The Application of Protein Concentrate Obtained from Green Leaf Biomass in Structuring Nanofibers for Delivery of Vitamin B12
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pumpkin Leaf Protein Concentrate (LPC)
2.3. Preparation of the Solutions
2.4. Characterization of the Gelatin-LPC Solutions
2.5. Electrospinning Processing
2.6. Nanofibers Morphology and Size
2.7. Encapsulation Efficiency
2.8. Fourier Transform Infrared Spectroscopy
2.9. Thermogravimetric Analysis
2.10. Differential Scanning Calorimetry (DSC)
2.11. Water Activity
2.12. Release Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
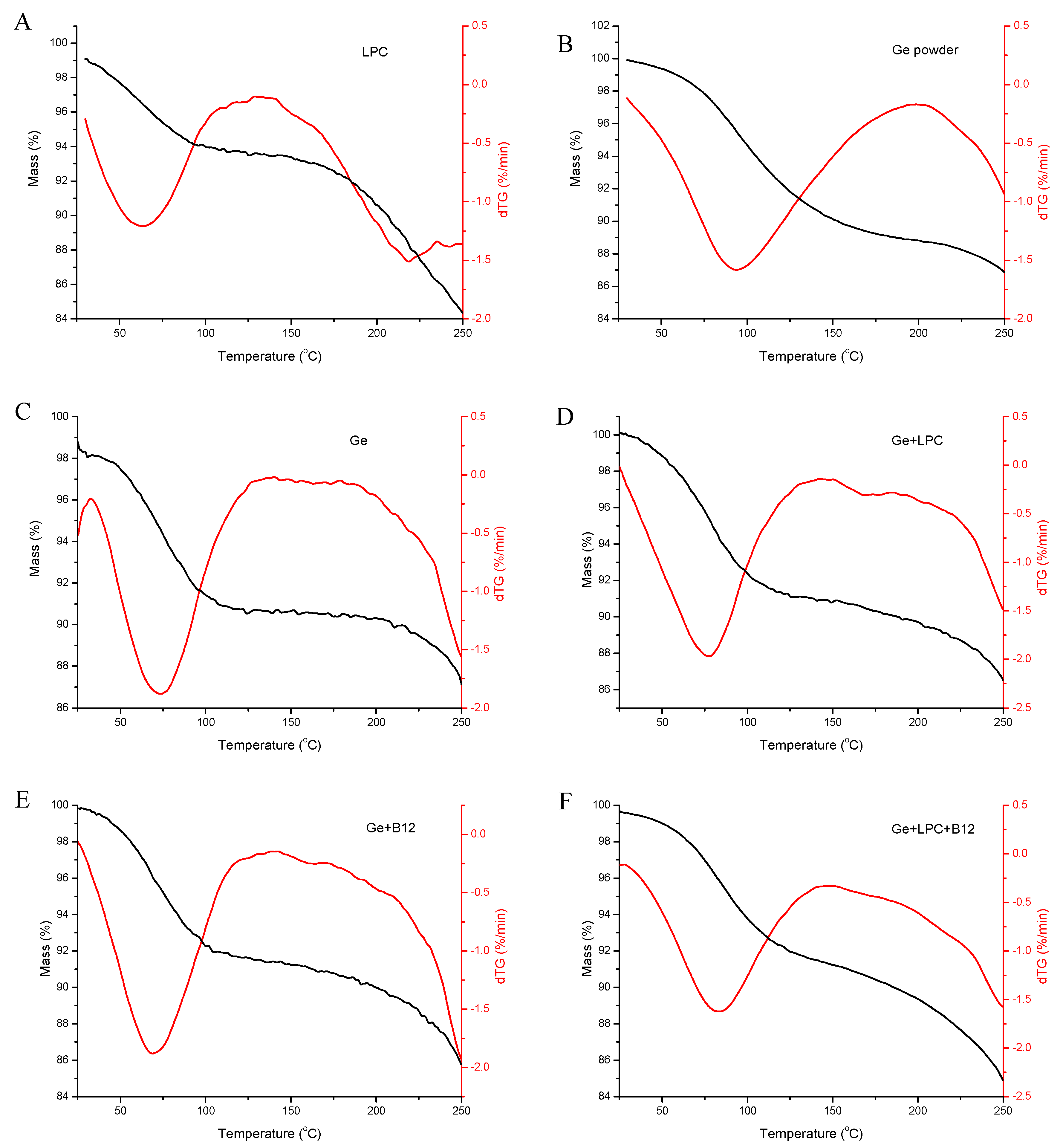
3.2. Thermal Stability Analysis
3.3. DSC
3.4. Water Activity (Aw)
3.5. Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gomez-Mascaraque, L.G.; Martínez-Sanz, M.; Lopez-Rubio, A. Electrospun curcumin-loaded protein nano fi ber mats as active/bioactive coatings for food packaging applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Safwat, S.; Hathout, R.M.; Ishak, R.A.; Mortada, N.D. Elaborated survey in the scope of nanocarriers engineering for boosting chemotherapy cytotoxicity: A meta-analysis study. Int. J. Pharm. 2021, 610, 121268. [Google Scholar] [CrossRef] [PubMed]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polymer Reviews 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Miao, X.; Wang, H.; Wang, W. Electrospun gelatin nanofibers encapsulated with peppermint and chamomile essential oils as potential edible packaging. J. Agric. Food. Chem. 2019, 67, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, V.; Fathi, M.; Shahedi, M.; Soltanizadeh, N.; Emam-Djomeh, Z. Fast-dissolving antioxidant nanofibers based on Spirulina protein concentrate and gelatin developed using needleless electrospinning. Food Biosci. 2022, 47, 101759. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; van den Eijnden, P.; Henket, J.T.M.M.; Langelaan, M.L.P.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Food-grade electrospinning of proteins. Innov. Food Sci. Emerg. Technol. 2013, 20, 269–275. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Gieteling, J.; de Jong, G.A.H.; Boom, R.M.; van der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Lübeck, M. Production of leaf protein concentrates in green biorefineries as alternative feed for monogastric animals. Anim. Feed Sci. Technol. 2020, 268, 114605. [Google Scholar] [CrossRef]
