Effect of Combined Chitosan and Hyperbranched Poly-L-Lysine Based Coating on Prolonging the Shelf Life of Oyster Mushroom (Pleurotus ostreatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagent
2.3. Instruments and Equipment
2.4. Sample Treatment
2.4.1. Preparation of Preservative
2.4.2. Sample Treatment
2.5. Determination Indexes and Methods
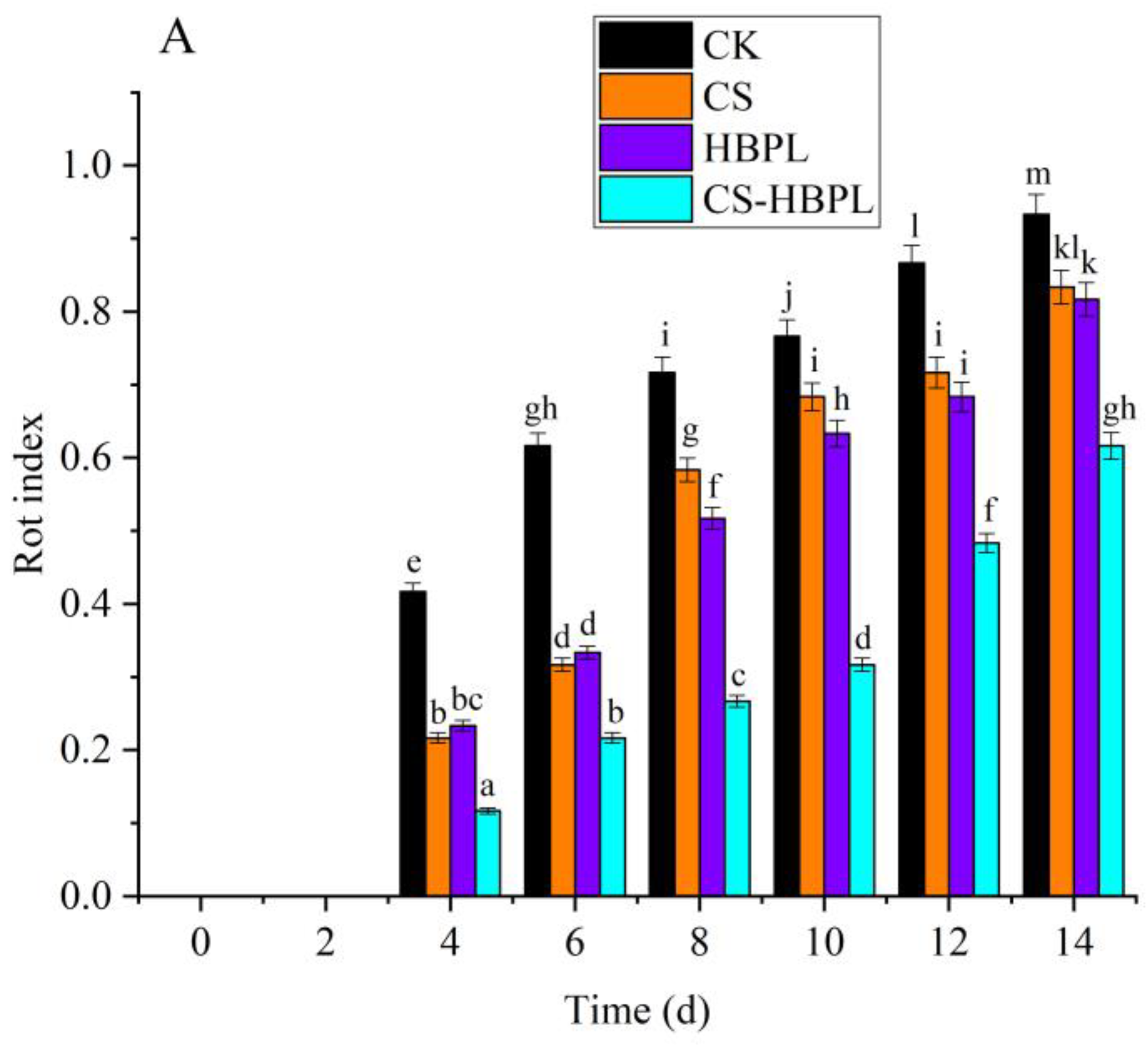
2.5.1. Rot Index
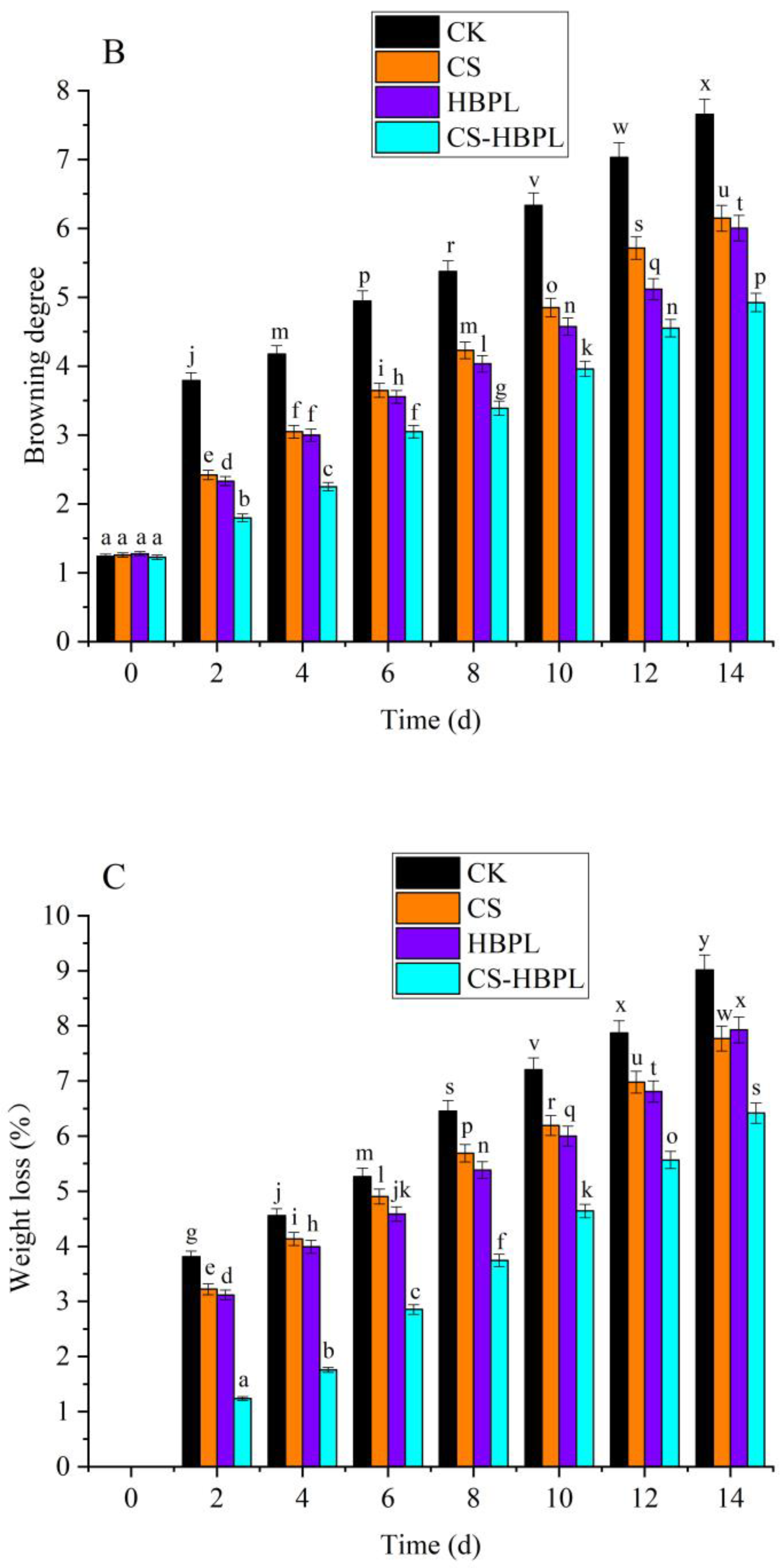
2.5.2. Browning Degree
2.5.3. Weight Loss
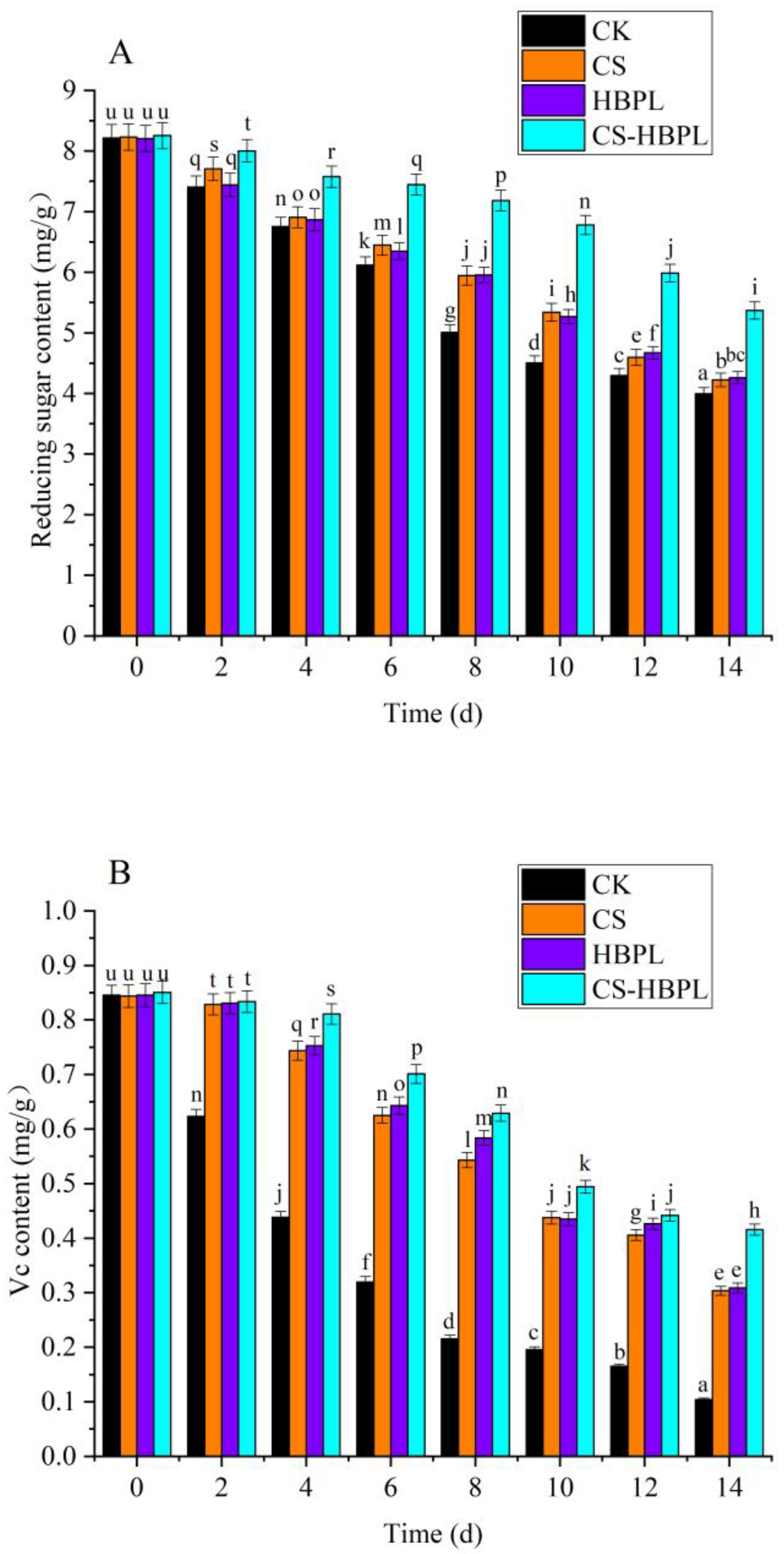
2.5.4. Reducing Sugar Content
2.5.5. Vitamin C Content
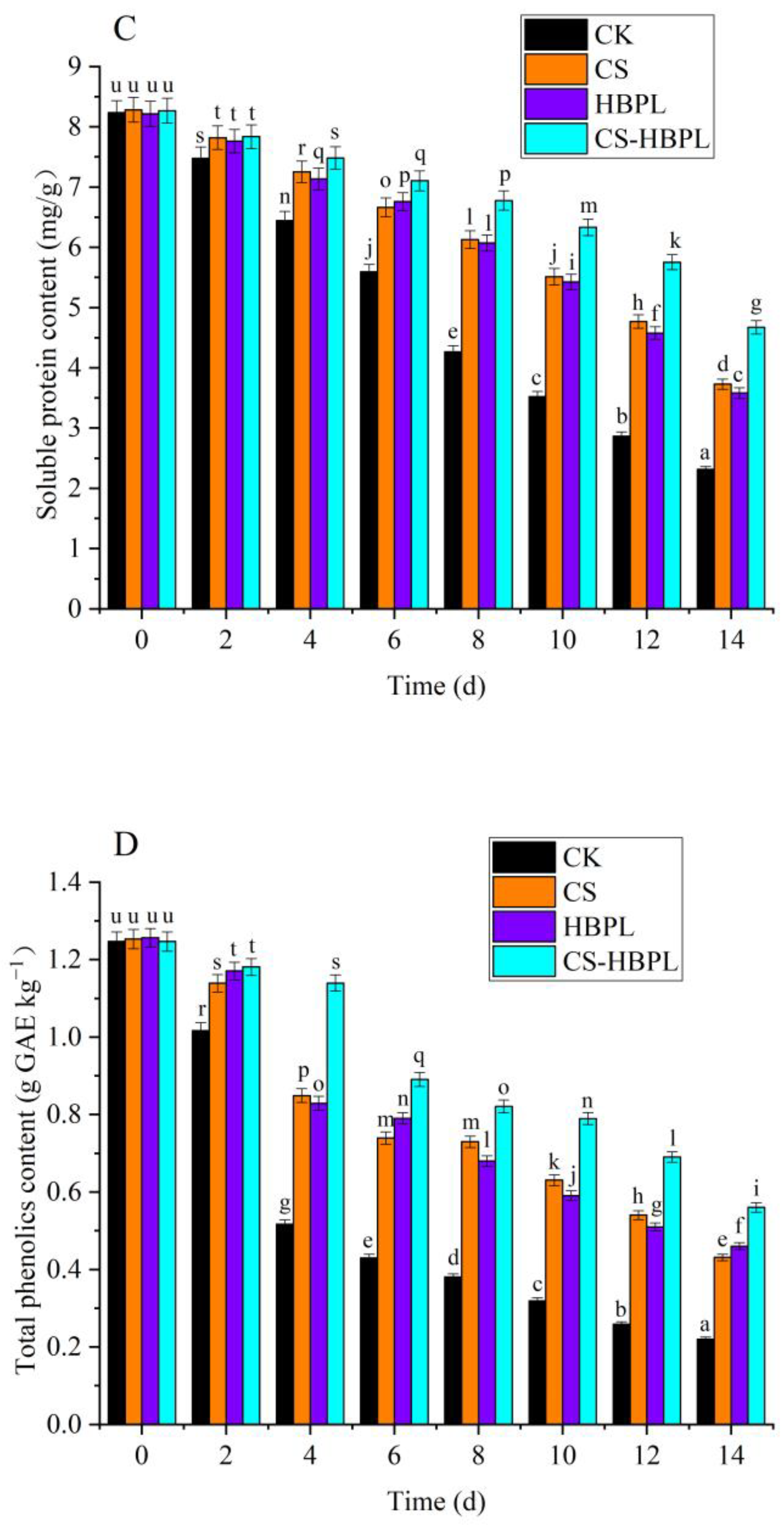
2.5.6. Total Phenols Content
2.5.7. Soluble Protein Content
2.5.8. Malondialdehyde (MDA)
2.5.9. Electrolyte Leakage Rate
2.5.10. Enzyme Activity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of CS and HBPL Treatment on Quality Characteristics during Oyster Mushroom Storage
3.2. Effects of CS and HBPL Treatment on Reducing Sugar, Soluble Protein, Total Phenol, and Vc Content during Oyster Mushroom Storage
3.3. Effects of CS and HBPL Treatment on MDA and Relative Electrolyte Leakage Rate during Oyster Mushroom Storage
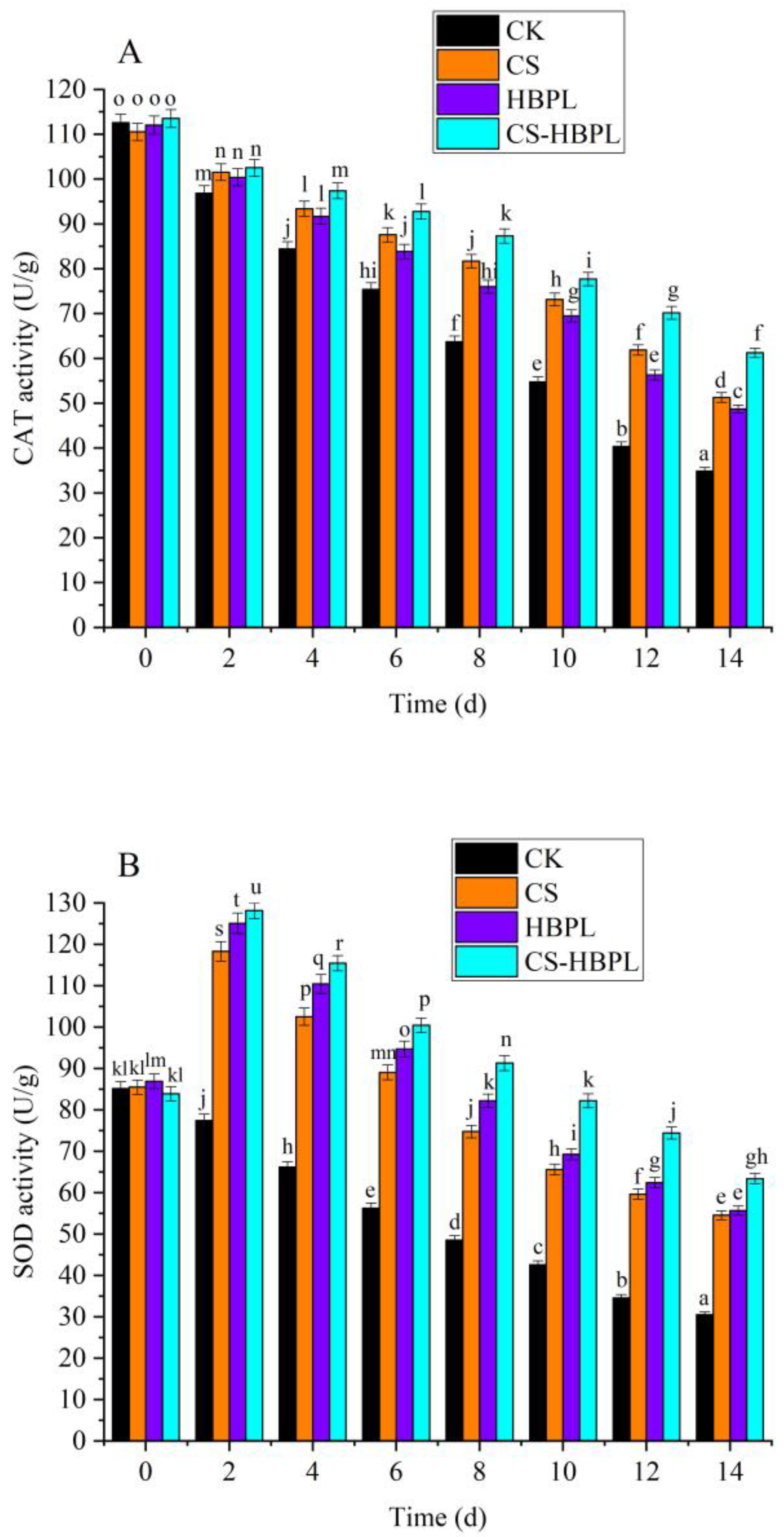
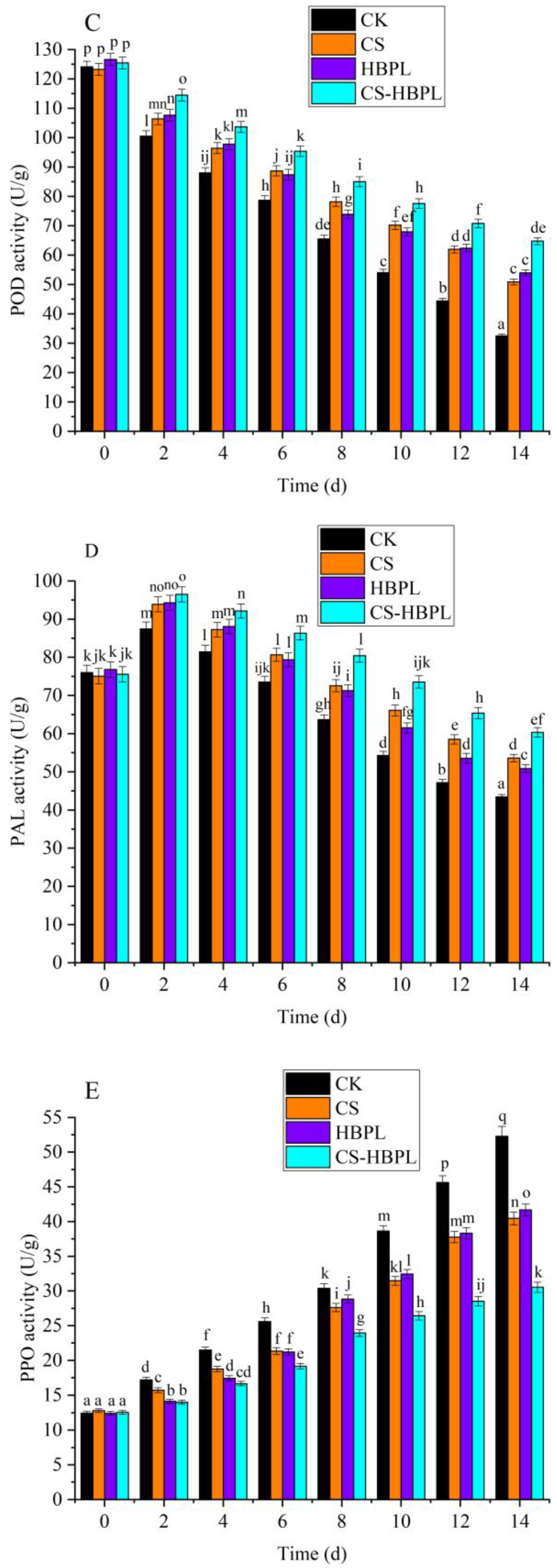
