Comparative Metabolome Profiling for Revealing the Effects of Different Cooking Methods on Glutinous Rice Longjing57 (Oryza sativa L. var. Glutinosa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Material and Cooking Treatments
2.2. Phenotype Determination and Color Measurement of Seed Appearance
2.3. Widely Targeted Metabolome Sampling and Analysis
2.4. Determination of Free Amino Acids and Carbohydrates
2.5. Statistical Analysis
3. Results
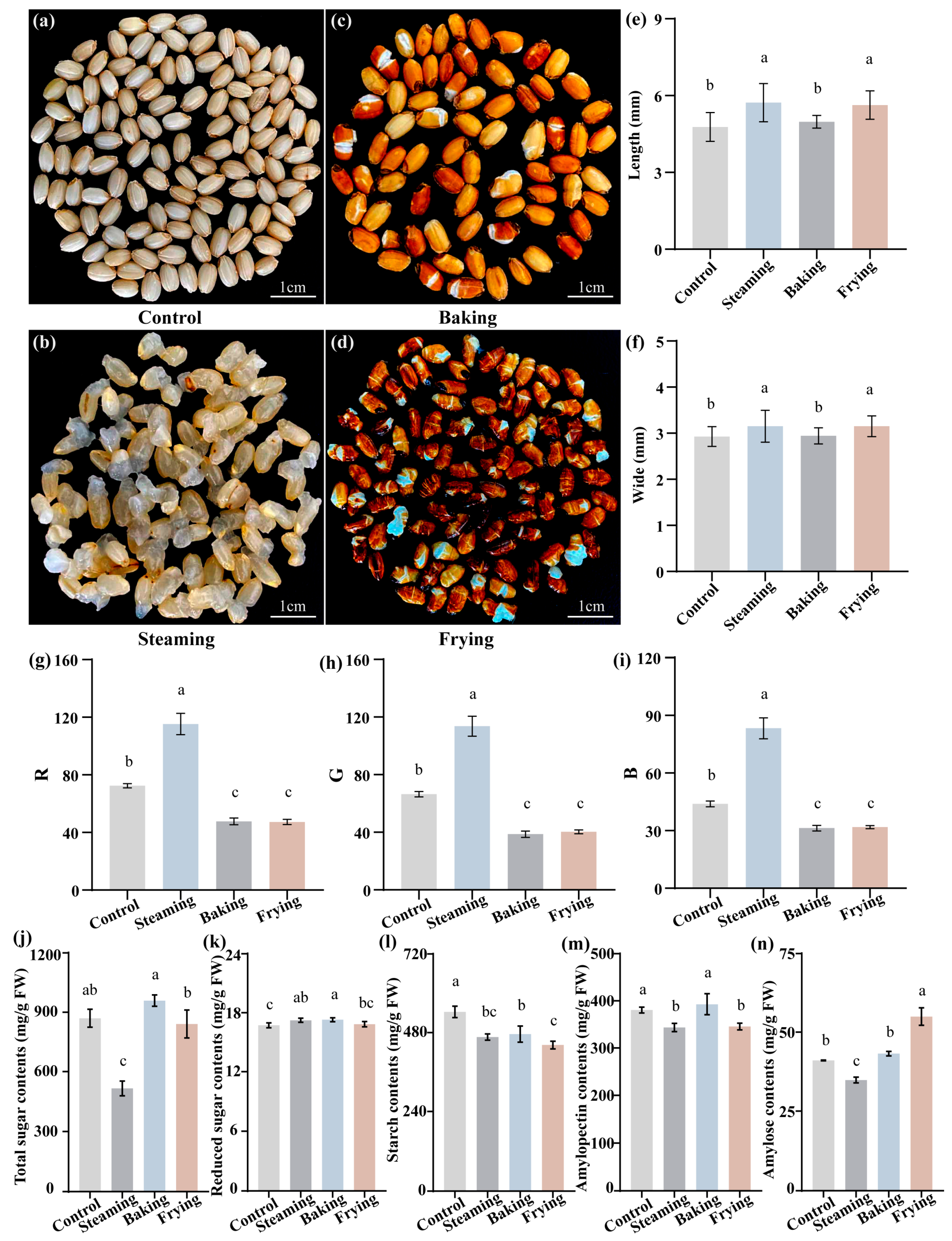
3.1. Investigation of Phenotypes and Qualities of GR Obtained Using Different Cooking Methods
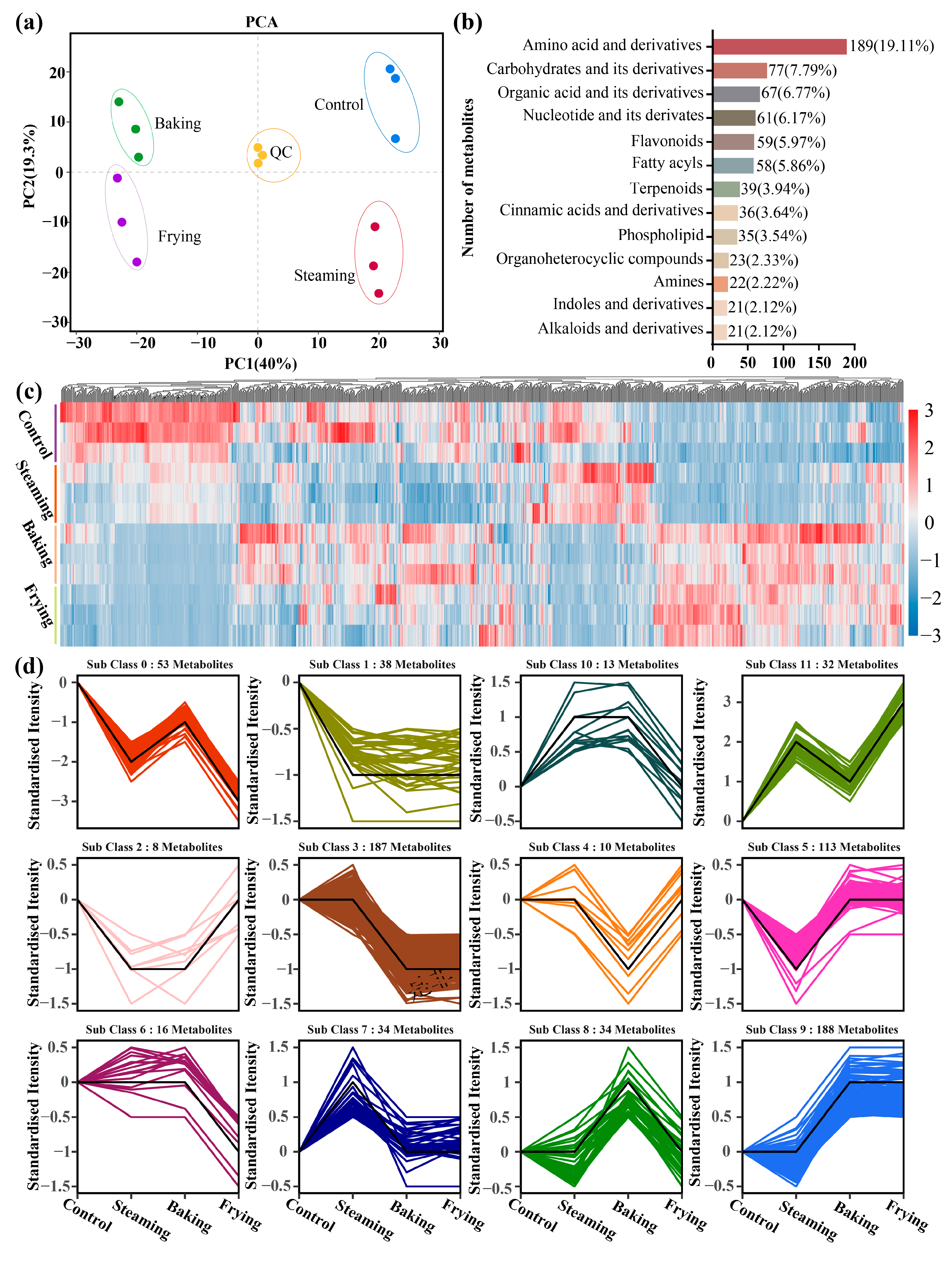
3.2. Identification of the Metabolic Profiles of GR
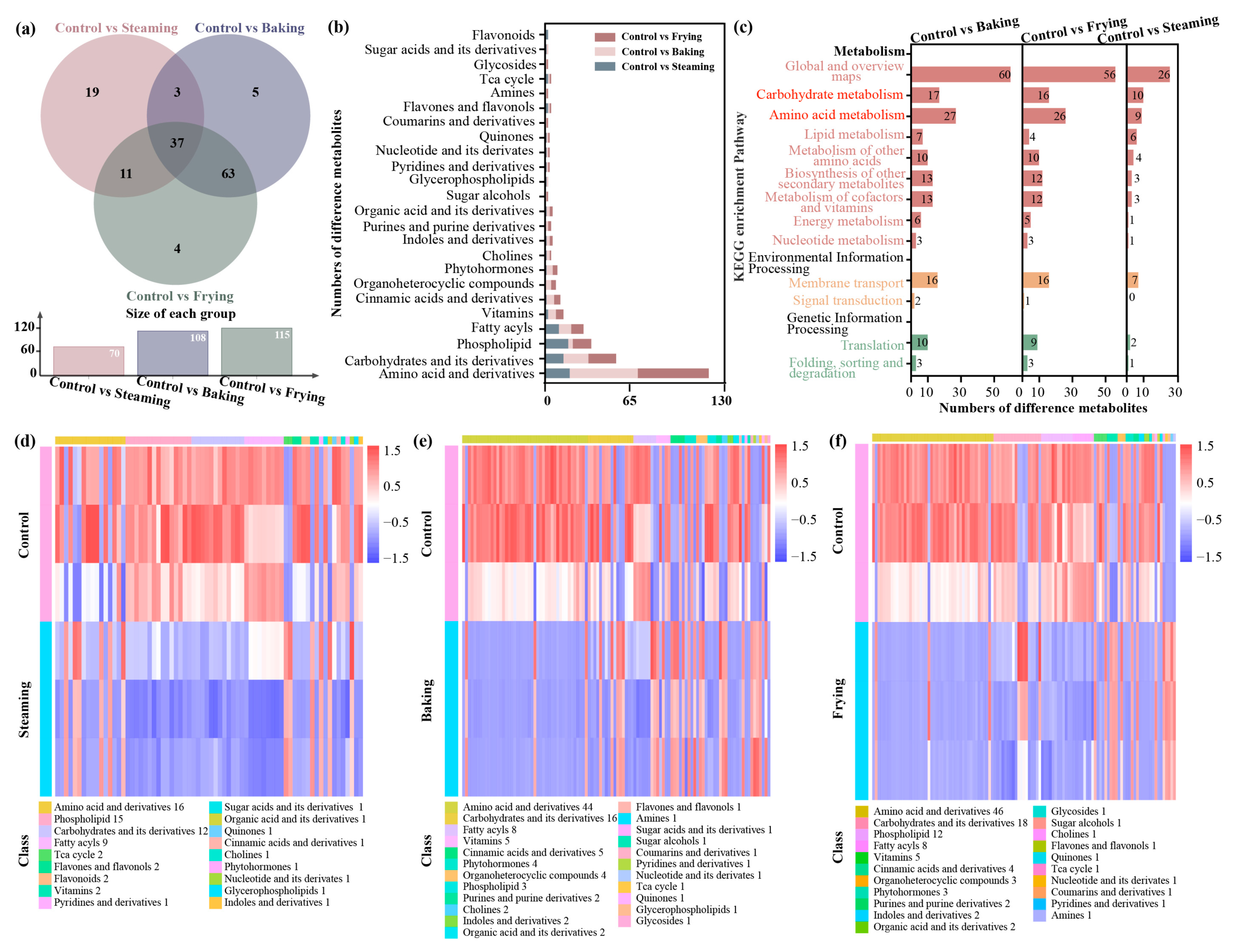
3.3. Differentially Cooking-Derived Compounds Analysis
3.4. DCCs Identified in Amino Acids and Carbohydrates
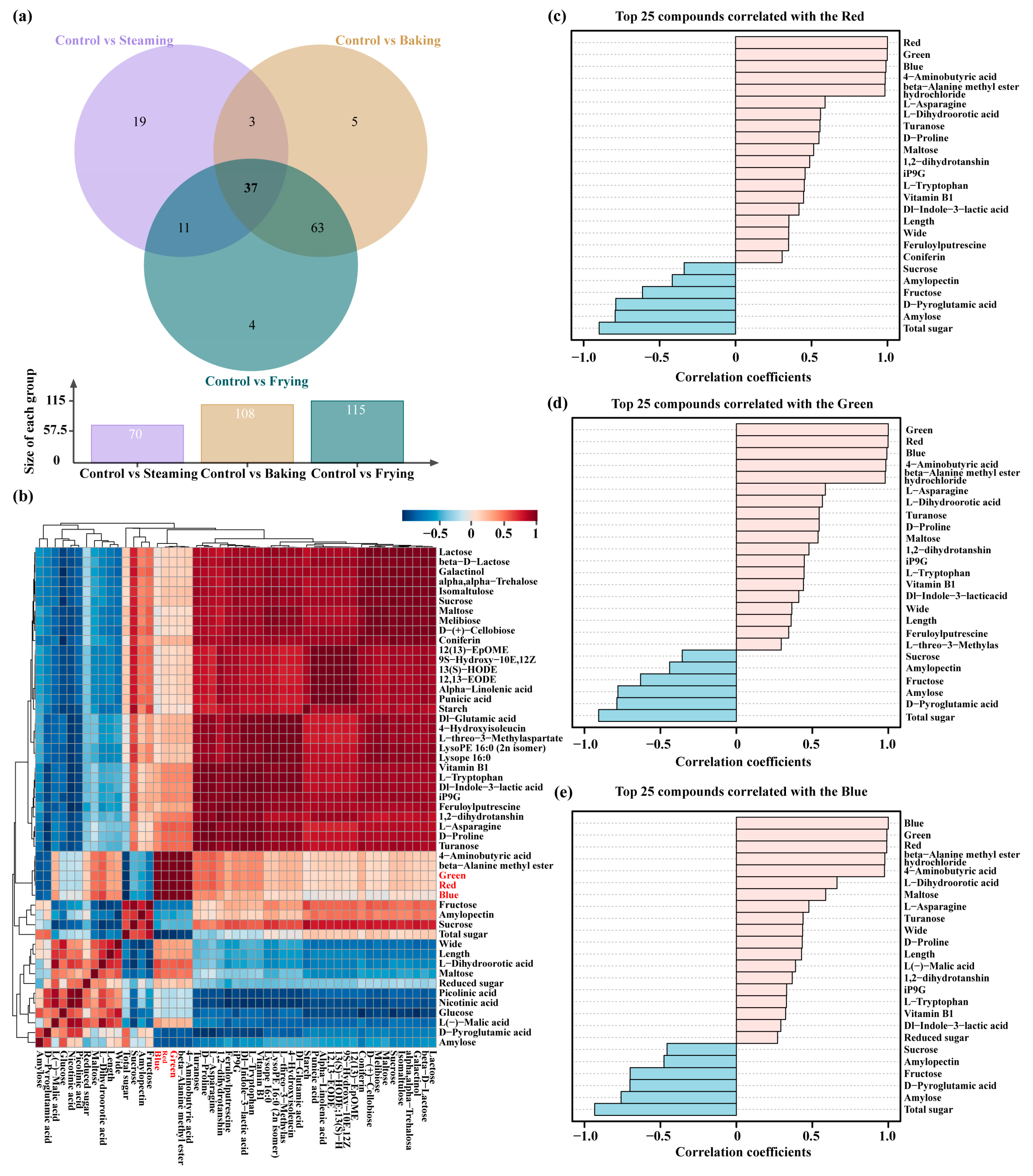
3.5. DCCs Correlated with Color and Quality Parameters
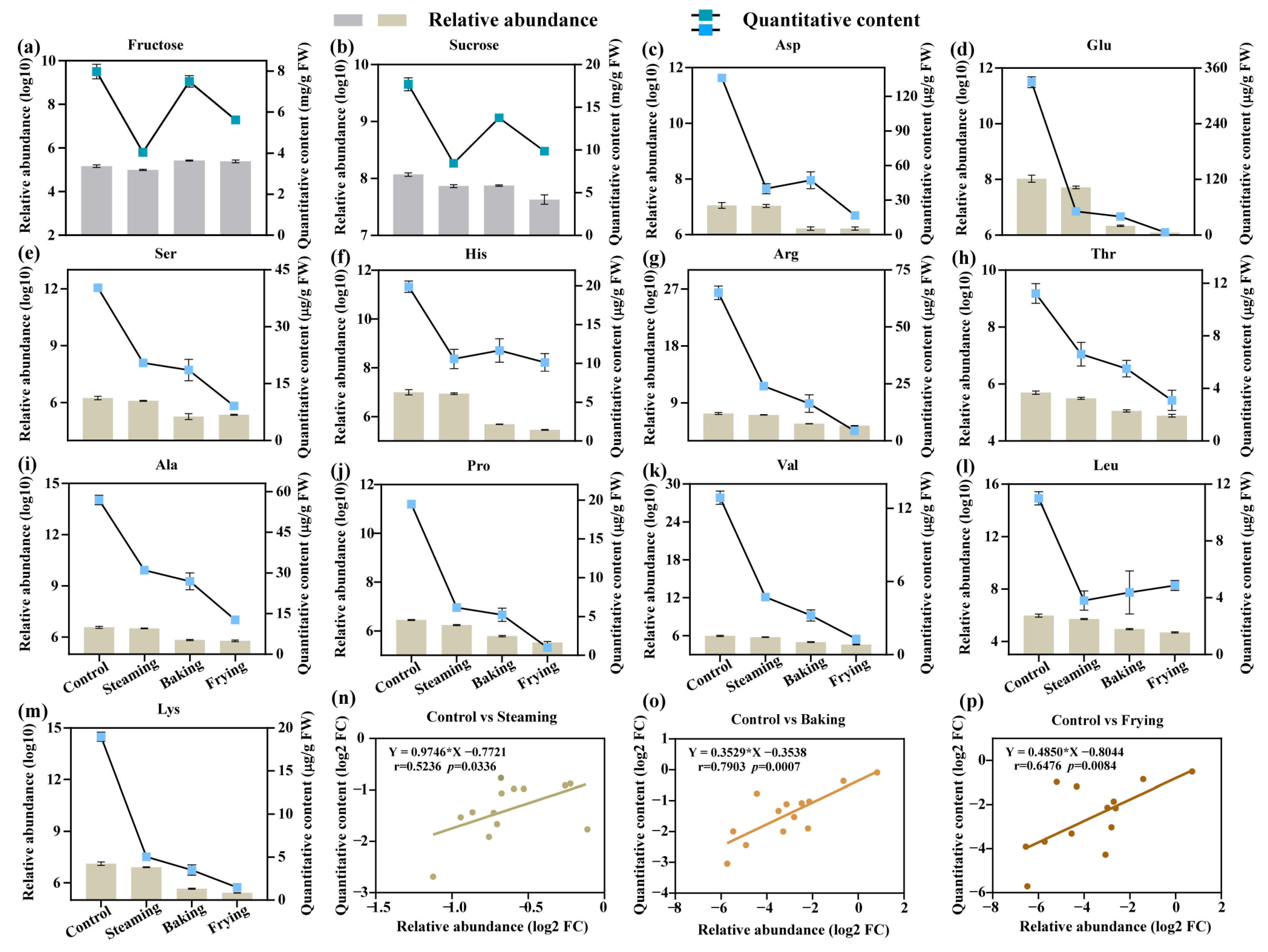
3.6. Quantification of Amino Acids and Carbohydrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Yang, X.; Chen, G.; Wang, X. Application of glutamic acid improved As tolerance in aromatic rice at early growth stage. Chemosphere 2023, 322, 138173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhu, J.K. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018, 60, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, J.; Shi, Q.; Zhang, Y.; Sun, C.; Li, A.; Lu, W.; Hu, J.; Zhou, N.; Wei, H.; et al. The key metabolites associated with nutritional components in purple glutinous rice. Food Res. Int. 2022, 160, 111686. [Google Scholar] [CrossRef]
