Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review
Abstract
1. Introduction
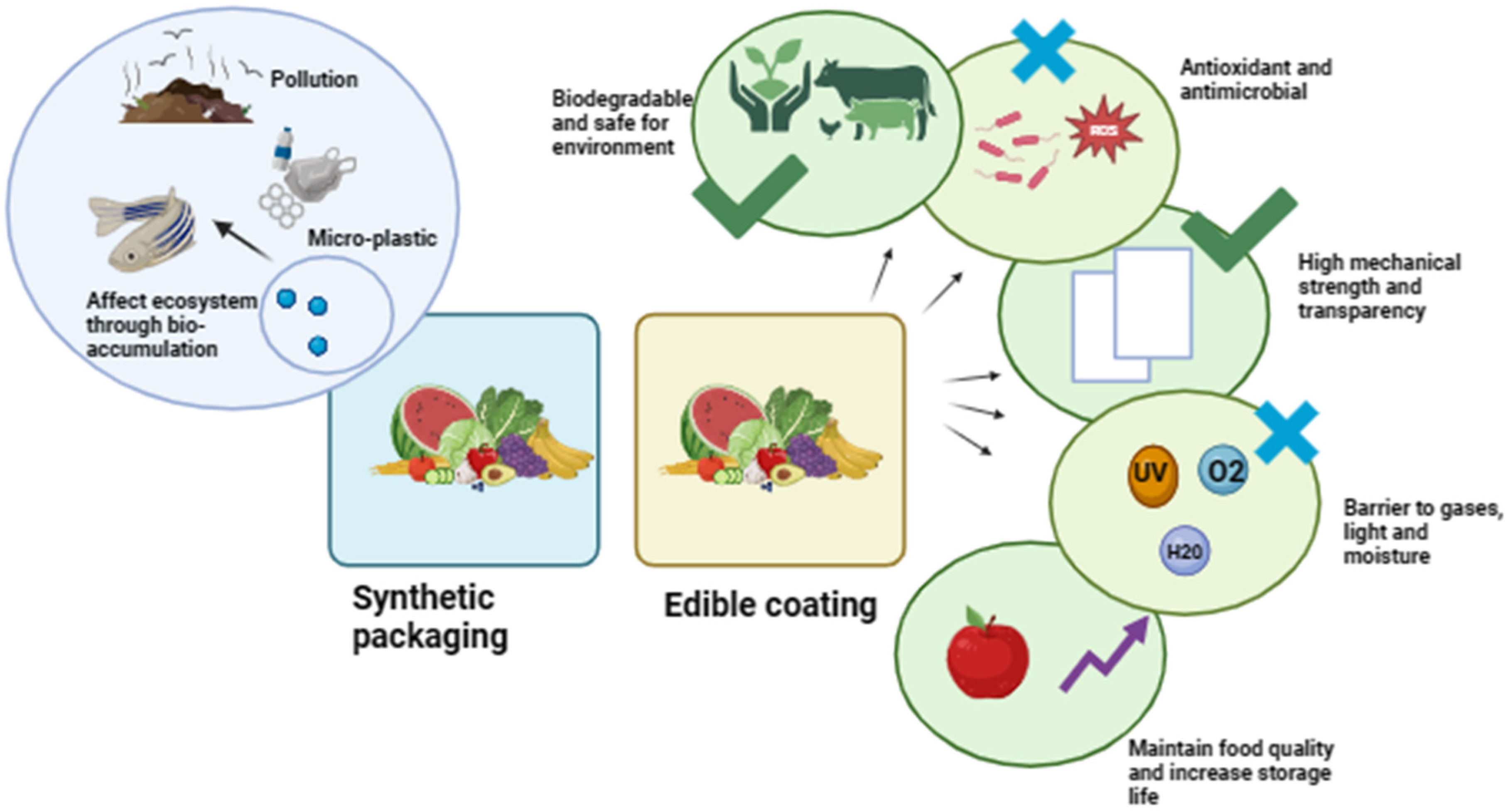
2. Fruit Preservation Techniques and Their Limitations
2.1. Physical Preservation
2.2. Chemical Preservation
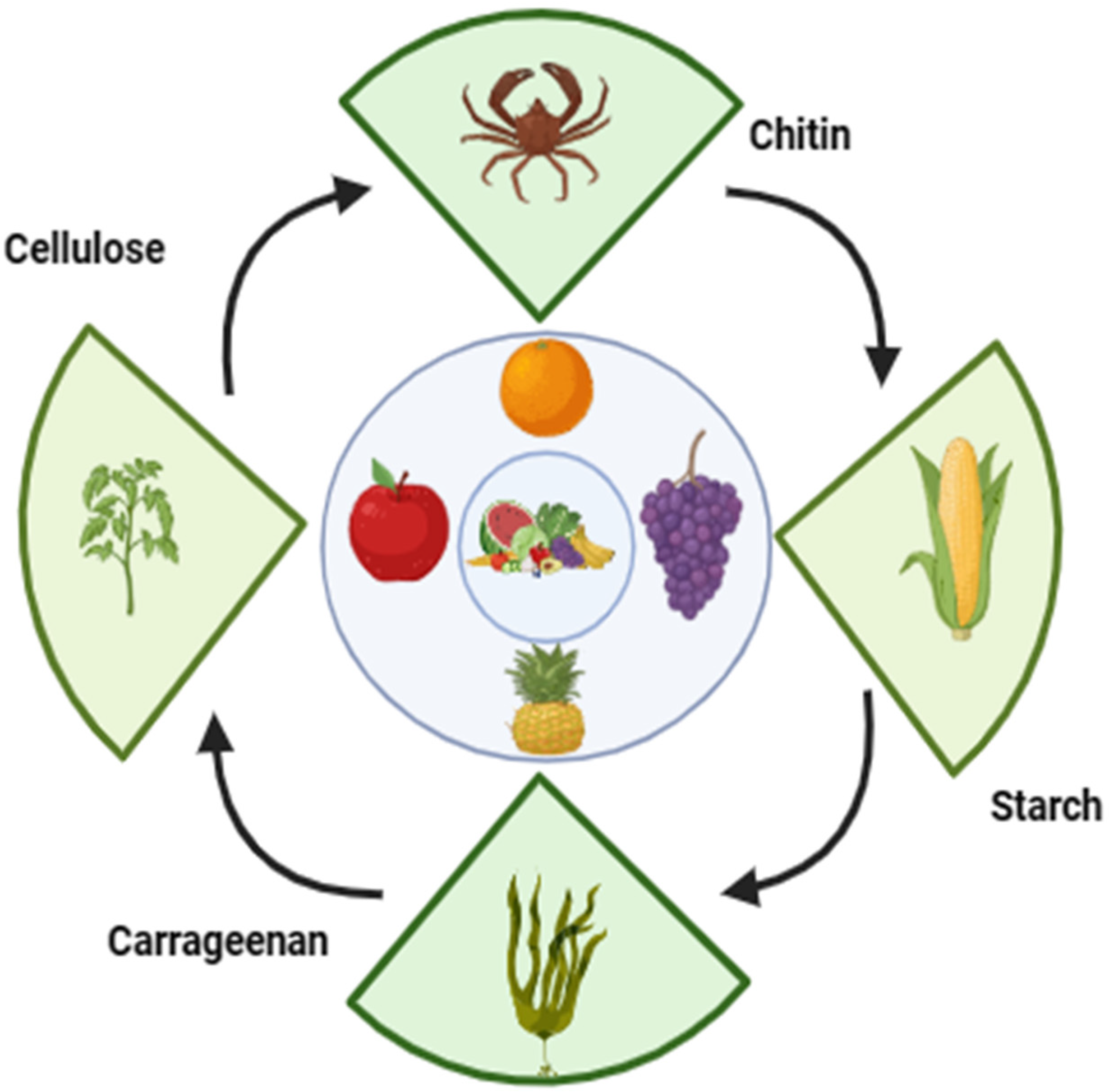
3. Different Polysaccharide-Based Edible Coatings for Fruit Preservation
3.1. Cellulose
3.2. Pectin
3.3. Starch
3.4. Chitosan
3.5. Carrageenan
3.6. Alginate
3.7. Pullulan
3.8. Natural Gum
3.9. Use of Biopolymer Nanostructure for the Preparation of Edible Coating
| Polysaccharide Based Biopolymer | Sources | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Cellulose | Plant cell wall and algal cell wall | Abundant in nature, Increased water holding capacity, High mechanical strength, non-toxicity, high crystalline property, and high molecular weight | High water absorption capacity reduces the water resistance in packaging | [69] |
| Chitosan | Insect exoskeleton and crustaceans | High antimicrobial activity, antioxidant and pigment absorption, biocompatible | Solubility in aqueous solution is poor | [54] |
| Alginate | Algae | Biocompatibility, High structural integrity and long-term storage capacity, thickening capacity, emulsifier, and stabilizer | Limitation in moisture barrier property, unpleasant odor, and cause precipitation at less pH | [70] |
| Starch | Plant sources such as cereals and potatoes, cassava | Reduced cost, biodegradable and abundant in nature, high mechanical property, selective permeability to gases | Requires plasticizers to improve the adhesion property | [71] |
| Pullulan | Fungal source | Barrier to oxygen and high thermal stability, good structural flexibility, water-soluble, high adhesion property | High cost, breakability, and high hydrophilicity | [72] |
| Carrageenan | Extracted from red seaweed | Biocompatibility and bio-adhesives | High hydrophilicity and poor mechanical strength | [73] |
| Pectin | Dicotyledonous plants and fruit peel like apple | Gel formation, biodegradability, emulsifier, and prebiotic properties | Hydrophilicity | [74,75] |
| Natural gum | Seeds and guar | Techno-functional properties, biocompatibility, thickening agent, and emulsifier | Limitation in mechanical and structural characteristics in raw form | [76,77] |
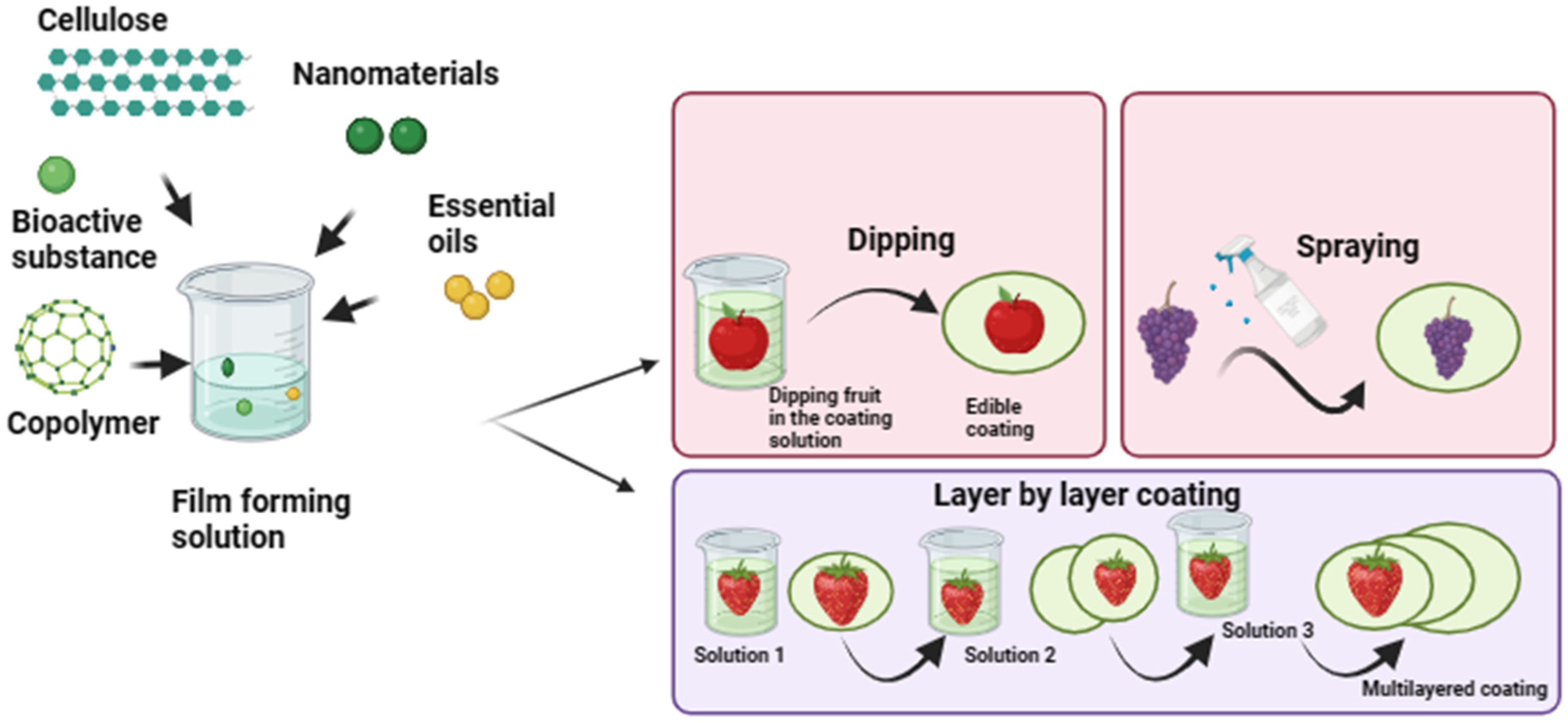
4. Techniques for the Synthesis of Biopolymer-Based Edible Coatings for Fruit Preservation
4.1. Dipping Method
4.2. Layer-by-Layer Edible Coating
4.3. Spraying Method
4.4. Panning Method
4.5. Fluidized Bed Coating
5. Properties of Polysaccharide-Based Edible Coatings for Fruit Preservation
5.1. Barrier Properties
5.2. Optical Properties
5.3. Structural Properties
5.4. Thermal Properties
5.5. Antimicrobial Properties
5.6. Antioxidant Properties
5.7. Enzymatic Browning
5.8. Adhesion
5.9. Fruits’ Firmness and Texture
6. Constraints of Utilizing Polysaccharide Edible Coating
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera Indica L.) Polyphenols: Anti-Inflammatory Intestinal Microbial Health Benefits, and Associated Mechanisms of Actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef] [PubMed]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.F.; Khalid, W.; Akram, S.; Khalid, M.A.; Zubair, M.; Kauser, S.; Abdelsamea Mohamedahmed, K.; Aziz, A.; Anusha Siddiqui, S. Bioactive Profile and Functional Food Applications of Banana in Food Sectors and Health: A Review. Int. J. Food Prop. 2022, 25, 2286–2300. [Google Scholar] [CrossRef]
- Cervantes, L.; Martinez-Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of Phenolic Compounds in Strawberry, Raspberry and Blueberry: Insights for Breeding Programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar] [CrossRef]
- Anand, S.; Barua, M.K. Modeling the Key Factors Leading to Post-Harvest Loss and Waste of Fruits and Vegetables in the Agri-Fresh Produce Supply Chain. Comput. Electron. Agric. 2022, 198, 106936. [Google Scholar] [CrossRef]
- Corbo, M.R.; Speranza, B.; Campaniello, D.; D’amato, D.; Sinigaglia, M. Fresh-Cut Fruits Preservation: Current Status and Emerging Technologies. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1143–1154. [Google Scholar]