3.4. Effects of CS and HBPL Treatment on Enzyme Activity during Oyster Mushroom Storage
3.5. Correlation Analysis of All the Indexes during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toro, G.V.D.; Vega, R.C.; Garin-Aguilar, M.E.; Lara, H.L. Biological quality of proteins from three strains of Pleurotus spp. Food Chem. 2006, 94, 494–497. [Google Scholar] [CrossRef]
- Fernandes, A.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effect of gamma and electron beam irradiation on the physico-chemical and nutritional properties of mushrooms: A review. Food Chem. 2012, 135, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Wang, L.J.; Li, W.X.; Zhang, S.J.; Sun, S.J. Effect of different microvacuum pressures on quality preservation of Pleurotus ostrea. Food Sci. 2013, 34, 311–314. [Google Scholar]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Guo, J.; Deng, D.; He, G.L.; Ji, C.L.; Wang, R.T.; Long, H.; Wang, J.T. Effect of shiitake mushrooms polysaccharide and chitosan coating on softening and browning of shiitake mushrooms (Lentinus edodes) during postharvest storage. Int. J. Biol. Macromol. 2022, 218, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Hadidi, M.; Karaca, A.C.; Hedayati, S.; Tarahi, M.; Assadpour, E.; Jafari, S.M. Chitosan-grafted phenolic acids as an efficient biopolymer for food packaging films/coatings. Carbohyd. Polym. 