- Naseer, B.; Naik, H.R.; Hussain, S.Z.; Zargar, I.; Beenish; Bhat, T.A.; Nazir, N. Effect of carboxymethyl cellulose and baking conditions on in-vitro starch digestibility and physico-textural characteristics of low glycemic index gluten-free rice cookies. LWT-Food Sci. Technol. 2021, 141, 110885. [Google Scholar] [CrossRef]
- Hu, X.; Fang, C.; Lu, L.; Hu, Z.; Zhang, W.; Chen, M. Dynamic changes in volatiles, soluble sugars, and fatty acids in glutinous rice during cooking. Foods 2023, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fang, C.; Zhang, W.; Lu, L.; Guo, Z.; Li, S.; Chen, M. Change in volatiles, soluble sugars and fatty acids of glutinous rice, japonica rice and indica rice during storage. LWT-Food Sci. Technol. 2023, 174, 114416. [Google Scholar] [CrossRef]
- Zhu, W.; Tie, Y.; Zhu, Z.; Yang, Y.; Feng, S.; Liu, J. The effects of an innovative pulping technique of synchronously pulping and gelatinizing treatment on raw materials properties, oenological parameters, fermentation process, and flavor characteristics of glutinous rice wine. Food Sci. Biotechnol. 2022, 31, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, R.; Takahashi, K.; Ichikawa, S.; Wakayama, M.; Kobayashi, A.; Miyagawa, S.; Uchimura, T. Using SPME-GC/REMPI-TOFMS to measure the volatile odor-active compounds in freshly cooked rice. ACS Omega 2020, 5, 20638–20642. [Google Scholar] [CrossRef]
- Sharafi, K.; Yunesian, M.; Nodehi, R.N.; Hossein Mahvi, A.; Pirsaheb, M.; Nazmara, S. The reduction of toxic metals of various rice types by different preparation and cooking processes—Human health risk assessment in Tehran households, Iran. Food Chem. 2019, 280, 294–302. [Google Scholar] [CrossRef]