- Alzamora, S.M.; Guerrero, S.N.; López-Malo, A.; Welti-Chanes, J.; Palou, E.; Argaiz, A.; Tapia, M.S. Combined Preservation Techniques for Fresh Fruit. In Improving the Safety of Fresh Fruit and Vegetables; Elsevier: Amsterdam, The Netherlands, 2005; pp. 599–630. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life. Agriengineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Kokkuvayil Ramadas, B.; Rhim, J.-W.; Roy, S. Recent Progress of Carrageenan-Based Composite Films in Active and Intelligent Food Packaging Applications. Polymers 2024, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Montaño-Leyva, B.; Aguirre-Güitrón, L.; Moreno-Hernández, C.L.; Fonseca-Cantabrana, Á.; del Carmen Romero-Islas, L.; González-Estrada, R.R. Impact of Edible Coatings on Quality of Fruits: A Review. Food Control 2022, 139, 109063. [Google Scholar] [CrossRef]
- Kumar, R.; Kapur, S. Morpholine: A Glazing Agent for Fruits and Vegetables Coating/Waxing. Int. J. Sci. Technol. Eng. 2016, 2, 694–697. [Google Scholar]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3823–3838. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An Overview of Fruit and Vegetable Edible Packaging Materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Lomonaco, T.; Manco, E.; Corti, A.; La Nasa, J.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Modugno, F.; Ceccarini, A.; Fuoco, R.; et al. Release of Harmful Volatile Organic Compounds (VOCs) from Photo-Degraded Plastic Debris: A Neglected Source of Environmental Pollution. J. Hazard. Mater. 2020, 394, 122596. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Roy, S.; Purohit, S.D.; Ghosh, T. Biopolymers for food packaging and biomedical applications: Options or obligations? Coatings 2022, 12, 1261. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz, Ł. Recent progress on UV-light barrier food packaging films–a systematic review. Innov. Food Sci. Emerg. Technol. 2023, 91, 103550. [Google Scholar] [CrossRef]
- Roy, S.; Chawla, R.; Santhosh, R.; Thakur, R.; Sarkar, P.; Zhang, W. Agar-based edible films and food packaging application: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104198. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose Nanomaterials Emulsion Coatings for Controlling Physiological Activity, Modifying Surface Morphology, and Enhancing Storability of Postharvest Bananas (Musa acuminate). Food Chem. 2017, 232, 359–368. [Google Scholar] [CrossRef]
- Mondal, K.; Ghosh, T.; Bhagabati, P.; Katiyar, V. Chapter 8—Sustainable Nanostructured Materials in Food Packaging. In Dynamics of Advanced Sustainable Nanomaterials and Their Related Nanocomposites at the Bio-Nano Interface; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–213. ISBN 978-0-12-819142-2. [Google Scholar] [CrossRef]
- Ghosh, T.; Katiyar, V. Nanochitosan Functionalized Hydrophobic Starch/Guar Gum Biocomposite for Edible Coating Application with Improved Optical, Thermal, Mechanical, and Surface Property. Int. J. Biol. Macromol. 2022, 211, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Melo, N.F.C.B.; de MendonçaSoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.P.; da Costa Tavares-Filho, J.H.; Galembeck, A.; Stamford, T.L.M.; Stamford-Arnaud, T.M.; et al. Effects of Fungal Chitosan Nanoparticles as Eco-Friendly Edible Coatings on the Quality of Postharvest Table Grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; Albiero, B.R.; Calisto, Í.H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Junior, S.B.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-Nanocomposite Edible Coatings Based on Arrowroot Starch/Cellulose Nanocrystals/Carnauba Wax Nanoemulsion Containing Essential Oils to Preserve Quality and Improve Shelf Life of Strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of Polysaccharides-Based Edible Coatings for Citrus Fruits: A Layer-by-Layer Approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of Chitosan-Based Apple Peel Polyphenols Edible Coating on the Preservation of Strawberry (Fragaria ananassa Cv Hongyan) Fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/Carboxymethyl Cellulose/Starch-Based Active Coating with Grapefruit Seed Extract to Extend the Shelf Life of Green Chilli. Ind. Crops Prod. 2023, 199, 116752. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of Nitric Oxide and Melatonin in Improving Postharvest Fruit Quality and the Separate and Crosstalk Biochemical Mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. Multiple 1-MCP Treatment More Effectively Alleviated Postharvest Nectarine Chilling Injury than Conventional One-Time 1-MCP Treatment by Regulating ROS and Energy Metabolism. Food Chem. 2020, 330, 127256. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Opara, U.L. Recent Developments on Dynamic Controlled Atmosphere Storage of Apples—A Review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV Treatment Improved the Quality of Postharvest Fruits and Vegetables by Inducing Resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Zhang, J.; Sheng, Z.; Cao, J.; Jiang, W. Different Molecular Weights Chitosan Coatings Delay the Senescence of Postharvest Nectarine Fruit in Relation to Changes of Redox State and Respiratory Pathway Metabolism. Food Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sabale, V.; Sabale, P.M.; Lakhotiya, C.L. Comparative Evaluation of Rice Bran Wax as an Ointment Base with Standard Base. Indian J. Pharm. Sci. 2009, 71, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; Zhang, Z. The Role of Different Natural Organic Acids in Postharvest Fruit Quality Management and Its Mechanism. Food Front. 2023, 4, 1127–1143. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Singh, G.P.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Tseng, Y.-K.; Lin, T.-H.; Wang, T.-C.; Tseng, Y.-H.; Chang, Y.-H.; Wu, M.-C.; Su, W.-F. Effect of Cellulose Compositions and Fabrication Methods on Mechanical Properties of Polyurethane-Cellulose Composites. Carbohydr. Polym. 2022, 291, 119549. [Google Scholar] [CrossRef]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin Doped Functionalized Cellulose Nanofibers Based Edible Chitosan Coating on Kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Mahna, N. Pectin-Based Edible Coating Preserves Antioxidative Capacity of Plum Fruit during Shelf Life. Food Sci. Technol. Int. 2020, 26, 583–592. [Google Scholar] [CrossRef]
- Menezes, J.; Athmaselvi, K.A. Study on Effect of Pectin Based Edible Coating on the Shelf Life of Sapota Fruits. Biosci. Biotechnol. Res. Asia 2016, 13, 1195–1199. [Google Scholar] [CrossRef]
- Hernández-Carrillo, J.; Orta-Zavalza, E.; González-Rodríguez, S.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.; Ortiz-Rivera, Y. Evaluation of the Effectivity of Reuterin in Pectin Edible Coatings to Extend the Shelf-Life of Strawberries during Cold Storage. Food Packag. Shelf Life 2021, 30, 100760. [Google Scholar] [CrossRef]
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-Based Edible Coating for Shelf-Life Extension of Ataulfo Mango. J. Food Process Eng. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Lauer, M.K.; Smith, R.C. Recent Advances in Starch-Based Films toward Food Packaging Applications: Physicochemical, Mechanical, and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef] [PubMed]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Aguilar-Méndez, M.A.; Martín-Martínez, E.S.; Tomás, S.A.; Cruz-Orea, A.; Jaime-Fonseca, M.R. Gelatine–Starch Films: Physicochemical Properties and Their Application in Extending the Post-Harvest Shelf Life of Avocado (Persea americana). J. Sci. Food Agric. 2008, 88, 185–193. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Rodríguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.F.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings 2018, 8, 318. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Starch-Based Coatings: Effect on Refrigerated Strawberry (Fragaria ananassa) Quality. J. Sci. Food Agric. 1998, 76, 411–420. [Google Scholar] [CrossRef]
- Othman, S.H.; Othman, N.F.L.; Shapi’i, R.A.; Ariffin, S.H.; Yunos, K.F.M. Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers 2021, 13, 390. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Zahabi, N.; Rezaee, Z.; Maherani, Z.; Boghori, P.; Keshavarz, Z. Evaluation of a Starch-Based Edible Film as Carrier of a Diantum Capillus-Veneris Extract to Improve the Shelf Life of Fresh-Cut Pears. J. Food Saf. 2016, 36, 291–298. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-García, M.D. Carrageenan Polysaccharides for Food Packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 594–609. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the Use of Carrageenan Matrices for the Development of Antiviral Edible Coatings of Interest in Berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Falcó, I.; Flores-Meraz, P.L.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. Antiviral Activity of Alginate-Oleic Acid Based Coatings Incorporating Green Tea Extract on Strawberries and Raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M.G. Active Edible Polysaccharide-Based Coating for Preservation of Fresh Figs (Ficus carica L.). Foods 2020, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of Alginate Edible Coating on Preserving Fruit Quality in Four Plum Cultivars during Postharvest Storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Hasan, K.; Islam, R.; Hasan, M.; Sarker, S.H.; Biswas, M.H. Effect of Alginate Edible Coatings Enriched with Black Cumin Extract for Improving Postharvest Quality Characteristics of Guava (Psidium guajava L.) Fruit. Food Bioprocess Technol. 2022, 15, 2050–2064. [Google Scholar] [CrossRef]
- Synowiec, A.; Gniewosz, M.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Węglarz, Z. Antimicrobial and Antioxidant Properties of Pullulan Film Containing Sweet Basil Extract and an Evaluation of Coating Effectiveness in the Prolongation of the Shelf Life of Apples Stored in Refrigeration Conditions. Innov. Food Sci. Emerg. Technol. 2014, 23, 171–181. [Google Scholar] [CrossRef]
- Kumar, N.; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Gao, X.; Pourramezan, H.; Ramezan, Y.; Roy, S.; Zhang, W.; Assadpour, E.; Zhou, J.; Jafari, S.M. Application of gums as techno-functional hydrocolloids in meat processing and preservation: A review. Int. J. Biolog. Macromol. 2024, 268, 131614. [Google Scholar] [CrossRef]
- Zhang, L.; Kou, X.; Huang, X.; Li, G.; Liu, J.; Ye, J. Peach-Gum: A Promising Alternative for Retarding the Ripening and Senescence in Postharvest Peach Fruit. Postharvest Biol. Technol. 2020, 161, 111088. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, L.; Tanwar, R.; Singh, S.; Gaikwad, K.K. Active Edible Coating Based on Guar Gum with Mint Extract and Antibrowning Agents for Ber (Ziziphus mauritiana) Fruits Preservation. J. Food Meas. Charact. 2023, 17, 129–142. [Google Scholar] [CrossRef]
- Khafar, E.A.A.; Zidan, N.S.; Aboul-Anean, H.E.D. The Effect of Nano Materials on Edible Coating and Films’ Improvement. Int. J. Pharm. Res. Allied Sci. 2018, 7, 20–41. [Google Scholar]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A Review on Nanomaterials and Nanohybrids Based Bio-Nanocomposites for Food Packaging. Food Chem. 2022, 376, 131912. [Google Scholar] [CrossRef]
- Bizymis, A.-P.; Tzia, C. Edible Films and Coatings: Properties for the Selection of the Components, Evolution through Com posites and Nanomaterials, and Safety Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 8777–8792. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef] [PubMed]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-Based Films and Food Coatings: An Overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan–Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-Based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Van Audenhove, J.; Bernaerts, T.; de Wilde d’Estmael, H.; Hendrickx, M.E.; Van Loey, A.M. Structural and Emulsion Stabilizing Properties of Pectin Rich Extracts Obtained from Different Botanical Sources. Food Res. Int. 2021, 141, 110087. [Google Scholar] [CrossRef] [PubMed]
- Eghbaljoo, H.; Sani, I.K.; Sani, M.A.; Rahati, S.; Mansouri, E.; Molaee-Aghaee, E.; Fatourehchi, N.; Kadi, A.; Arab, A.; Sarabandi, K.; et al. Advances in Plant Gum Polysaccharides; Sources, Techno-Functional Properties, and Applications in the Food Industry-A Review. Int. J. Biol. Macromol. 2022, 222, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Priya, H.; Kumar, S.R.; Trivedi, D.; Prasad, N.; Ahmad, F.; Chengaiyan, J.G.; Haque, S.; Rana, S.S. Gum Ghatti: A Comprehensive Review on Production, Processing, Remarkable Properties, and Diverse Applications. ACS Omega 2024, 9, 9974–9990. [Google Scholar] [CrossRef] [PubMed]
- Tufan, E.G.; Borazan, A.A.I.; Koçkar, Ö.M. A Review on Edible Film and Coating Applications for Fresh and Dried Fruits and Vegetables. Bilecik Şeyh Edebali Üniversitesi Fen Bilim. Derg. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Biswas, D.; Roy, S.; Jamróz, E. Advances in biopolymer-based multi-layer film preparations and food packaging applications. Food Package. Shelf Life 2023, 35, 101033. [Google Scholar] [CrossRef]