2023, 314, e120901. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohyd. Polym. 2023, 309, e120666. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.F.; Deng, S.G.; Xu, C.H.; Kang, Y.F.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, e123716. [Google Scholar] [CrossRef]
- Ye, R.S.; Xu, H.Y.; Wan, C.X.; Peng, S.S.; Wang, L.J.; Xu, H.; Aguilar, Z.P.; Xiong, Y.H.; Zeng, Z.L.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-L-lysine. Biochem. Bioph. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef]
- Song, Z.Y.; Li, F.; Guan, H.; Xu, Y.F.; Fu, Q.J.; Li, D.P. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Ge, Y.H.; Wei, M.L.; Li, C.Y.; Chen, Y.R.; Lv, J.Y.; Meng, K.; Wang, W.H.; Li, J.R. Reactive oxygen species metabolism and phenylpropanoid pathway involved in disease resistance against Penicillium expansum in apple fruit induced by ε-poly-l-lysine. J. Sci. Food Agric. 2018, 98, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhu, J.J.; Yu, Y.S.; Hoffman, L.; Yang, X.K. Hyperbranched lysine-arginine copolymer for gene delivery. J. Biomat. Sci.-Polym. Ed. 2015, 26, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Wolf, M.; Scholl, M.; Kadlecova, Z.; Perrier, T.; Klok, H.A.; Saulnier, P.; Lenarz, T.; Stöver, T. Potential novel drug carriers for inner ear treatment: Hyperbranched polylysine and lipid nanocapsules. Nanomedicine 2009, 4, 623–635. [Google Scholar] [CrossRef]
- Zu, G.Y.; Liu, M.; Zhang, K.C.; Hong, S.N.; Dong, J.J.; Cao, Y.; Jiang, B.; Luo, L.Q.; Pei, R.J. Functional Hyperbranched Polylysine as Potential Contrast Agent Probes for Magnetic Resonance Imaging. Biomacromolecules 2016, 17, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Song, L. A Stable, Safe, and Efficient Food Preservative. CN Patent 2022,105,988,55.3, 6 September 2022. [Google Scholar]
- Yang, Z.J.; Xi, Y.; Bai, J.; Jiang, Z.W.; Wang, S.Q.; Zhang, H.L.; Dai, W.; Chen, C.C.; Gou, Z.R.; Yang, G.L.; et al. Covalent grafting of hyperbranched poly-L-lysine on Ti-based implants achieves dual functions of antibacteria and promoted osteointegration in vivo. Biomaterials 2021, 269, e120534. [Google Scholar] [CrossRef]
- Li, Y.N.; Ye, Q.Q.; Hou, W.F.; Zhang, G.Q. Development of antibacterial ε-polylysine/chitosan hybrid films and the effect on citrus. Int. J. Biol. Macromol. 2018, 118, 2051–2056. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zheng, Y.; Li, R.M. Effects of chitosan-based coatings incorporated with e-polylysine and ascorbic acid on the shelf-life of pork. Food Chem. 2022, 390, e133206. [Google Scholar] [CrossRef]
