Characterization of Antioxidant Bioactive Compounds and Rheological, Color and Sensory Properties in 3D-Printed Fruit Snacks
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Preparation of Fruit Material for 3D Printing
2.3. Three-Dimensional Printing of Fruit Snacks
2.4. Extraction of Antioxidant Bioactive Compounds
2.5. Determination of Polyphenolic Compounds
2.5.1. Determination of Total Phenolic Content (TPC)
2.5.2. Determination of Total Flavonoids (TF)
2.5.3. Determination of Total Hydroxycinnamic Acids (HCAs) and Total Flavonols (FLs)
2.5.4. Determination of Condensed Tannins (CTs)
2.6. Determination of Pigments
2.6.1. Determination of Total Monomeric Anthocyanins (ANTs)
2.6.2. Determination of Total Carotenoids (CARs), Chlorophyll A (CHL A) and Chlorophyll B (CHL B)
2.7. In Vitro Antioxidant Capacity (AOC)
2.7.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Scavenging Activity Assay
2.7.2. FRAP (Ferric-Reducing Antioxidant Power) Assay
2.8. Determination of Rheological Properties of 3DP Snacks
2.8.1. Texture Analysis
Forward Extrusion Test
Penetration Test
2.8.2. Dimension Measurements
2.8.3. Particle Size Distribution
2.9. Determination of Instrumental Color
2.10. Sensory Evaluation of 3D-Printed Snacks
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Polyphenolic Compounds, Pigments and Antioxidant Capacity in 3D-Printed Snacks
3.2. Characterization of Rheological Properties in 3D-Printed Snacks
3.2.1. Texture Analysis (Forward Extrusion and Penetration Test)
3.2.2. Dimension Measurements of 3D-Printed Snacks
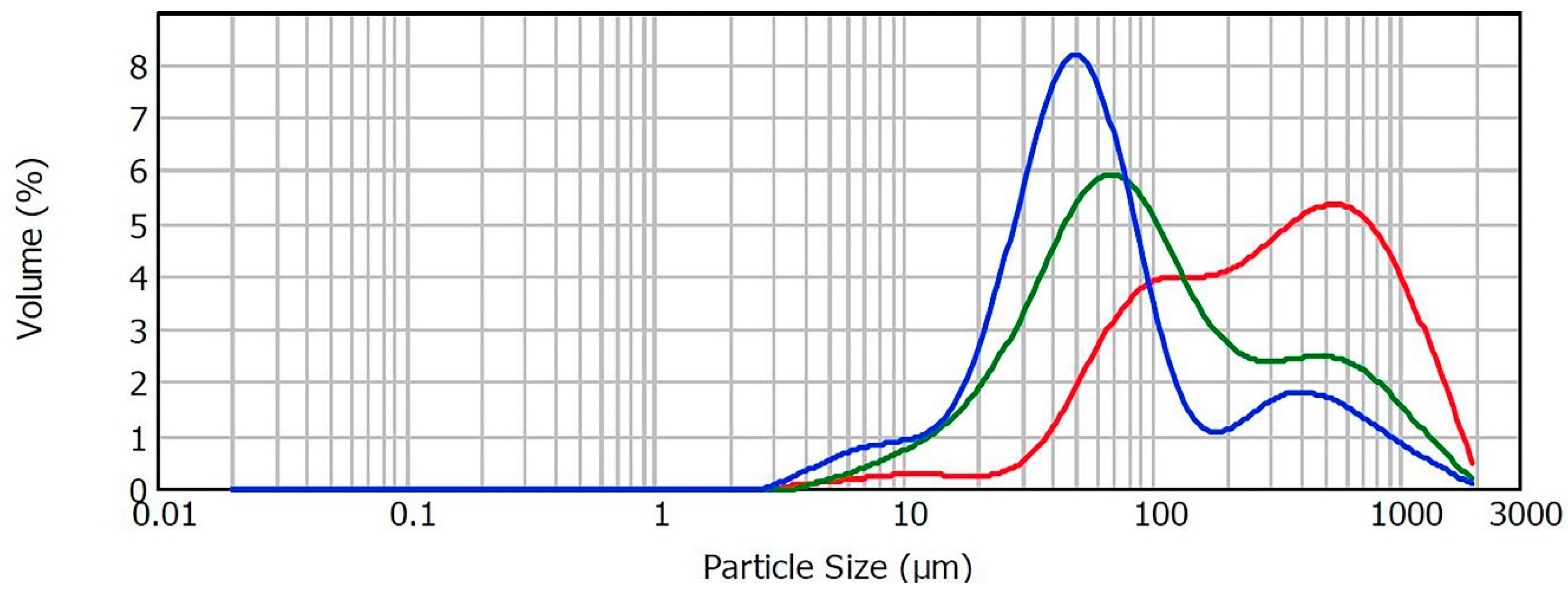
3.2.3. Particle Size Distribution
3.3. Characterization of Color Properties in 3D-Printed Snacks
3.4. Characterization of the Sensory Properties of 3D-Printed Snacks
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alsubhi, M.; Blake, M.; Nguyen, T.; Majmudar, I.; Moodie, M.; Ananthapavan, J. Consumer willingness to pay for healthier food products: A systematic review. Obes. Rev. 2022, 24, e13525. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Brdar, D.; Putnik, P.; Bosiljkov, T.; Durgo, K.; Huđek Turković, A.; Brčić Karačonji, I.; Jurica, K.; Pavlić, B.; Granato, D.; et al. Strawberry tree fruits (Arbutus unedo L.): Bioactive composition, cellular antioxidant activity, and 3D printing of functional foods. Food Chem. 2024, 433, 137287. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Sagbas, H.I.; Ilhan, G.; Zitouni, H.; Anjum, M.A.; Hanine, H.; Necas, T.; Ondrasek, I.; Ercisli, S. Morphological and biochemical characterization of diverse strawberry tree (Arbutus unedo L.) genotypes from northern Turkey. Agronomy 2020, 10, 1581. [Google Scholar] [CrossRef]
- Colak, A.M. Morphological and biochemical diversity in fruits of Arbutus unedo L. from east aegean region in Turkey. Erwerbs-Obstbau 2019, 61, 379–383. [Google Scholar] [CrossRef]
- Boussalah, N.; Boussalah, D.; Cebadera-Miranda, L.; Fernández-Ruiz, V.; Barros, L.; Ferreira, I.C.F.R.; Cortes Sanchez Mata, M.; Madani, K. Nutrient composition of Algerian strawberry-tree fruits (Arbutus unedo L.). Fruits 2018, 73, 283–297. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Djekić, I.; Velebit, B.; Pavlić, B.; Putnik, P.; Šojić Merkulov, D.; Bebek Markovinović, A.; Bursać Kovačević, D. Food quality 4.0: Sustainable food manufacturing for the twenty-first century. Food Eng. Rev. 2023, 15, 577–608. [Google Scholar] [CrossRef]
