Electronic Nose and Gas Chromatograph Devices for the Evaluation of the Sensory Quality of Green Coffee Beans
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sensory Analysis
2.3. Analysis of Volatile Compounds
2.4. E-Nose Measurements
2.5. Multivariate Data Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Sensory Analysis of Green Coffee Beans
3.2. VOCs of Green Coffee Beans
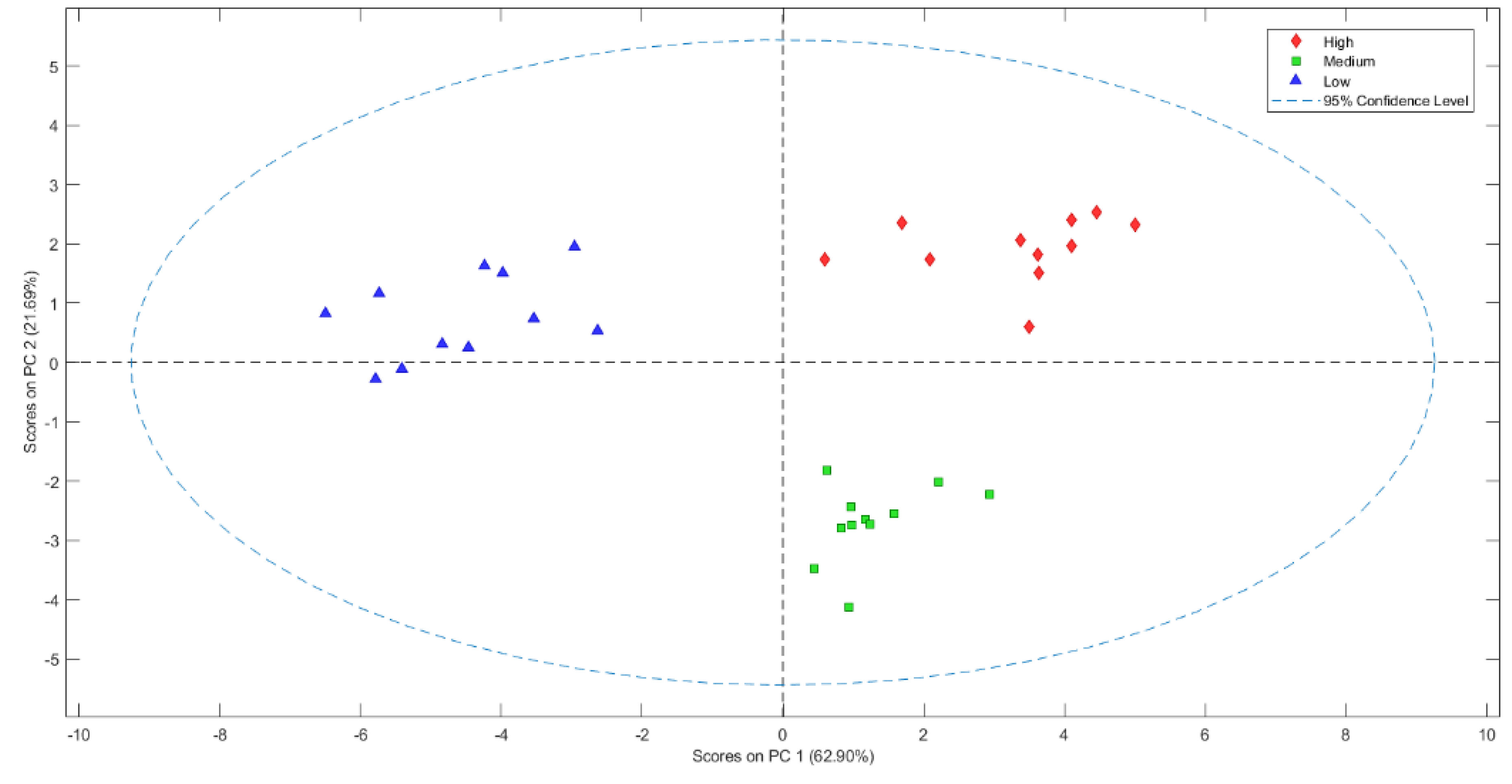
3.3. E-Nose Application to Discriminate Quality from Green Coffee Beans
3.4. Relation between E-Nose Data and Coffee Bean Aroma
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alves, R.C.; Casal, S.; Oliveira, M.B.P.P. Tocopherols in coffee brews: Influence of coffee species roast degree and brewing procedure. J. Food Compos. Anal. 2010, 23, 802–808. [Google Scholar] [CrossRef]
- Di Bella, G.; Potortì, A.G.; Turco, V.L.; Saitta, M.; Dugo, G. Plasticizer residues by HRGC–MS in espresso coffees from capsules, pods and moka pots. Food Control 2014, 41, 185–192. [Google Scholar] [CrossRef]
- Stokes, C.N.; O’Sullivan, M.G.; Kerry, J.P. Hedonic and descriptive sensory evaluation of instant and fresh coffee products European. Food Res. Technol. 2017, 243, 331–340. [Google Scholar] [CrossRef]
- Puerta, G.I. Composición química de una taza de café Centro Nacional de Investigaciones de Café (Cenicafé). Prog. Investig. Cient. 2013, 414, 10. [Google Scholar]
- Holscher, W.; Vitzthum, O.G.; Steinhart, H. Identification and sensorial evaluation of aroma-impact-compounds in roasted Colombian coffee. AGRIS 1990, 34, 205–212. [Google Scholar]
- Gotteland, M.; Saturnino de Pablo, V. Some trues concerning coffee. Rev. Chil. Nutr. 2007, 34, 105. [Google Scholar]
- Wei, F.; Tanokura, M. Chemical changes in the components of coffee beans during roasting. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. [Google Scholar] [CrossRef]
- Gutierrez, N.; Barrera, O. Selección y entrenamiento de un panel en análisis sensorial de café Coffea arabica L. Rev. Cienc. Agrar. 2015, 32, 77–87. [Google Scholar] [CrossRef][Green Version]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Evaluation of the olfactory pattern of black olives stuffed with flavored hydrocolloids. LWT-Food Sci. Technol. 2022, 163, 113556. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.; Thomas, H.A. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Martínez Gila, D.M.; Gámez García, J.; Bellincontro, A.; Mencarelli, F.; Gómez Ortega, J. Fast tool based on electronic nose to predict olive fruit quality after harvest. Postharvest Biol. Technol. 2020, 160, 111058. [Google Scholar] [CrossRef]