- Eakkanaluksamee, K.; Anuntagool, J. Optimization of high-protein glutinous rice flour production using response surface method. Rice Sci. 2020, 27, 75–80. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Jiang, Y. Amino acids in rice grains and their regulation by polyamines and phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, transport, and regulation of amino acids: What is missing in rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Jin, Q.; Wang, X. Influence of oryzanol and tocopherols on thermal oxidation of rice bran oil during the heating process at Chinese cooking temperatures. LWT-Food Sci. Technol. 2021, 142, 111022. [Google Scholar] [CrossRef]
- Xu, D.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effect of high pressure steam on the eating quality of cooked rice. LWT-Food Sci. Technol. 2019, 104, 100–108. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, H.; Zhang, T.; Tamogami, S.; Chen, J.Y. Analysis of flavor volatiles of glutinous rice during cooking by combined gas chromatography–mass spectrometry with modified headspace solid-phase microextraction method. J. Food Compos. Anal. 2009, 22, 347–353. [Google Scholar] [CrossRef]
- Hu, S.; Liu, C.; Liu, X. Innovative application of metabolomics on bioactive ingredients of foods. Foods 2022, 11, 2974. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Zhang, J.; Zhang, Y.T.; Sun, J.Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT-Food Sci. Technol. 2023, 182, 114853. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT-Food Sci. Technol. 2023, 178, 114586. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging applications of metabolomics in food science and future trends. Food Che. X 2022, 16, 100500. [Google Scholar] [CrossRef]
- Sheng, Q.; Yao, X.; Chen, H.; Tang, H.; Lu, L. Identification of nutritional ingredients and medicinal components of hawk tea and insect tea using widely targeted secondary metabolomics. Horticulturae 2023, 9, 457. [Google Scholar] [CrossRef]
- Tao, N.; Wang, R.; Xu, X.; Dong, T.; Zhang, S.; Liang, M.; Wang, Q. Xanthosine is a novel anti-browning compound in potato identified by widely targeted metabolomic analysis and in vitro test. Postharvest Biol. Technol. 2021, 171, 111367. [Google Scholar] [CrossRef]
- Sangpong, L.; Khaksar, G.; Pinsorn, P.; Oikawa, A.; Sasaki, R.; Erban, A.; Watanabe, M.; Wangpaiboon, K.; Tohge, T.; Kopka, J.; et al. Assessing dynamic changes of taste-related primary metabolism during ripening of durian pulp using metabolomic and transcriptomic analyses. Front Plant Sci. 2021, 12, 687799. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Du, P.; Liang, X.; Li, H.; Lu, Q.; Li, S.; Liu, H.; Hong, Y.; Varshney, R.K.; et al. Impact of different cooking methods on the chemical profile of high-oleic acid peanut seeds. Food Chem. 2022, 379, 131970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, X.; Lin, D.; Wu, K.; Wu, Z.; Zhang, Z.; Peng, X. Grain quality affected by introducing photorespiratory bypasses into rice. Agronomy 2022, 12, 566. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Y.; Ma, R.; An, J.; Huang, W.; Wu, Y.; Hou, J.; Zhang, D.; Lin, F.; Xu, R.; et al. Genetic dissection of seed appearance quality using recombinant inbred lines in soybean. Mol. Breed. 2021, 41, 72. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Chen, H.; Li, G.; Su, K.; Song, M.; Duan, Z.; Li, X.; Cao, X.; Huang, J.; Huang, S.; et al. Comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut processed with different methods. Food Chem. 2021, 335, 127662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q.; Pei, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformatics 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S.; Han, X.; Li, C.; Ni, Y.; Hao, J. Physico-chemical properties and free amino acids profiles of six wolfberry cultivars in Zhongning. J. Food Compos. Anal. 2020, 88, 103460. [Google Scholar] [CrossRef]
- Hu, X.; Fang, C.; Lu, L.; Hu, Z.; Shao, Y.; Zhu, Z. Determination of soluble sugar profile in rice. J Chromatogr. B. 2017, 1058, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Hosny, A.; Saad-Allah, K. Reducing nitrogen leaching while enhancing growth, yield performance and physiological traits of rice by the application of controlled-release urea fertilizer. Paddy Water Environ. 2021, 19, 173–188. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, B.; Tan, C.P.; Fu, X.; Huang, Q. Effects of limited moisture content and storing temperature on retrogradation of rice starch. Int. J. Biol. Macromol. 2019, 137, 1068–1075. [Google Scholar] [CrossRef]