- Ducrocq, M.; Boire, A.; Anton, M.; Micard, V.; Morel, M.H. Rubisco: A promising plant protein to enrich wheat-based food without impairing dough viscoelasticity and protein polymerisation. Food Hydrocoll. 2020, 109, 106101. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; De Jong, G.A.H. Characterization of heat-set gels from RuBisCO in comparison to those from other proteins. J. Agric. Food. Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, M.; Geerdink, P.; Engelen-Smit, N.P.; Van Der Meer, I.M.; America, A.H.P.; Mes, J.J.; Kootstra, A.M.J.; Henket, J.T.M.M.; Mulder, W.J. Isolation and gelling properties of duckweed protein concentrate. ACS Food Sci. Technol. 2021, 1, 908–916. [Google Scholar] [CrossRef]
- Udousoro, I.; Ekanem, P. Assessment of proximate compositions of twelve edible vegetables in Nigeria. Int. J. Modern Chem. 2013, 4, 79–89. [Google Scholar]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, S.O.; Taiwo, K.A.; Oyedele, J.D.; Adebooye, O.C.; Aluko, R.E. In Vitro Characterization of Fluted Pumpkin Leaf Protein Hydrolysates and Ultrafiltration of Peptide Fractions: Antioxidant and Enzyme-Inhibitory Properties. Pol. J. Food Nutr. Sci. 2020, 70, 429–443. [Google Scholar] [CrossRef]
- Tanimowo Fadupin, G.; Ariyo, O. Effect of blanching on nutrient and anti-nutrient content of pumpkin (cucurbita pepo) leaves. West Afr. J. Food Nutr. 2014, 12. [Google Scholar]
- Zhang, X.; Tan, L.; Taxipalati, M.; Deng, L. Fabrication and characterization of fast dissolving glycerol monolaurate microemulsion encapsulated gelatin nanofibers with antimicrobial activity. J. Sci. Food Agric. 2021, 101, 5660–5670. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinia, H.; Ghorani, B.; Emadzadeh, B.; Mohebbi, M. Prolonged-release of menthol through a superhydrophilic multilayered structure of balangu (Lallemantia royleana)-gelatin nanofibers. Mater. Sci. Eng. C 2020, 115, 111115. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Hossein Goli, S.A.; Milad Fathi, F. Encapsulation of Orange Essential Oil Using Cross-linked Electrospun Gelatin Nanofibers. Food Bioprocess Technol. 2018, 11, 427–434. [Google Scholar] [CrossRef]
- Maiorova, L.A.; Erokhina, S.I.; Pisani, M.; Barucca, G.; Marcaccio, M.; Koifman, O.I.; Salnikov, D.S.; Gromova, O.A.; Astolfi, P.; Ricci, V.; et al. Encapsulation of vitamin B12 into nanoengineered capsules and soft matter nanosystems for targeted delivery. Colloids Surf. B. 2019, 182, 110366. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Study of microencapsulation and controlled release of modified chitosan microparticles containing vitamin B12. Powder Technol. 2017, 318, 162–169. [Google Scholar] [CrossRef]