- Portalo-Calero, F.; Arroyo, P.; Suárez, J.I.; Lozano, J. Triangular test of amanita mushrooms by using electronic nose and sensory panel. Foods 2019, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Escuderos, M.E.; García, M.; Jiménez, A.; Horrillo, M.C. Edible and non-edible olive oils discrimination by the application of a sensory olfactory system based on tin dioxide sensors. Food Chem. 2013, 136, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Boselli, E.; Fernández, A.; Arroyo, P.; Lozano, J.; Martín-Vertedor, D. Determination of the masking effect of the ‘zapateria’ defect in flavoured stuffed olives using E-nose. Molecules 2022, 27, 4300. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef]
- Barea-Ramos, J.D.; Cascos, G.; Mesías, M.; Lozano, J.; Martín-Vertedor, D. Evaluation of the Olfactory Quality of Roasted Coffee Beans Using a Digital Nose. Sensors 2022, 22, 8654. [Google Scholar] [CrossRef]
- Ceballos, D.A.C.; Meneses, J.A.M.; López, C.A.G.; Narváez, J.A.G.; Luna, D.A.R.; García, J.H. Estudio de fragancia y aroma del café tostado con la nariz electrónica Coffee-NOSE. In Proceedings of the 2020 IX International Congress of Mechatronics Engineering and Automation (CIIMA), Cartagena, Colombia, 4–6 November 2020; pp. 1–6. [Google Scholar]
- Oates, M.J.; Abu-Khalaf, N.; Molina-Cabrera, C.; Ruiz-Canales, A.; Ramos, J.; Bahder, B.W. Detection of Lethal Bronzing Disease in Cabbage Palms (Sabal palmetto) Using a Low-Cost Electronic Nose. Comput. Electron. Agriculture 2020, 155, 348–358. [Google Scholar]
- Illy, A.; Viani, R. Espresso Coffee: The Science of Quality; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-phenylethanol (rose aroma) production potential of an isolated pichia kudriavzevii through solid-state fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Celeghini, R.M.; Debien, I.C.; Nogueira, G.C.; Meireles, M.A.A. Phenolic compounds in coffee compared to other beverages. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 137–142. [Google Scholar]
- Moreira, R.F.A.; Trugo, L.C.; De Maria, C.A.B. Volatile components in roasted coffee Part II Aliphatic alicyclic and aromatic compounds. Quim. Nova 2000, 23, 195–203. [Google Scholar] [CrossRef]