- Huang, S.; Rao, G.; Ashraf, U.; Deng, Q.; Dong, H.; Zhang, H.; Mo, Z.; Pan, S.; Tang, X. Ultrasonic seed treatment improved morpho-physiological and yield traits and reduced grain Cd concentrations in rice. Ecotox. Environ. Safe. 2021, 214, 112119. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Yu, T.; Zeng, Z.; Ashraf, U.; Shi, Q.; Huang, S.; Lian, T.; Chen, J.; Muzaffar, W.; Shen, W. Silicon application modulates the growth, rhizosphere soil characteristics, and bacterial community structure in sugarcane. Front. Plant Sci. 2021, 12, 710139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Rashid, M.A.R.; Zhang, Y.; Zhao, Y.; Pan, Y. Genome wide association study on development and evolution of glutinous rice. BMC Genomic Data 2022, 23, 33. [Google Scholar] [CrossRef]
- Burešová, I.; Červenka, L.; Šebestíková, R.; Augustová, M.; Jarošová, A. Applicability of flours from pigmented and glutinous rice in gluten-free bread baking. Foods 2023, 12, 1324. [Google Scholar] [CrossRef]
- Yu, L.; Turner, M.S.; Fitzgerald, M.; Stokes, J.R.; Witt, T. Review of the effects of different processing technologies on cooked and convenience rice quality. Trends Food Sci. Technol. 2017, 59, 124–138. [Google Scholar] [CrossRef]
- Paesani, C.; Gómez, M. Effects of the pre-frying process on the cooking quality of rice. LWT-Food Sci. Technol. 2021, 140, 110743. [Google Scholar] [CrossRef]
- Syafutri, M.I.; Pratama, F.; Syaiful, F.; Faizal, A. Effects of varieties and cooking methods on physical and chemical characteristics of cooked rice. Rice Sci. 2016, 23, 282–286. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Ray, C.A. Control of maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, L.; Xia, Y.; Wu, Y.; Zhou, Y.; Wang, H. Effects of frying on starch structure and digestibility of glutinous rice cakes. J. Cereal Sci. 2018, 83, 196–203. [Google Scholar] [CrossRef]
