The Effect of Acid Hydrolysis on the Pickering Emulsifying Capacity of Tartary Buckwheat Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Hydrolyzed Tartary Buckwheat Flour (HTBF)
2.3. Determination of Particle Size
2.4. Determination of Interfacial Tension
2.5. Determination of Contact Angle
2.6. Preparation of Pickering HIPEs
2.7. Microscopic Observation of Pickering HIPEs
2.8. Gel Strength Measurement of Pickering HIPEs
2.9. Microrheological Determination of Pickering HIPEs
2.10. Statistical Analysis
3. Results and Discussion
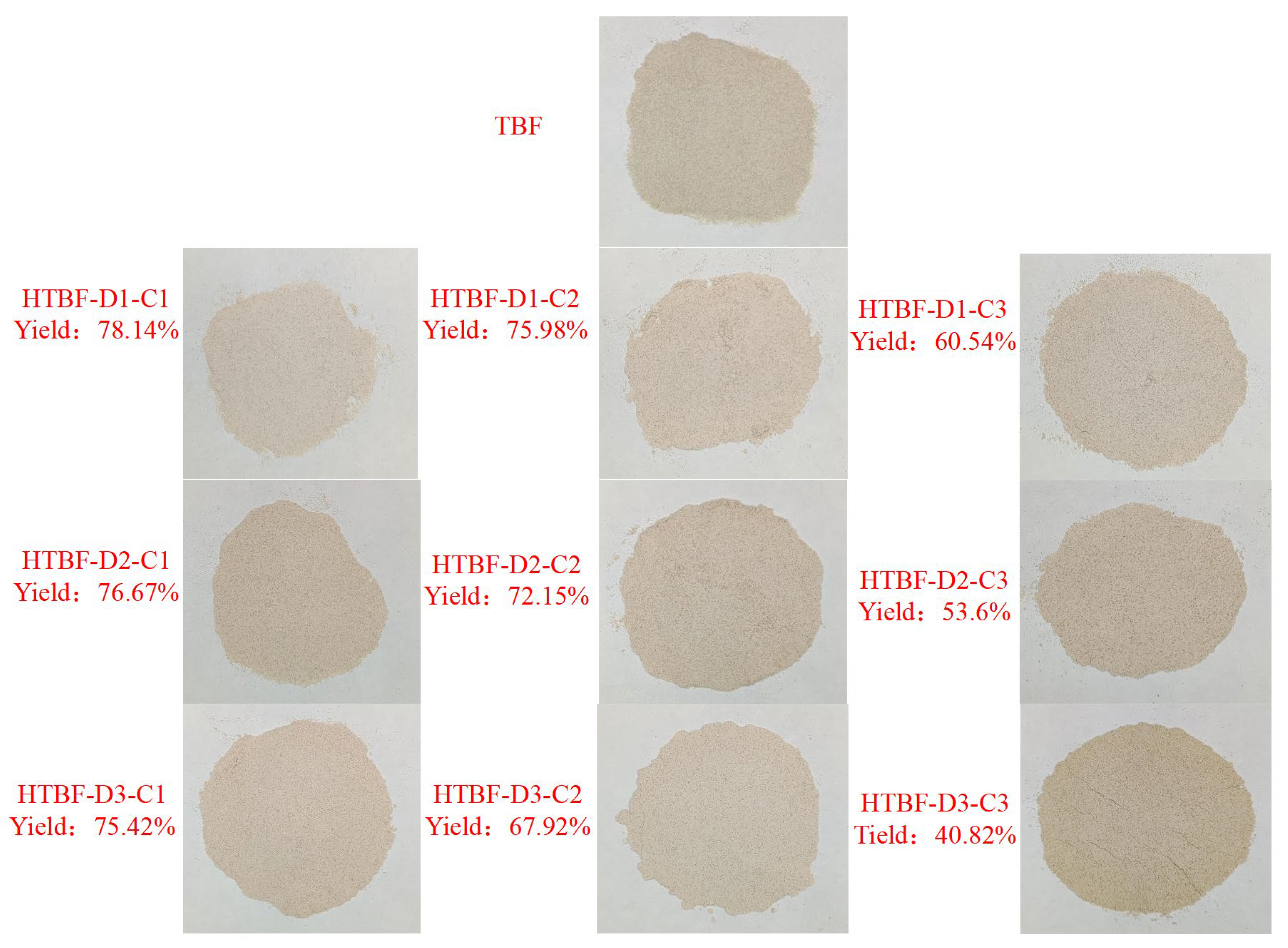
3.1. Appearance and Yield of HTBF
3.2. Particle Size Analysis
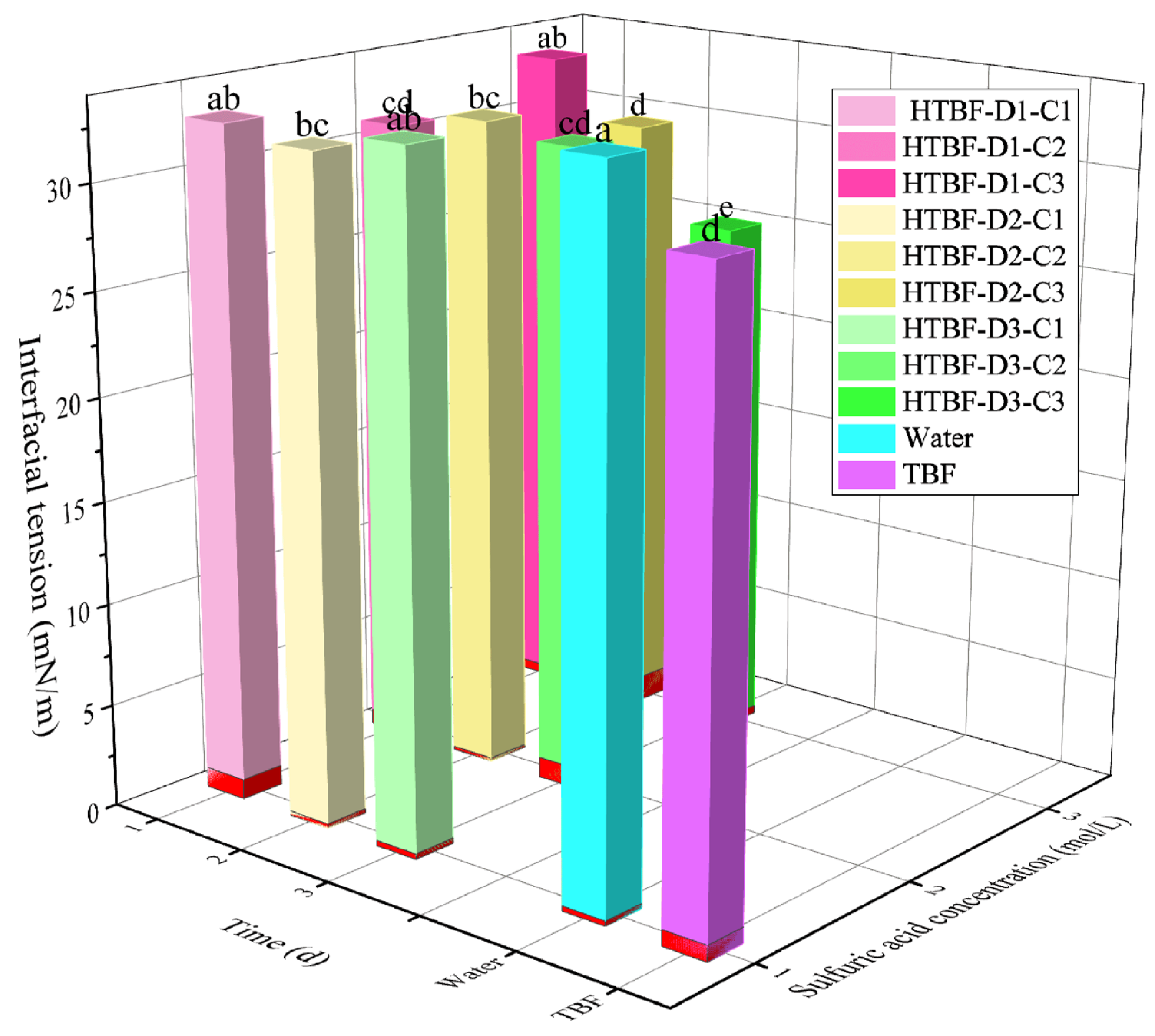
3.3. Interface Tension Analysis
3.4. Contact Angle Analysis
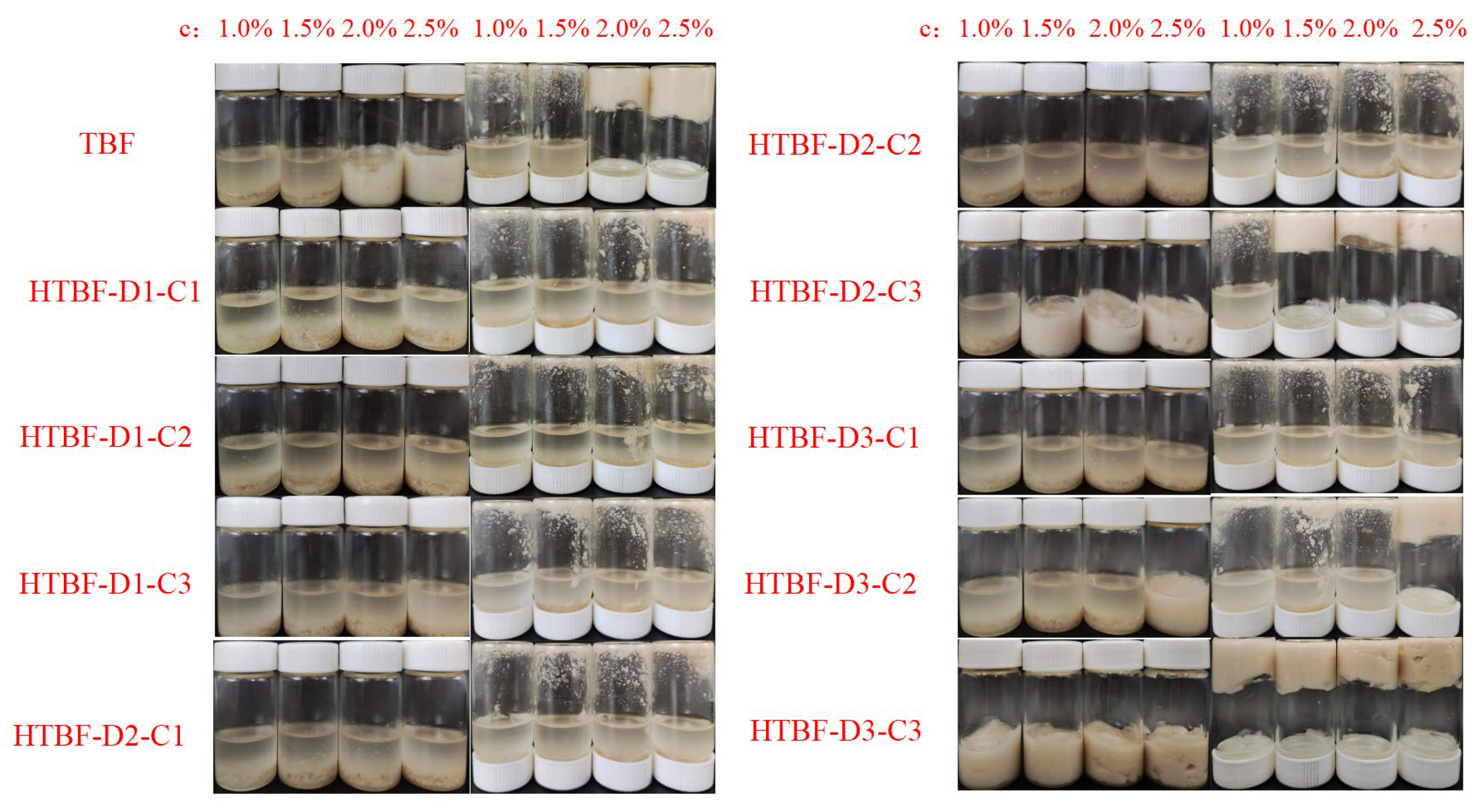
3.5. Formation of Pickering HIPEs
3.6. Microscopic Analysis of Pickering HIPEs
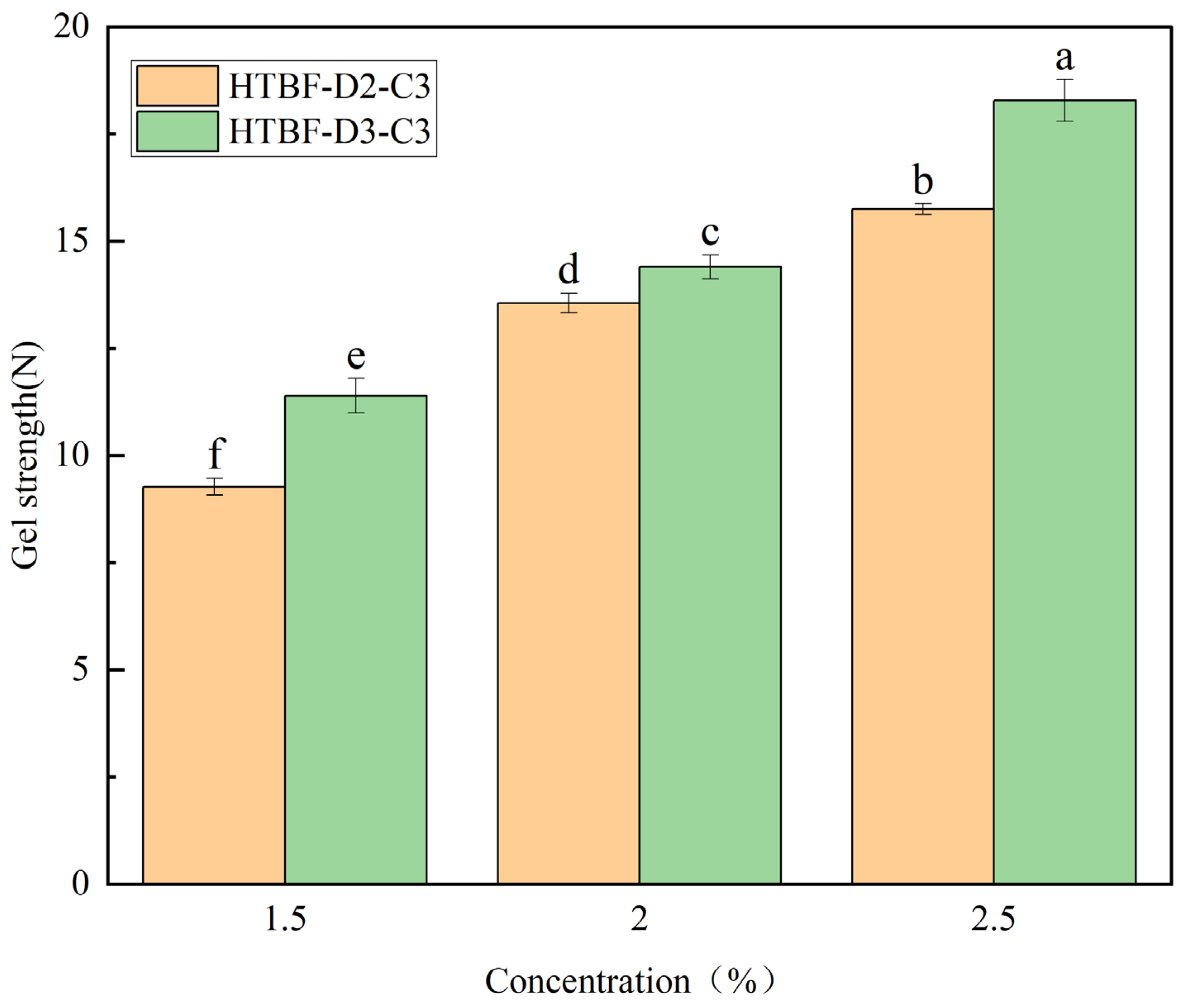
3.7. Gel Strength Analysis of Pickering HIPEs
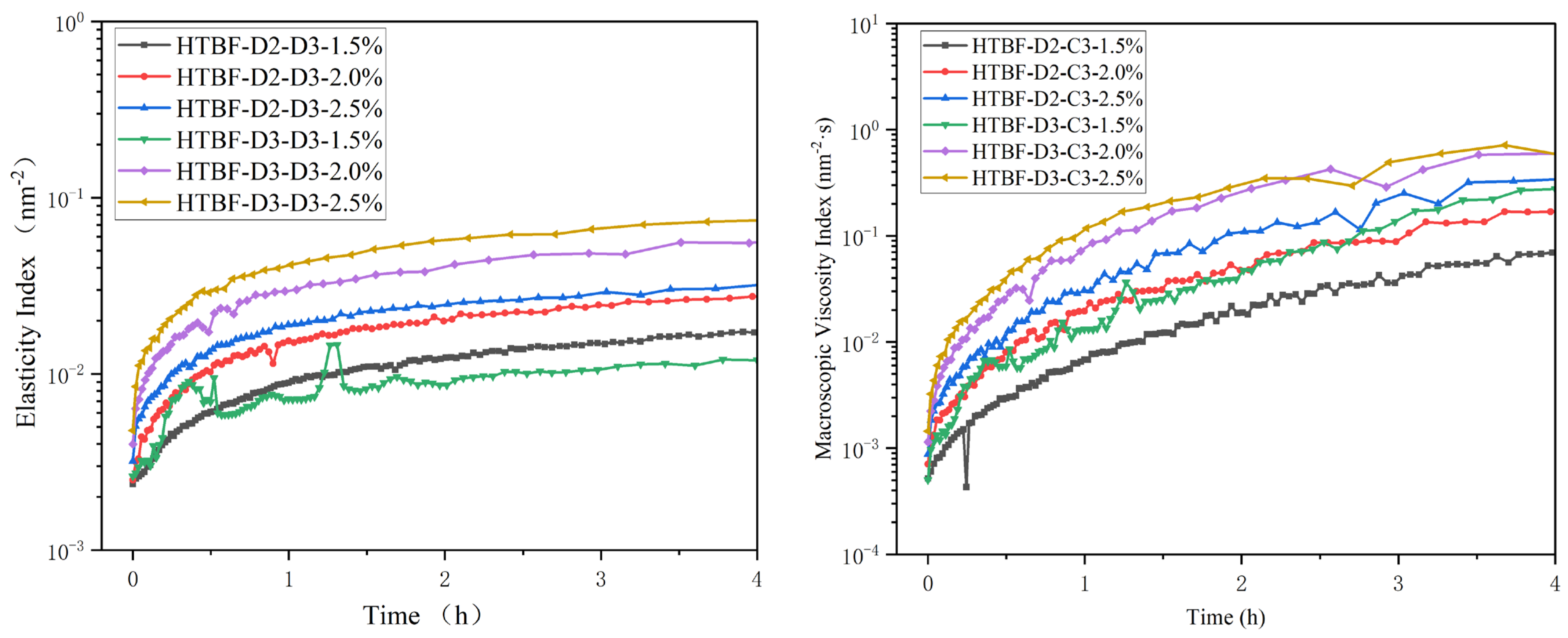
3.8. Microrheological Analysis of Pickering HIPEs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.Y.; Mhaske, P.; Farahnaky, A.; Kasapis, S.; Majzoobi, M. Cassava starch: Chemical modification and its impact on functional properties and digestibility, a review. Food Hydrocoll. 2022, 129, 107542. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, J.J.; An, N.N.; Li, D.; Wang, L.J.; Wang, Y. Succeeded high-temperature acid hydrolysis of granular maize starch by introducing heat-moisture pre-treatment. Int. J. Biol. Macromol. 2022, 222, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Yadav, B.S.; Yadav, R. Characterization of acid hydrolysis based nano-converted mung bean (Vigna radiata L.) starch for morphological, rheological and thermal properties. Int. J. Biol. Macromol. 2022, 211, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocoll. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Singh, V.; Ali, S.Z. Properties of starches modified by different acids. Int. J. Food Prop. 2008, 11, 495–507. [Google Scholar] [CrossRef]
- Królikowska, K.; Pietrzyk, S.; Łabanowska, M.; Kurdziel, M.; Pająk, P. The influence of acid hydrolysis on physicochemical properties of starch-oleic acid mixtures and generation of radicals. Food Hydrocoll. 2021, 118, 106780. [Google Scholar] [CrossRef]
- Fouladi, E.; Nafchi, A.M. Effects of acid-hydrolysis and hydroxypropylation on functional properties of sago starch. Int. J. Biol. Macromol. 2014, 68, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Y. Nanostarch: Preparation, modification, and application in pickering emulsions. J. Agric. Food Chem. 2021, 69, 6929–6942. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Saari, H.; Heravifar, K.; Rayner, M.; Wahlgren, M.; Sjöö, M. Preparation and characterization of starch particles for use in Pickering emulsions. Cereal. Chem. 2016, 93, 116–124. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Wei, T.; Shen, J.; Wang, M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT-Food. Sci. Technol. 2017, 86, 285–292. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, Y.; Li, H.; Fan, H.; Wang, M. In vitro digestibility and changes in physicochemical and textural properties of tartary buckwheat starch under high hydrostatic pressure. J. Food Eng. 2016, 189, 64–71. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Geng, S.; Liu, C.; Ma, H.; Liu, B. Effects of tartary buckwheat bran flour on dough properties and quality of steamed bread. Foods 2021, 10, 2052. [Google Scholar] [CrossRef] [PubMed]
- Bist, Y.; Kumar, Y.; Saxena, D. Enhancing the storage stability of Pickering emulsion using esterified buckwheat starch with improved structure and morphology. LWT-Food. Sci. Technol. 2022, 161, 113329. [Google Scholar] [CrossRef]
- Gonzalez, A.; Wang, Y. Effects of acid hydrolysis level prior to heat-moisture treatment on properties of starches with different crystalline polymorphs. LWT-Food. Sci. Technol. 2023, 187, 115302. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–5. [Google Scholar] [CrossRef]
- Alteraifi, A.M. Effects of solid–liquid interface on the interfacial tension measured by micropipet technique. J. Colloid Interf. Sci. 2005, 287, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.; Strobel, M.; Lyons, C.S.; Janis, S. A statistical comparison of contact angle measurement methods. J. Adhes. Sci. Technol. 2019, 33, 1758–1769. [Google Scholar] [CrossRef]
- Wang, K.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Stabilization of Pickering emulsions using starch nanocrystals treated with alkaline solution. Int. J. Biol. Macromol. 2020, 155, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Yang, Z.; He, X.; Zhang, B.; Fu, X.; Tan, C.; Huang, Q. Starch granules as Pickering emulsifiers: Role of octenylsuccinylation and particle size. Food Chem. 2019, 283, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Saikia, T.; Aultan, A.; Al Hamad, J. Effect of mixing method on the rheokinetics of pickering emulsified gel system used as disproportionate permeability reducer. Rheol. Acta 2020, 59, 875–885. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of surface charge density of bacterial cellulose nanofibrils on the rheology property of O/W Pickering emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Selma-Gracia, R.; Laparra, J.; Haros, C. Potential beneficial effect of hydrothermal treatment of starches from various sources on in vitro digestion. Food Hydrocoll. 2020, 103, 105687. [Google Scholar] [CrossRef]
- Tang, H.; Wang, L.; Li, Y.; Dong, S. Effect of acidolysis and oxidation on structure and properties of konjac glucomannan. Int. J. Biol. Macromol. 2019, 130, 378–387. [Google Scholar]