- Hira, N.; Mitalo, O.W.; Okada, R.; Sangawa, M.; Masuda, K.; Fujita, N.; Ushijima, K.; Akagi, T.; Kubo, Y. The Effect of Layer-by-Layer Edible Coating on the Shelf Life and Transcriptome of ‘Kosui’Japanese Pear Fruit. Postharvest Biol. Technol. 2022, 185, 111787. [Google Scholar] [CrossRef]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The Effect of the Layer-by-Layer (LBL) Edible Coating on Strawberry Quality and Metabolites during Storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Silva-Vera, W.; Zamorano-Riquelme, M.; Rocco-Orellana, C.; Vega-Viveros, R.; Gimenez-Castillo, B.; Silva-Weiss, A.; Osorio-Lira, F. Study of Spray System Applications of Edible Coating Suspensions Based on Hydrocolloids Containing Cellulose Nanofibers on Grape Surface (Vitis vinifera L.). Food Bioprocess Technol. 2018, 11, 1575–1585. [Google Scholar] [CrossRef]
- Basnur, J.; Putra, M.F.F.; Jayusman, S.V.A.; Zulhilmi, Z. Sustainable Packaging: Bioplastics as a Low-Carbon Future Step for the Sustainable Development Goals (SDGs). ASEAN J. Sci. Eng. Mater. 2024, 3, 51–58. [Google Scholar]
- Chhikara, S.; Kumar, D. Edible Coating and Edible Film as Food Packaging Material: A Review. J. Packag. Technol. Res. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z. Effect of Coating Thickness on Release Characteristics of Controlled Release Urea Produced in Fluidized Bed Using Waterborne Starch Biopolymer as Coating Material. Procedia Eng. 2016, 148, 282–289. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Adibi, A.; Trinh, B.M.; Mekonnen, T.H. Recent Progress in Sustainable Barrier Paper Coating for Food Packaging Applications. Prog. Org. Coat. 2023, 181, 107566. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q. V Development and Application of Rice Starch Based Edible Coating to Improve the Postharvest Storage Potential and Quality of Plum Fruit (Prunus salicina). Sci. Hortic. 2018, 237, 59–66. [Google Scholar] [CrossRef]
- Trinetta, V.; Cutter, C.N.; Floros, J.D. Effects of Ingredient Composition on Optical and Mechanical Properties of Pullulan Film for Food-Packaging Applications. LWT-Food Sci. Technol. 2011, 44, 2296–2301. [Google Scholar] [CrossRef]
- Dehghan Tanha, L.; Khoshkhoo, Z.; Azizi, M.H. Application of Edible Coating Made of Sturgeon Gelatin and Portulaca Oleracea Extract for Improving the Shelf Life of Fish Sausages. J. Food Meas. Charact. 2021, 15, 4306–4313. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in Barrier Coatings and Film Technologies for Achieving Sustainable Packaging of Food Products—A Review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio-and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure–Property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Gao, L.; Wang, Z.; Rao, W.; Du, M.; Zhang, D. Mechanical Properties, Thermal Stability, and Solubility of Sheep Bone Collagen–Chitosan Films. J. Food Process Eng. 2020, 43, e13086. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Biopolymers for Active Packaging: Demand, Development and Directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Xu, K.; Li, Q.; Xie, L.; Shi, Z.; Su, G.; Harper, D.; Tang, Z.; Zhou, J.; Du, G.; Wang, S. Novel Flexible, Strong, Thermal-Stable, and High-Barrier Switchgrass-Based Lignin-Containing Cellulose Nanofibrils/Chitosan Biocomposites for Food Packaging. Ind. Crops Prod. 2022, 179, 114661. [Google Scholar] [CrossRef]
- Senna, M.M.H.; Al-Shamrani, K.M.; Al-Arifi, A.S. Edible Coating for Shelf-Life Extension of Fresh Banana Fruit Based on Gamma Irradiated Plasticized Poly (Vinyl Alcohol)/Carboxymethyl Cellulose/Tannin Composites. Mater. Sci. Appl. 2014, 5, 45851. [Google Scholar] [CrossRef]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; de Almeida, M.M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of Edible Chitosan and Cinnamon Essential Oil Coatings on the Shelf Life of Minimally Processed Pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of Postharvest Quality of Plum (Prunus domestica L.) Using Polysaccharide-Based Edible Coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Bains, A.; Dhull, S.B.; Chawla, P.; Goksen, G. Effect of Aloe Vera and Carboxymethyl Cellulose-Derived Binary Blend Edible Coating on the Shelf Life of Fresh-Cut Apple. Food Sci. Nutr. 2023, 11, 6987–6999. [Google Scholar] [CrossRef] [PubMed]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Application of Pregelatinized Corn Starch and Basil Essential Oil Edible Coating with Cellulose Nanofiber as Pickering Emulsion Agent to Prevent Quality-Quantity Loss of Mandarin Orange. Food Packag. Shelf Life 2023, 35, 101010. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Khozemy, E.E.; El-Dein, A.E.; El-Sawy, N.M. Radiation Synthesis of Edible Coating Films of Nanocurcumin Based on Carboxymethyl Chitosan/Polyvinyl Alcohol to Extend the Shelf Life of Sweet Orange “Valencia”. J. Polym. Environ. 2023, 31, 3783–3802. [Google Scholar] [CrossRef]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of Hydroxyethyl Cellulose and Sodium Alginate Edible Coating Containing Asparagus Waste Extract on Postharvest Quality of Strawberry Fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid 62, 791–Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT-Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Basiak, E.; Geyer, M.; Debeaufort, F.; Lenart, A.; Linke, M. Relevance of Interactions between Starch-Based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis. Int. J. Mol. Sci. 2019, 20, 2220. [Google Scholar] [CrossRef]
- Leena, M.M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible Coating with Resveratrol Loaded Electrospun Zein Nanofibers with Enhanced Bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Cohen, Y.; Saidi, L.; Porat, R.; Poverenov, E. Covalent Linkage of Bioactive Volatiles to a Polysaccharide Support as a Potential Approach for Preparing Active Edible Coatings and Delivery Systems for Food Products. Food Chem. 2021, 338, 127822. [Google Scholar] [CrossRef]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.-R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal Edible Coatings Containing Argentinian Propolis Extract and Their Application in Raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Hernandez-Montiel, L.G.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Main Diseases in Postharvest Blueberries, Conventional and Eco-Friendly Control Methods: A Review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Batista, R.A.; Otoni, C.G.; Soares, N.F.F. Antimicrobial Food Packaging Incorporated with Triclosan: Potential Uses and Restrictions. In Antimicrobial Food Package; Academic Press: Cambridge, MA, USA, 2016; pp. 417–423. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible Coatings and Antimicrobial Nanoemulsions for Enhancing Shelf Life and Reducing Foodborne Pathogens of Fruits and Vegetables: A Review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Sánchez-Tamayo, M.; Ochoa-Martínez, C.; Critzer, F. Inactivation of Salmonella Enterica and Colletotrichum Gloeosporioides on Whole Mangoes by Application of an Antimicrobial Coating Containing Oregano Essential Oil. Horticulturae 2021, 7, 305. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Vazquez Gonzalez, Y.; Ragazzo-Sánchez, J.A. Application of Essential Oils and Polyphenols as Natural Antimicrobial Agents in Postharvest Treatments: Advances and Challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Neeraj; Pratibha; Singla, M. Enhancement of Storage Life and Quality Maintenance of Litchi (Litchi chinensis Sonn.) Fruit Using Chitosan: Pullulan Blend Antimicrobial Edible Coating. Int. J. Fruit Sci. 2020, 20, S1662–S1680. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral Nanoemulsion Incorporated Edible Coating to Extend the Shelf Life of Fresh Cut Pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Jiao, W.; Shu, C.; Li, X.; Cao, J.; Fan, X.; Jiang, W. Preparation of a Chitosan-Chlorogenic Acid Conjugate and Its Application as Edible Coating in Postharvest Preservation of Peach Fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Ackah, S.; Bi, Y. Post-Harvest Chitosan Treatment Suppresses Oxidative Stress by Regulating Reactive Oxygen Species Metabolism in Wounded Apples. Front. Plant Sci. 2022, 13, 959762. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Asi, M.R.; Hameed, A.; Bourquin, L.D. Effect of Postharvest Application of Aloe Vera Gel on Shelf Life, Activities of Anti-Oxidative Enzymes, and Quality of ‘Gola’Guava Fruit. Foods 2020, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Luo, Y. Enzymatic Browning and Its Control in Fresh-Cut Produce. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic Browning of Fruit and Vegetables: A Review. In Enzymes in Food Technology: Improvements and Innovations; Springer: Singapore, 2018; pp. 63–78. [Google Scholar]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’Apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef]