- Baik, H.W.; Russell, R.M. Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 1999, 19, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Akbari, N.; Assadpour, E.; Kharazmi, M.S.; Jafari, S.M. Encapsulation of Vitamin B12 by Complex Coacervation of Whey Protein Concentrate–Pectin; Optimization and Characterization. Molecules 2022, 27, 6130. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, V.; Matos, M.; Serrano, E.; Álvarez, J.R.; Marcet, I.; Blanco-López, M.C.; Gutiérrez, G. Lyophilised nanovesicles loaded with vitamin B12. J. Mol. Liq. 2022, 365, 120129. [Google Scholar] [CrossRef]
- Tan, Y.; Lee, P.W.; Martens, T.D.; McClements, D.J. Comparison of emulsifying properties of plant and animal proteins in oil-in-water emulsions: Whey, soy, and RuBisCo proteins. Food Biophys. 2022, 17, 409–421. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Kouidri, S.; Salahinejad, E.; Tcharkhtchi, A.; Bakir, F. Controlled release from polyurethane films: Drug release mechanisms. J. Appl. Polym. Sci. 2021, 138, 50083. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Rangkupan, R.; Jeeratawatchai, H.; Kanokpanont, S.; Damrongsakkul, S. Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. Int. J. Biol. Macromol. 2010, 47, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci.; Part B: Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J.M. Physicochemical, antioxidant and antimicrobial properties of electrospun poly (ε-caprolactone) films containing a solid dispersion of sage (Salvia officinalis L.) extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Salević-Jelić, A.; Lević, S.; Stojanović, D.; Jeremić, S.; Miletić, D.; Pantić, M.; Pavlović, V.; Ignjatović, I.S.; Uskoković, P.; Nedović, V. Biodegradable and active zein-gelatin-based electrospun mats and solvent-cast films incorporating sage extract: Formulation and comparative characterization. Food Packag. Shelf Life 2023, 35, 101027. [Google Scholar] [CrossRef]
- Ghosh, R.; Thomas, D.S.; Arcot, J. Molecular Recognition Patterns between Vitamin B12 and Proteins Explored through STD-NMR and In Silico Studies. Foods 2023, 12, 575. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on wettability, mechanical property and biocompatibility of electrospun gelatin/zein nanofibers cross-linked by glucose. Food Hydrocolloids 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Suryawanshi, D.; Wavhule, P.; Shinde, U.; Kamble, M.; Amin, P. Development, optimization and in-vivo evaluation of cyanocobalamin loaded orodispersible films using hot-melt extrusion technology: A quality by design (QbD) approach. J. Drug Deliv. Sci. Technol. 2021, 63, 102559. [Google Scholar] [CrossRef]