- Zuo, Y.; Gu, J.; Tan, H.; Qiao, Z.; Xie, Y.; Zhang, Y. The characterization of granule structural changes in acid-thinning starches by new methods and its effect on other properties. J. Adhes. Sci. Technol. 2014, 28, 479–489. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, G.; Hong, B.; Zhao, S.; Han, X.; Pera-Titus, M. Unraveling particle size and roughness effects on the interfacial catalytic properties of Pickering emulsions. Colloids Surf. A 2021, 599, 124800. [Google Scholar] [CrossRef]
- Guo, C.; Geng, S.; Shi, Y.; Yuan, C.; Liu, B. Effect of sulfuric acid hydrolysis on the structure and Pickering emulsifying capacity of acorn starch. Food Chem. X 2024, 22, 101277. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. Modeling of sedimentation and creaming in suspensions and Pickering emulsions. Fluids 2019, 4, 186. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Huang, Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends. Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Vázquez-García, H.G.; Méndez-Montealvo, G.; Basilio-Cortés, U.A.; Navarro-Cortés, R.; Rodríguez-Marín, M.L.; Castro-Rosas, J.; Gómez-Aldapa, C.A. Effect of acid hydrolysis and OSA esterification of waxy cassava starch on emulsifying properties in Pickering-type emulsions. LWT Food. Sci. Technol. 2018, 91, 258–264. [Google Scholar] [CrossRef]