- Tian, J.; Xie, S.; Zhang, P.; Wang, Q.; Li, J.; Xu, X. Attenuation of Postharvest Peel Browning and Chilling Injury of Banana Fruit by Astragalus Polysaccharides. Postharvest Biol. Technol. 2022, 184, 111783. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Yusof, N.M.; Jai, J.; Hamzah, F. Effect of Coating Adhesion on Turmeric Essential Oil Incorporated into Chitosan-Based Edible Coating. Mater. Sci. Forum 2017, 890, 204–208. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Bai, J.; Zhang, F.; Zhang, R.; Zhang, X.; Zhong, K.; Yan, B. Development of Mussel-Inspired Chitosan-Derived Edible Coating for Fruit Preservation. Carbohydr. Polym. 2023, 321, 121293. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hong, M.; Wang, L.; Meng, Q.; Ke, Q.; Kou, X. Bioadhesive and Antibacterial Edible Coating of EGCG-Grafted Pectin for Improving the Quality of Grapes during Storage. Food Hydrocoll. 2023, 136, 108255. [Google Scholar] [CrossRef]
- Cruz, V.; Rojas, R.; Saucedo-Pompa, S.; Martínez, D.G.; Aguilera-Carbó, A.F.; Alvarez, O.B.; Rodríguez, R.; Ruiz, J.; Aguilar, C.N. Improvement of Shelf Life and Sensory Quality of Pears Using a Specialized Edible Coating. J. Chem. 2015, 2015, 138707. [Google Scholar] [CrossRef]
- Toliba, A.O.; Rabie, M.A.; El-Araby, G.M. Extending the Shelf-Life of Cold Stored Strawberry by Chitosan and Carnauba Coatings. Zagazig J. Agric. Res. 2014, 41, 1067–1076. [Google Scholar]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Liu, Y.-P.; Liu, J.-L.; Nie, G.-W. Effect of Carboxymethyl Chitosan-Gelatin-Based Edible Coatings on the Quality and Antioxidant Properties of Sweet Cherry during Postharvest Storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
| Edible Coating | Active Ingredient | Fruit Preserved | Key Results | Shelf Life | Refs. |
|---|---|---|---|---|---|
| Chitosan | Apple peel polyphenols | Strawberry | Prevented fruit decay, maintained total phenol content, firmness, and anthocyanin, and reduced weight loss Pros and cons: High antimicrobial properties but limits in solubility | Increase in shelf life | [29] |
| Chitosan | Cinnamon essential oils | Pineapple | Antimicrobial activity Escherichia coli and Salmonella spp. maintained fruit firmness and prevented weight loss Pros and cons: High barrier property and prevent microbe proliferation, but limits against oxygen barrier | Increased shelf life up to 11 days | [100] |
| Carboxymethyl cellulose and pectin | Plum | Maintained firmness, reduction in weight loss titratable acidity, vitamin C, flavonoid, and antioxidant activity Pros and cons: High mechanical strength but causes high moisture permeability | Increase in shelf life | [101] | |
| Carboxymethylcellulose | Aloe vera | Apple | Prevented weight loss, and microbial growth, browning, increase in titratable acidity Pros and cons: Abundant in nature but limited in barrier properties and flexibility | Increased shelf life up to 10 days | [102] |
| Carrageenan | Green tea extract | Raspberries and blueberries | Anti-viral activity against murine norovirus and hepatitis A virus, preservation of firmness Pros and cons: High adhesiveness, good gelling properties but poor flexibility | Increase in shelf life | [56] |
| Chitosan and starch | Papaya | Maintained firmness, reduced weight loss, and microbial growth Pros and cons: Chitosan is effective in preventing microbes, and starch has good mechanical strength, but chitosan limits solubility, and starch is susceptible to recrystallization | Increased shelf life by 15 days | [50] | |
| Pectin | Plum | Reduced polyphenol oxidase activity, maintained polyphenol content and anthocyanin and antioxidant capacity Pros and cons: Good gel-forming properties and transparency but lack in stability at particular temperature conditions | Increase in shelf life | [43] | |