- Guillot, A.J.; Merino-Gutierrez, P.; Bocchino, A.; O’Mahony, C.; Giner, R.M.; Recio, M.C.; Garrigues, T.M.; Melero, A. Exploration of Microneedle-assisted skin delivery of cyanocobalamin formulated in ultraflexible lipid vesicles. Eur. J. Pharm. Biopharm. 2022, 177, 184–198. [Google Scholar] [CrossRef]
- Chronakis, I.S. Gelation of edible blue-green algae protein isolate (Spirulina platensis strain Pacifica): Thermal transitions, rheological properties, and molecular forces involved. J. Agric. Food. Chem. 2001, 49, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Delahaije, R.J.; Wierenga, P.A.; Giuseppin, M.L.; Gruppen, H. Comparison of heat-inducedaggregation of globular proteins. J. Agric. Food. Chem. 2015, 63, 5257–5265. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Sun, X. Physicochemical and structural properties of 8S and/or 11S globulins from mungbean [Vigna radiata (L.) Wilczek] with various polypeptide constituents. J. Agric. Food. Chem. 2010, 58, 6395–6402. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.A.; Furuta, M. Influence of phase behavior and miscibility on mechanical, thermal and micro-structure of soluble starch-gelatin thermoplastic biodegradable blend films. Food Nutr. Sci. 2014, 5. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Chirife, J.; del Pilar Buera, M.; Labuza, T.P. Water activity, water glass dynamics, and the control of microbiological growth in foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 465–513. [Google Scholar] [CrossRef]
- Rather, J.A.; Majid, S.D.; Dar, A.H.; Amin, T.; Makroo, H.A.; Mir, S.A.; Barba, F.J.; Dar, B.N. Extraction of Gelatin From Poultry Byproduct: Influence of Drying Method on Structural, Thermal, Functional, and Rheological Characteristics of the Dried Gelatin Powder. Front. Nutr. 2022, 9, 895197. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Ghorani, B.; Emadzadeh, B.; Rezaeinia, H.; Russell, S.J. Improvements in gelatin cold water solubility after electrospinning and associated physicochemical, functional and rheological properties. Food Hydrocoll. 2020, 104, 105740. [Google Scholar] [CrossRef]
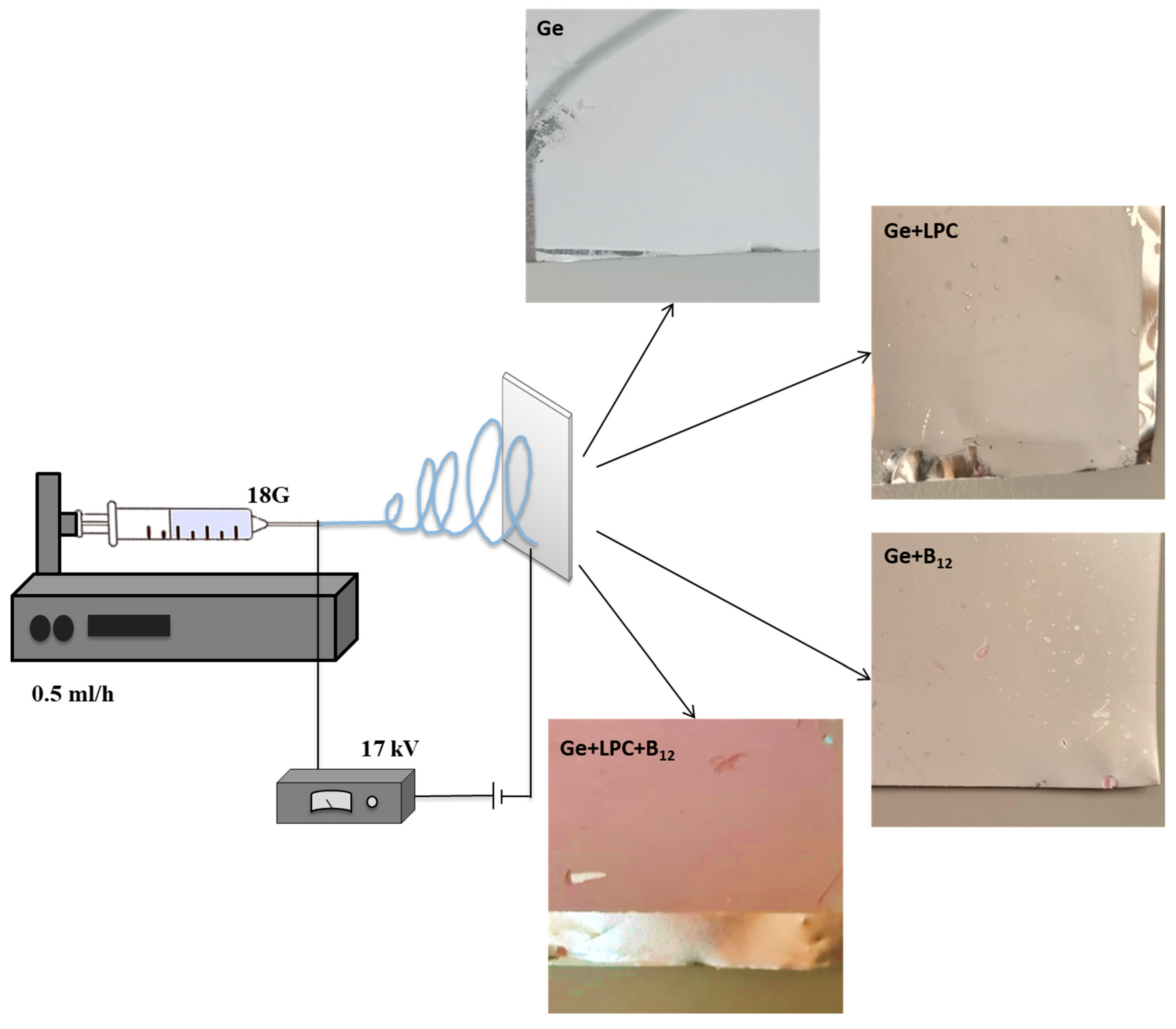




| Sample | Viscosity (mPa s) | Surface Tension (mN/m) | Density (g/mL) | Conductivity (mS/cm) |
|---|---|---|---|---|
| LPC | 1.15 ± 0.08 a | 33.4 ± 0.06 c | 0.999 ± 0.000 a | 2.76 ± 0.01 b |
| Ge | 35.00 ± 0.04 c | 33.5 ± 0.08 c | 1.082 ± 0.000 d | 4.10 ± 0.00 d |