- Toci, A.T.; Farah, A.; Deliza, R. Investigação da composição volátil dos defeitos intriínsecos do café em relação aos grãos de boa qualidade. Quim. Nova 2007, 32, 1–5. [Google Scholar]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on factors affecting coffee volatiles: From seed to cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef]
- Clarke, R.J. The Flavour of Coffee Dev. Food Sci. 1986, 3B, 1–47. [Google Scholar]
- Bejarano Roncancio, J.J.; Suárez Latorre, L.M. Algunos peligros químicos y nutricionales del consumo de los alimentos de venta en espacios públicos. Rev. Univ. Ind. Santander Salud 2015, 47, 349–360. [Google Scholar]
- European Commission. Commission Recommendation (EC) No 2007/196 of 28 March 2007 Concerning on the Monitoring of the Presence of Furan in Foodstuffs, L 88/56; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Yeretzian, C.; Jordan, A.; Badoud, R.; Lindinger, W. From the green bean to the cup of coffee: Investigating coffee roasting by on-line monitoring of volatiles. Eur. Food Res. Technol. 2002, 214, 92–104. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.E.; Blanc, R.J.; Gorna, G. Phenylethyl Alcohol: An Aroma Profile Perfumer. Flavorist 1998, 23, 43–46. [Google Scholar]
- Gutiérrez, M.C.; Droguet, M. La cromatografía de gases y la espectrometría de masas: Identificación de compuestos causantes de mal olor. BoletÍN Intexter 2022, 122, 35–41. [Google Scholar]
- Rodríguez, J.; Durán, C.; Reyes, A. Electronic nose for quality control of Colombian coffee through the detection of defects in “Cup Tests”. Sensors 2009, 10, 36–46. [Google Scholar] [CrossRef]
- Gardner, J.W.; Shurmer, H.V.; Tan, T.T. Application of an electronic nose to the discrimination of coffees. Sens. Actuators B Chem. 1992, 6, 71–75. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Dwulit, A. Recognition of coffee using differential electronic nose. IEEE Trans. Instrum. Meas. 2012, 61, 1803–1810. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a low-cost electronic nose and machine learning modelling to assess coffee aroma profile and intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef] [PubMed]


| High | Medium | Low | |
|---|---|---|---|
| High | --- | p < 0.05 | p < 0.05 |
| n = 5 | n = 5 | ||
| 95% | 98% | ||
| Medium | p < 0.05 | --- | p < 0.05 |
| n = 5 | n = 5 | ||
| 100% | 95% | ||
