, 8, 901. [Google Scholar] [CrossRef]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript Abundance Patterns of 9- and 13-Lipoxygenase Subfamily Gene Members in Response to Abiotic Stresses (Heat, Cold, Drought or Salt) in Tomato (Solanum lycopersicum L.) Highlights Member-Specific Dynamics Relevant to Each Stress. Genes 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.; Petriccione, M. Melatonin Treatment Reduces Qualitative Decay and Improves Antioxidant System in Highbush Blueberry Fruit during Cold Storage. J. Sci. Food Agric. 2022, 102, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin Alleviates Pericarp Browning in Litchi Fruit by Regulating Membrane Lipid and Energy Metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Ismail, H.A.; Richard, I.; Ramaiya, S.D.; Zakaria, M.H.; Lee, S.Y. Browning in Relation to Enzymatic Activities and Phytochemical Content in Terap Peel (Artocarpus odoratissimus Blanco) during Postharvest Ripening. Horticulturae 2023, 9, 57. [Google Scholar] [CrossRef]
- González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Class III Peroxidases (POD) in Pepper (Capsicum annuum L.): Genome-Wide Identification and Regulation during Nitric Oxide (NO)-Influenced Fruit Ripening. Antioxidants 2023, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.; Doğan, M.; Arslan, O. Enzymatic Browning in Foods and Its Prevention. 2009. Available online: https://dspace.balikesir.edu.tr/xmlui/handle/20.500.12462/9199 (accessed on 21 December 2023).
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, J.; Wu, C.; He, J.; Wang, B. Effects of Melatonin Treatment on Browning Alleviation of Fresh-Cut Foods. J. Food Biochem. 2021, 45, e13798. [Google Scholar] [CrossRef]
- Wei, D.; Yang, J.; Xiang, Y.; Meng, L.; Pan, Y.; Zhang, Z. Attenuation of Postharvest Browning in Rambutan Fruit by Melatonin Is Associated With Inhibition of Phenolics Oxidation and Reinforcement of Antioxidative Process. Front. Nutr. 2022, 9, 905006. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K.; Charoenphun, N.; Lekjing, S.; Noonim, P. Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong. Foods 2024, 13, 72. https://doi.org/10.3390/foods13010072
Venkatachalam K, Charoenphun N, Lekjing S, Noonim P. Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong. Foods. 2024; 13(1):72. https://doi.org/10.3390/foods13010072
Chicago/Turabian StyleVenkatachalam, Karthikeyan, Narin Charoenphun, Somwang Lekjing, and Paramee Noonim. 2024. "Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong" Foods 13, no. 1: 72. https://doi.org/10.3390/foods13010072
APA StyleVenkatachalam, K., Charoenphun, N., Lekjing, S., & Noonim, P. (2024). Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong. Foods, 13(1), 72. https://doi.org/10.3390/foods13010072