- Wang, X.; Tian, R.; Yang, H.; Shen, C.; Han, X. Effect of baking technique for rice wine production and the characteristics of baked rice wine. J. Sci. Food Agr. 2022, 102, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Liyanaarachchi, G.V.V.; Mahanama, K.R.R.; Somasiri, H.P.P.S.; Punyasiri, P.A.N.; Ranatunga, M.A.B. Variation in amino acid composition of rice (Oryza sativa L.) as affected by the cooking technique. J. Food Process. Pres. 2022, 46, e16781. [Google Scholar] [CrossRef]
- Nishimura, M.; Yoshida, S.; Haramoto, M.; Mizuno, H.; Fukuda, T.; Kagami-Katsuyama, H.; Tanaka, A.; Ohkawara, T.; Sato, Y.; Nishihira, J. Effects of white rice containing enriched gamma-aminobutyric acid on blood pressure. J. Tradit. Complement. Med. 2016, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sirisoontaralak, P.; Nakornpanom, N.N.; Koakietdumrongkul, K.; Panumaswiwath, C. Development of quick cooking germinated brown rice with convenient preparation and containing health benefits. LWT-Food Sci. Technol. 2015, 61, 138–144. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.A.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2024, 438, 137994. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. Formation of Maillard reaction products in bread crust-like model system made of different whole cereal flours. Eur. Food Res. Technol. 2020, 246, 1207–1218. [Google Scholar] [CrossRef]