| Low | p < 0.05 | p < 0.05 | --- |
| n = 5 | n = 5 | ||
| 100% | 96% |
| Quality | Positive Attributes | ||||
| Fruity | Herbal | Sweet | Nutty | Spiced | |
| High | 7.8 ± 0.5 a | 4.5 ± 0.4 a | 3.2 ± 0.3 a | 2.3 ± 0.2 a | 2.2 ± 0.2 a |
| Medium | 5.6 ± 0.7 b | 3.4 ± 0.2 b | 2.5 ± 0.1 b | 1.6 ± 0.3 b | 1.4 ± 0.2 b |
| Low | 3.6 ± 0.4 c | 2.6 ± 0.3 c | 2.0 ± 0.1 b | 1.5 ± 0.2 b | 1.1 ± 0.2 b |
| Quality | Negative Attributes | ||||
| Roasted | Smoky | Fermented | Earthy | ||
| High | 0.6 ± 0.2 c | 0.5 ± 0.1 c | n.d. | n.d. | |
| Medium | 2.5 ± 0.2 b | 1.5 ± 0.3 b | 1.0 ± 0.1 b | 1.5 ± 0.3 b | |
| Low | 3.6 ± 0.2 a | 2.6 ± 0.1 a | 2.6 ± 0.3 a | 2.3 ± 0.2 a | |
| R.T. (Min) | CAS Number | Volatile Compounds | Quantities | ||
|---|---|---|---|---|---|
| High | Medium | Low | |||
| Furans | |||||
| 9.0 | 108-29-2 | Dihydro-5-methyl-2(3H)-furanone | 0.70 a | 0.76 a | 0.28 b |
| 12.4 | 3777-69-3 | 2-Methyl-furan | 1.04 b | 2.07 a | 2.24 a |
| 22.0 | 51080-20-7 | Furan | 0.81 b | 2.33 a | 2.27 a |
| 38.1 | 104-61-0 | Dihydro-2-methyl-2(3H)-furanone | 1.00 ns | 0.00 | 0.00 |
| Pyrazines and pyridines | |||||
| 13.7 | 110-86-1 | Pyridine | 1.52 c | 1.94 b | 6.97 a |
| 15.9 | 75354-36-8 | (E)-7-Methyl-1,6-dioxaspiro [4.5]decane | 0.08 c | 0.26 b | 0.90 a |
| 24.1 | 24683-00-9 | 2,5-Dimethyl-pyridine | 2.46 c | 3.44 b | 4.88 a |
| Pyrroles | |||||
| 24.9 | 3146-84-7 | 3,4-Dihydro-2,2,3-trimethyl-2H-pyrrole 1-oxide | 0.00 ns | 0.00 | 1.28 |
| 28.0 | 20189-42-8 | 1H-Pyrrole-2,5-dione, 3-ethyl-4-methyl- | 1.31 a | 1.17 a | 0.54 b |
| 30.5 | 1H-Pyrrole | 1.30 ns | 1.40 | 0.00 | |
| 32.4 | 120-72-9 | 1H-Indole | 0.00 ns | 0.00 | 0.32 |
| Aldehydes | |||||
| 2.0 | 123-72-8 | Butanal | 1.49 ns | 0.00 | 0.00 |
| 2.8 | 96-17-3 | 2-Methyl-butanal | 2.27 a | 1.27 c | 1.99 b |
| 5.3 | 66-25-1 | Hexanal | 2.03 c | 2.99 b | 4.29 a |
| 10.9 | 100-52-7 | Benzaldehyde | 0.00 ns | 0.68 | 0.80 |
| 16.0 | 2548-87-0 | (E)-2-Octenal | 0.00 ns | 0.00 | 0.84 |
| 18.9 | 124-19-6 | Nonanal | 7.46 a | 7.07 a | 4.17 b |
| 22.9 | 56114-69-3 | Benzaldehyde, 2,5-bis[(trimethylsilyl)oxy]- | 2.00 a | 1.40 b | 2.02 a |
| 22.6 | 18829-56-6 | (E)-2-Nonenal | 0.00 ns | 0.00 | 1.62 |
| 26.6 | 5910-87-2 | (E,E)-2,4-Nonadienal | 0.00 ns | 0.00 | 0.66 |
| 40.7 | 121-33-5 | 4-Hydroxy-3-methoxybenzaldehyde (Vanilene) | 2.49 a | 2.78 a | 0.65 b |
| Ketones | |||||
| 20.2 | 1000302-96-9 | Propenone, 1-(4-nitrophenyl)-3-phenylamino- | 3.17 ns | 0.00 | 0.00 |