- Saari, H.; Rayner, M.; Wahlgren, M. T Effects of starch granules differing in size and morphology from different botanical sources and their mixtures on the characteristics of Pickering emulsions. Food Hydrocoll. 2019, 89, 844–855. [Google Scholar] [CrossRef]
- Jia, Y.; Kong, L.; Zhang, B.; Fu, X.; Huang, Q. FFabrication and characterization of Pickering high internal phase emulsions stabilized by debranched starch-capric acid complex nanoparticles. Int. J. Biol. Macromol. 2022, 207, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Han, F.; Lv, X.; Zhang, S.; Ma, H.; Liu, B. Formation mechanism of Pickering emulsion gels stabilized by proanthocyanidin particles: Experimental and molecular dynamics studies. Food Chem. 2023, 418, 135904. [Google Scholar] [CrossRef] [PubMed]
- Durgut, E.; Zhou, M.; Dikici, B.A.; Foudazi, R.; Claeyssens, F. Modifying pickering polymerized high internal phase emulsion morphology by adjusting particle hydrophilicity. Colloids Surf. A 2024, 680, 132629. [Google Scholar] [CrossRef]
- Lv, X.; Guo, C.; Ma, Y.; Liu, B. Effect of citric acid esterification on the structure and physicochemical properties of tigernut starch. Int. J. Biol. Macromol. 2022, 222, 2833–2842. [Google Scholar] [CrossRef]



| Sample | L* | a* | b* |
|---|---|---|---|
| TBF | 79.31 ± 0.81 cd | 1.08 ± 0.08 h | 14.55 ± 0.06 e |