| Chitosan/pullulan | Pomegranate peel extract | Mango | Increase in fruit firmness, texture, antioxidant activity, TSS, and reduced weight loss Pros and cons: Pullulan is highly transparent, excellent oxygen barrier material but has limited availability and is highly expensive | Increase in shelf life 18 days | [62] |
| Gum | Peach | Retarded ethylene production, weight loss, softening of fruit, and maintained nutritional content Pros and cons: Excellent thickening agent, but an excess amount can cause a gummy texture | Increased storage time by maintaining the quality of the peach fruit | [64] | |
| Starch | Cellulose nanofibers and basil essential oil | Mandarin orange | Prevented weight loss and maintained fruit color Pros and cons: Highly accessible and cost-effective but limits in stability and barrier properties | Increased storage life for 12 days | [103] |
| Carboxymethyl cellulose/chitosan/Polyvinyl alcohol | Nano curcumin | Sweet orange | Maintained fruit freshness, reduced weight loss, and antimicrobial properties against Bacillus subtills, Staphylococcus aureus and Escherichia coli Pros and cons: Polyvinyl alcohol is transparent and has good film-forming properties but is highly water-sensible | Increased storage life for 56 days | [104] |
| Hydroxy methyl cellulose and sodium alginate | Asparagus extract | Strawberry | Antifungal against Penicillium italicum, reduced weight loss, and increased phenol and flavonoid content Pros and cons: Sodium alginate has excellent gelling properties, and hydroxymethyl cellulose is transparent but lacks stability and mechanical properties | Increase in shelf life | [105] |
| Alginate | Black cumin extract | Guava | Antibacterial against Staphylococcus hominis and Escherichia coli, reduced respiration rate and weight loss, retained vitamin C, phenols, and flavonoids Pros and cons: High thermal resistance and gel-forming properties but can interact with other ingredients | Increase in shelf life | [60] |
| Xanthan | Cinnamic acid | Pears | Inhibit the activity of browning enzymessuch as peroxidases (POD) and polyphenol oxidase (PPO), prevent the oxidation of phenols into melanincompounds Pros and cons: Good adhesion and flexibility but can affect sensorial characteristics of food | Increased storage time by maintaining the quality | [106] |
| Starch | Whey protein | Plum | Reduce the respiration rate and weight loss retention. Pros and cons: Flexible and transparent but has a poor barrier and mechanical properties, also Whey protein is expensive | Increased shelf life | [107] |
| Zein | Resveratrol | Apple slices | Reduced moisture loss and increased color retention Pros and cons: Forms a glossy appearance, but an excess amount can cause brittle and sensitivity to pH | Increased storage time by quality retention and nutrient delivery | [108] |
| Chitosan | Vanillin, cinnamaldehyde, and mandarin extract | Melon | Antimicrobial activity, good sensory quality, maintained fruit quality Pros and cons: Strong and flexible but can cause allergy due to shellfish in certain people | Increased shelf-life by enhanced quality and sensory properties | [109] |
| Zein | Argentinian propolis extracts | Raspberries | Maintained fruit quality, freshness, firmness, and antimicrobial activity Pros and cons: Highly transparent and glossy appearance but can cause allergy problems | Increased shelf life up to 11 days | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillai, A.R.S.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review. Foods 2024, 13, 1529. https://doi.org/10.3390/foods13101529
Pillai ARS, Eapen AS, Zhang W, Roy S. Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review. Foods. 2024; 13(10):1529. https://doi.org/10.3390/foods13101529
Chicago/Turabian StylePillai, Athira R. S., Ansu Sara Eapen, Wanli Zhang, and Swarup Roy. 2024. "Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review" Foods 13, no. 10: 1529. https://doi.org/10.3390/foods13101529
APA StylePillai, A. R. S., Eapen, A. S., Zhang, W., & Roy, S. (2024). Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review. Foods, 13(10), 1529. https://doi.org/10.3390/foods13101529