| 27.6 | 2758-18-1 | 3-Methyl-2-cyclopenten-1-one | 1.19 ns | 0.00 | 0.00 |
| 45.4 | 105-86-2 | Geraniol | 1.23 b | 1.71 a | 0.81 c |
| 71.3 | 502-69-2 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.73 c | 4.26 b | 5.07 a |
| Esters | |||||
| 8.4 | 141-32-2 | 2-Propenoic acid, butyl ester | 0.44 ns | 0.38 | 0.26 |
| 22.2 | 103-09-3 | Acetic acid, 2-ethylhexyl ester | 2.02 a | 1.59 b | 0.32 c |
| 22.2 | 72218-58-7 | 3-Methylheptyl acetate | 0.84 ns | 0.00 | 0.00 |
| 26.1 | 109-15-9 | 2-Methyl-propanoic acid, octyl ester | 4.33 ns | 0.00 | 0.00 |
| 54.3 | 1000298-25-6 | 1,3-Dimethylbutyl isopropylphosphonofluoridate | 0.66 ns | 0.00 | 0.00 |
| 74.2 | 628-97-7 | Hexadecanoic acid, ethyl ester | 1.41 a | 1.43 a | 0.88 b |
| 80.7 | 544-35-4 | Linoleic acid ethyl ester | 0.00 ns | 0.53 | 0.59 |
| Acids derivates | |||||
| 6.9 | 503-74-2 | 3-Methyl-butanoic acid | 0.34 c | 2.32 b | 2.84 a |
| 6.7 | 4536-23-6 | 2-Methyl-hexanoic acid | 1.51 a | 1.02 b | 0.39 c |
| 7.2 | 116-53-0 | 2-Methyl-butanoic acid | 0.00 ns | 0.00 | 1.80 |
| 8.2 | 541-47-9 | 3-Methyl-2-butenoic acid | 1.34 a | 0.69 b | 0.38 c |
| Carboxilic acids | |||||
| 2.3 | 64-19-7 | Acetic acid | 0.12 c | 1.02 b | 1.32 a |
| 6.5 | 109-52-4 | Pentanoic acid | 0.00 | 0.59 b | 1.80 a |
| 12.1 | 142-62-1 | Hexanoic acid | 0.00 ns | 0.00 | 1.06 |
| 12.1 | 107-92-6 | Butanoic acid | 0.00 ns | 0.00 | 1.29 |
| 24.9 | 124-07-2 | Octanoic acid | 0.83 c | 1.99 b | 5.72 a |
| 31.6 | 112-05-0 | Nonanoic acid | 2.43 c | 3.42 b | 4.80 a |
| Lactones | |||||
| 9.0 | 96-48-0 | γ-Butyrolactone | 0.00 ns | 0.00 | 0.39 |
| Aromatics | |||||
| 12.0 | 108-95-2 | Phenol | 2.34 a | 1.54 b | 0.00 |
| 14.2 | 5989-27-5 | (D-Limonene) | 1.98 a | 2.01 a | 1.36 b |
| 17.8 | 90-05-1 | 2-Methoxy-phenol (Guaiacol) | 0.66 ns | 0.00 | 0.00 |
| 34.2 | 7786-61-0 | 2-Methoxy-4-vinylphenol | 9.92 a | 7.01 b | 2.27 c |
| 37.2 | 584-84-9 | Benzene, 2,4-diisocyanato-1-methyl- | 2.48 a | 1.79 b | 0.00 |
| 75.1 | 33777-97-8 | 3-Phenyl-4-azafluorene | 0.00 ns | 0.00 | 0.33 |
| 80.2 | 15089-22-2 | N-Benzyl-N-ethyl-p-isopropylbenzamide | 0.00 ns | 0.00 | 1.38 |
| Alcohols | |||||
| 3.8 | 123-51-3 | 3-Methyl-1-butanol, | 1.51 b | 2.33 a | 2.29 a |
| 4.5 | 71-41-0 | 1-Pentanol | 0.56 ns | 0.00 | 0.00 |
| 4.8 | 513-85-9 | 2,3-Butanediol | 0.00 ns | 0.00 | 0.92 |
| 7.4 | 111-27-3 | 1-Hexanol | 2.66 a | 2.38 b | 0.65 c |