| HTBF-D1-C1 | 82.69 ± 0.22 a | 3.50 ± 0.10 f | 14.52 ± 0.07 e |
| HTBF-D1-C2 | 81.22 ± 0.16 b | 3.60 ± 0.26 e | 13.91 ± 0.11 f |
| HTBF-D1-C3 | 78.19 ± 0.54 d | 3.79 ± 0.15 c | 14.07 ± 0.07 f |
| HTBF-D2-C1 | 79.63 ± 0.37 c | 4.06 ± 0.06 b | 16.76 ± 0.07 b |
| HTBF-D2-C2 | 78.59 ± 0.64 cd | 3.68 ± 0.26 de | 15.49 ± 0.53 d |
| HTBF-D2-C3 | 76.66 ± 0.21 e | 3.64 ± 0.03 de | 16.34 ± 0.03 c |
| HTBF-D3-C1 | 78.79 ± 0.43 cd | 4.57 ± 0.02 a | 16.20 ± 0.08 c |
| HTBF-D3-C2 | 78.41 ± 0.19 d | 3.66 ± 0.05 de | 14.64 ± 0.14 e |
| HTBF-D3-C3 | 75.28 ± 0.39 f | 3.06 ± 0.04 g | 18.21 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Guo, C.; Liu, B. The Effect of Acid Hydrolysis on the Pickering Emulsifying Capacity of Tartary Buckwheat Flour. Foods 2024, 13, 1543. https://doi.org/10.3390/foods13101543
Zhang S, Guo C, Liu B. The Effect of Acid Hydrolysis on the Pickering Emulsifying Capacity of Tartary Buckwheat Flour. Foods. 2024; 13(10):1543. https://doi.org/10.3390/foods13101543
Chicago/Turabian StyleZhang, Shijie, Changsheng Guo, and Benguo Liu. 2024. "The Effect of Acid Hydrolysis on the Pickering Emulsifying Capacity of Tartary Buckwheat Flour" Foods 13, no. 10: 1543. https://doi.org/10.3390/foods13101543
APA StyleZhang, S., Guo, C., & Liu, B. (2024). The Effect of Acid Hydrolysis on the Pickering Emulsifying Capacity of Tartary Buckwheat Flour. Foods, 13(10), 1543. https://doi.org/10.3390/foods13101543