- Kathuria, D.; Hamid; Gautam, S.; Thakur, A. Maillard reaction in different food products: Effect on product quality, human health and mitigation strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, G.; Li, Y. Bread characteristics and antioxidant activities of Maillard reaction products of white pan bread containing various sugars. LWT-Food Sci. Technol. 2018, 95, 308–315. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Cai, L.; Liu, C.; Zhang, Y.; Wang, L.; Liu, H.; Feng, Y.; Pan, G.; Ma, W. Comparative Metabolome Profiling for Revealing the Effects of Different Cooking Methods on Glutinous Rice Longjing57 (Oryza sativa L. var. Glutinosa). Foods 2024, 13, 1617. https://doi.org/10.3390/foods13111617
Guo Z, Cai L, Liu C, Zhang Y, Wang L, Liu H, Feng Y, Pan G, Ma W. Comparative Metabolome Profiling for Revealing the Effects of Different Cooking Methods on Glutinous Rice Longjing57 (Oryza sativa L. var. Glutinosa). Foods. 2024; 13(11):1617. https://doi.org/10.3390/foods13111617
Chicago/Turabian StyleGuo, Zhenhua, Lijun Cai, Chuanxue Liu, Yunjiang Zhang, Linan Wang, Hao Liu, Yanjiang Feng, Guojun Pan, and Wendong Ma. 2024. "Comparative Metabolome Profiling for Revealing the Effects of Different Cooking Methods on Glutinous Rice Longjing57 (Oryza sativa L. var. Glutinosa)" Foods 13, no. 11: 1617. https://doi.org/10.3390/foods13111617
APA StyleGuo, Z., Cai, L., Liu, C., Zhang, Y., Wang, L., Liu, H., Feng, Y., Pan, G., & Ma, W. (2024). Comparative Metabolome Profiling for Revealing the Effects of Different Cooking Methods on Glutinous Rice Longjing57 (Oryza sativa L. var. Glutinosa). Foods, 13(11), 1617. https://doi.org/10.3390/foods13111617