| 11.9 | 3391-86-4 | 1-Octen-3-ol | 0.96 b | 1.29 a | 1.24 a |
| 14.4 | 104-76-7 | 2-Ethyl-1-hexanol | 1.81 a | 1.97 a | 1.25 b |
| 14.5 | 100-51-6 | Benzyl alcohol | 2.02 a | 2.06 a | 1.26 b |
| 19.3 | 60-12-8 | Phenylethyl Alcohol | 11.60 a | 11.82 a | 4.85 b |
| 22.0 | 768-95-6 | 1-Adamantanol | 0.98 c | 2.14 a | 1.53 b |
| 23.6 | 143-08-8 | 1-Nonanol | 1.07 ns | 0.00 | 0.00 |
| 26.9 | 122-99-6 | 2-Phenoxy-ethanol | 1.45 b | 1.80 a | 0.67 c |
| Hydrocarbons | |||||
| 18.6 | 78-70-6 | 3,7-Dimethyl-1,6-octadien-3-ol (Linalool) | 0.73 a | 0.65 a | 0.36 b |
| 23.6 | 124-11-8 | 1-Nonene | 0.00 ns | 0.00 | 0.90 |
| 25.7 | 112-40-3 | Dodecane | 0.00 ns | 0.00 | 0.53 |
| 41.4 | 629-59-4 | Tetradecane | 0.85 c | 3.55 a | 3.35 b |
| Sulfur compounds | |||||
| 1.9 | 75-18-3 | Dimethyl sulfide | 0.00 | 1.98 b | 5.48 a |
| Thiophenes | |||||
| 16.8 | 2557-78-0 | o-Fluorothiophenol | 2.88 ns | 2.99 | 0.00 |
| 25.2 | 1708-32-3 | 2,5-Dihydro-thiophene | 0.33 c | 1.67 b | 2.79 a |
| Others | |||||
| 8.1 | 100-42-5 | Styrene | 1.01 a | 0.52 n | 0.14 c |
| 11.6 | 127-91-3 | Beta-Pinene | 1.67 ns | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos, G.; Lozano, J.; Montero-Fernández, I.; Marcía-Fuentes, J.A.; Aleman, R.S.; Ruiz-Canales, A.; Martín-Vertedor, D. Electronic Nose and Gas Chromatograph Devices for the Evaluation of the Sensory Quality of Green Coffee Beans. Foods 2024, 13, 87. https://doi.org/10.3390/foods13010087
Cascos G, Lozano J, Montero-Fernández I, Marcía-Fuentes JA, Aleman RS, Ruiz-Canales A, Martín-Vertedor D. Electronic Nose and Gas Chromatograph Devices for the Evaluation of the Sensory Quality of Green Coffee Beans. Foods. 2024; 13(1):87. https://doi.org/10.3390/foods13010087
Chicago/Turabian StyleCascos, Gema, Jesús Lozano, Ismael Montero-Fernández, Jhunior Abrahan Marcía-Fuentes, Ricardo S. Aleman, Antonio Ruiz-Canales, and Daniel Martín-Vertedor. 2024. "Electronic Nose and Gas Chromatograph Devices for the Evaluation of the Sensory Quality of Green Coffee Beans" Foods 13, no. 1: 87. https://doi.org/10.3390/foods13010087
APA StyleCascos, G., Lozano, J., Montero-Fernández, I., Marcía-Fuentes, J. A., Aleman, R. S., Ruiz-Canales, A., & Martín-Vertedor, D. (2024). Electronic Nose and Gas Chromatograph Devices for the Evaluation of the Sensory Quality of Green Coffee Beans. Foods, 13(1), 87. https://doi.org/10.3390/foods13010087