- Ma, H.F.; Lu, S.U.; Han, Q.J.; Li, W. Determination of vtamin C content in five kinds of fruits and vegetables by UV spectrophotometry. Chem. Bioeng. 2012, 29, 92–94. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, W.L.; Hu, S.J.; Kang, Y.C.; Zhang, Y.T.; Ng, T.B. Effects of Oudemansiella radicata polysaccharide on postharvest quality of oyster mushroom (Pleurotus ostreatus) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2020, 166, e111207. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from Southeast Asia. Molecules 2020, 25, e1972. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, e108849. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.J.; Luo, Z.S.; Ying, T.J. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Qin, X.Y.; Tian, P.P.; Wang, J. Toughening and its association with the postharvest quality of king oyster mushroom (Pleurotus eryngii) stored at low temperature. Food Chem. 2016, 196, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.J.; Luo, S.S.; Chen, Q.P.; Shen, L.R.; Ying, T.J. Effect of integrated application of gamma irradiation and modified atmosphere packaging on physicochemical and microbiological properties of shiitake mushroom (Lentinus edodes). Food Chem. 2010, 122, 761–767. [Google Scholar] [CrossRef]
- Xiong, Q.L.; Xing, Z.T.; Feng, Z.Y.; Tan, Q.; Bian, Y.B. Effect of 60Co γ-irradiation on postharvest quality and selected enzyme activities of Pleurotus nebrodensis. LWT-Food Sci. Technol. 2009, 42, 157–161. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, e125093. [Google Scholar] [CrossRef]
- Shi, D.F.; Zhou, R.R.; Feng, X.; Dong, X.B.; Gao, H.; Fan, X.Z.; Yin, C.M.; Qiao, Y.; Liu, Y.; Huang, W. Effects of low-dose gamma-irradiation on the water state of fresh Lentinula edodes. LWT-Food Sci. Technol. 2020, 118, e108764. [Google Scholar] [CrossRef]
- Benoit, M.A.; D’Aprano, G.; Lacroix, M. Effect of gamma-irradiation on phenylalanine ammonia-lyase activity, total phenolic content, and respiration of mushrooms (Agaricus bisporus). J. Agric. Food. Chem. 2000, 48, 6312–6316. [Google Scholar] [CrossRef]
- Jang, J.H.; Moon, K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.; Zhang, J.; Liu, W.; Guan, W.; Wang, Z. Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2017, 123, 112–118. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit. Postharvest Biol. Technol. 2019, 157, e110960. [Google Scholar] [CrossRef]