| B12 solution * | 1.20 ± 0.04 a | 35.5 ± 0.02 e | 1.037 ± 0.000 b | 1.81 ± 0.07 a |
| Ge+LPC | 40.00 ± 0.02 e | 30.9 ± 0.01 b | 1.085 ± 0.000 e | 4.51 ± 0.02 e |
| Ge+B12 | 36.63 ± 0.05 d | 29.1 ± 0.00 a | 1.069 ± 0.001 c | 3.83 ± 0.15 c |
| Ge+LPC+B12 | 30.17 ± 0.03 b | 34.4 ± 0.47 d | 1.070 ± 0.000 c | 5.46 ± 0.07 f |
| Sample | Temperature | ΔH (J/g) | ||
|---|---|---|---|---|
| Onset | Peak | Offset | ||
| LPC | 58.79 | 96.11 | 124.71 | 161.65 |
| GE powder | 76.25 | 118.24 | 168.86 | 251.32 |
| Ge | 67.75 | 90.24 | 161.72 | 490.02 |
| Ge+LPC | 66.38 | 95.53 | 130.93 | 310.53 |
| Ge+B12 | 33.81 | 57.39 | 88.12 | 138.58 |
| Ge+LPC+B12 | 63.33 | 89.99 | 124.32 | 232.39 |
| Sample | Aw |
|---|---|
| LPC | 0.393 ± 0.031 a |
| Ge powder | 0.392 ± 0.042 a |
| Ge | 0.336 ± 0.041 a |
| Ge+LPC | 0.341 ± 0.001 a |
| Ge+B12 | 0.361 ± 0.001 a |
| Ge+LPC+B12 | 0.376 ± 0.033 a |
| Model | Parameter | Ge+LPC+B12 |
|---|---|---|
| Higuchi | k | 15.44 |
| R2 | 0.935 | |
| RMSE | 5.476 | |
| Ritger-Peppas | k | 20.93 |
| n | 0.389 | |
| R2 | 0.947 | |
| RMSE | 4.943 | |
| Kopcha | A | 19.32 |
| B | −0.877 | |
| R2 | 0.943 | |
| RMSE | 5.101 | |
| Pepas–Sahlin | k1 | 20.156 |
| k2 | 1.055 | |
| m | 0.354 | |
| R2 | 0.944 | |
| RMSE | 5.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balanč, B.; Salević-Jelić, A.; Đorđević, V.; Bugarski, B.; Nedović, V.; Petrović, P.; Knežević-Jugović, Z. The Application of Protein Concentrate Obtained from Green Leaf Biomass in Structuring Nanofibers for Delivery of Vitamin B12. Foods 2024, 13, 1576. https://doi.org/10.3390/foods13101576
Balanč B, Salević-Jelić A, Đorđević V, Bugarski B, Nedović V, Petrović P, Knežević-Jugović Z. The Application of Protein Concentrate Obtained from Green Leaf Biomass in Structuring Nanofibers for Delivery of Vitamin B12. Foods. 2024; 13(10):1576. https://doi.org/10.3390/foods13101576
Chicago/Turabian StyleBalanč, Bojana, Ana Salević-Jelić, Verica Đorđević, Branko Bugarski, Viktor Nedović, Predrag Petrović, and Zorica Knežević-Jugović. 2024. "The Application of Protein Concentrate Obtained from Green Leaf Biomass in Structuring Nanofibers for Delivery of Vitamin B12" Foods 13, no. 10: 1576. https://doi.org/10.3390/foods13101576
APA StyleBalanč, B., Salević-Jelić, A., Đorđević, V., Bugarski, B., Nedović, V., Petrović, P., & Knežević-Jugović, Z. (2024). The Application of Protein Concentrate Obtained from Green Leaf Biomass in Structuring Nanofibers for Delivery of Vitamin B12. Foods, 13(10), 1576. https://doi.org/10.3390/foods13101576