- Huque, R.; Wills, R.B.H.; Pristijono, P.; Golding, J.B. Effect of nitric oxide (NO) and associated control treatments on the metabolism of fresh-cut apple slices in relation to development of surface browning. Postharvest Biol. Technol. 2013, 78, 16–23. [Google Scholar] [CrossRef]


| RD | BR | WL | RS | Vc | TP | SP | MDA | REL | CAT | SOD | POD | PAL | PPO | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD | 1 | 0.947 ** | 0.930 ** | −0.972 ** | −0.941 ** | −0.954 ** | −0.960 ** | 0.939 ** | 0.821 ** | −0.956 ** | −0.840 ** | −0.955 ** | −0.869 ** | 0.949 ** |
| BR | 1 | 0.969 ** | −0.959 ** | −0.967 ** | −0.961 ** | −0.972 ** | 0.960 ** | 0.865 ** | −0.975 ** | −0.828 ** | −0.979 ** | −0.811 ** | 0.956 ** | |
| WL | 1 | −0.962 ** | −0.907 ** | −0.940 ** | −0.930 ** | 0.904 ** | 0.772 ** | −0.955 ** | −0.719 ** | −0.974 ** | −0.742 ** | 0.924 ** | ||
| RS | 1 | 0.920 ** | 0.932 ** | 0.969 ** | −0.934 ** | −0.841 ** | 0.973 ** | 0.797 ** | 0.967 ** | 0.860 ** | −0.962 ** | |||
| Vc | 1 | 0.964 ** | 0.958 ** | −0.958 ** | −0.842 ** | 0.943 ** | 0.899 ** | 0.939 ** | 0.838 ** | −0.918 ** | ||||
| TP | 1 | 0.939 ** | −0.933 ** | −0.778 ** | 0.941 ** | 0.823 ** | 0.949 ** | 0.776 ** | −0.902 ** | |||||
| SP | 1 | −0.972 ** | −0.920 ** | 0.987 ** | 0.847 ** | 0.967 ** | 0.883 ** | −0.975 ** | ||||||
| MDA | 1 | 0.911 ** | −0.948 ** | −0.841 ** | −0.940 ** | −0.835 ** | 0.941 ** | |||||||
| REL | 1 | −0.883 ** | −0.804 ** | −0.839 ** | −0.864 ** | 0.915 ** | ||||||||
| CAT | 1 | 0.800 ** | 0.986 ** | 0.856 ** | −0.978 ** | |||||||||
| SOD | 1 | 0.776 ** | 0.911 ** | −0.824 ** | ||||||||||
| POD | 1 | 0.816 ** | −0.967 ** | |||||||||||
| PAL | 1 | −0.897 ** | ||||||||||||
| PPO | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Ren, R.; Yao, L.; Li, J.; Tong, L.; Yuan, J.; Wang, D. Effect of Combined Chitosan and Hyperbranched Poly-L-Lysine Based Coating on Prolonging the Shelf Life of Oyster Mushroom (Pleurotus ostreatus). Foods 2024, 13, 77. https://doi.org/10.3390/foods13010077
Sun J, Ren R, Yao L, Li J, Tong L, Yuan J, Wang D. Effect of Combined Chitosan and Hyperbranched Poly-L-Lysine Based Coating on Prolonging the Shelf Life of Oyster Mushroom (Pleurotus ostreatus). Foods. 2024; 13(1):77. https://doi.org/10.3390/foods13010077
Chicago/Turabian StyleSun, Jianrui, Ruirui Ren, Linlin Yao, Jinglan Li, Li Tong, Jiangfeng Yuan, and Dahong Wang. 2024. "Effect of Combined Chitosan and Hyperbranched Poly-L-Lysine Based Coating on Prolonging the Shelf Life of Oyster Mushroom (Pleurotus ostreatus)" Foods 13, no. 1: 77. https://doi.org/10.3390/foods13010077