- Sundarsingh, A.; Zhang, M.; Mujumdar, A.S.; Li, J. Research progress in printing formulation for 3D printing of healthy future foods. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Clary, T.; Rosales, A.; Ubeyitogullari, A. Upcycling imperfect broccoli and carrots into healthy snacks using an innovative 3D food printing approach. Food Sci. Nutr. 2023, 12, 84–93. [Google Scholar] [CrossRef]
- Zhu, S.; Vazquez Ramos, P.; Heckert, O.R.; Stieger, M.; van der Goot, A.J.; Schutyser, M. Creating protein-rich snack foods using binder jet 3D printing. J. Food Eng. 2022, 332, 111124. [Google Scholar] [CrossRef]
- Radoš, K.; Pastor, K.; Kojić, J.; Drakula, S.; Dujmić, F.; Novotni, D.; Čukelj Mustač, N. Influence of infill level and post-processing on physical parameters and betaine content of enriched 3D-printed sweet snacks. Foods 2023, 12, 4417. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montero, C.; Vicente-Jurado, D.; Igual, M.; Martínez-Monzó, J.; García-Segovia, P. Fiber enrichment of 3D printed apricot gel snacks with orange by-products. Gels 2023, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.D.; Lee, C.P.; Foo, S.Y.; Tan, J.C.W.; Tan, S.S.Y.; Ong, E.S.; Leo, C.H.; Hashimoto, M. 3D printability and biochemical analysis of revalorized orange peel waste. Int. J. Bioprint. 2023, 9, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Radoš, K.; Benković, M.; Čukelj Mustač, N.; Habuš, M.; Voučko, B.; Pavičić, T.V.; Ćurić, D.; Ježek, D.; Novotni, D. Powder properties, rheology and 3D printing quality of gluten-free blends. J. Food Eng. 2023, 338, 111251. [Google Scholar] [CrossRef]
- Letras, P.; Oliveira, S.; Varela, J.; Nunes, M.C.; Raymundo, A. 3D printed gluten-free cereal snack with incorporation of Spirulina (Arthrospira platensis) and/or Chlorella vulgaris. Algal Res. 2022, 68, 102863. [Google Scholar] [CrossRef]
- Chen, X.-h.; Zhang, M.; Teng, X.-x.; Mujumdar, A.S. Internal structure design for improved shape fidelity and crispness of 3D printed pumpkin-based snacks after freeze-drying. Food Res. Int. 2022, 157, 111220. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, I.; Putnik, P.; Valjak, F.; Pavlić, B.; Šojić, B.; Bebek Markovinović, A.; Bursać Kovačević, D. 3D printing as novel tool for fruit-based functional food production. Curr. Opin. Food Sci. 2021, 41, 138–145. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Putnik, P.; Bosiljkov, T.; Kostelac, D.; Frece, J.; Markov, K.; Žigolić, A.; Kaurinović, J.; Pavlić, B.; Duralija, B.; et al. 3D printing of functional strawberry snacks: Food design, texture, antioxidant bioactive compounds, and microbial stability. Antioxidants 2023, 12, 436. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2020, 10, 3. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA). Clin. Biochem. 1996, 29, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Vahčić, N.; Hruškar, M.; Marković, K. Quantitative descriptive analysis method in sensory evaluation of yoghurt. Mljekarstvo 2000, 50, 279–296. [Google Scholar]
- Cloud Software Group, Inc. Data Science Workbench, Version 14.1. Available online: http://tibco.com (accessed on 5 March 2024).
- Bebek Markovinović, A.; Putnik, P.; Duralija, B.; Krivohlavek, A.; Ivešić, M.; Mandić Andačić, I.; Palac Bešlić, I.; Pavlić, B.; Lorenzo, J.M.; Bursać Kovačević, D. Chemometric Valorization of Strawberry (Fragaria x ananassa Duch.) cv. ‘Albion’ for the Production of Functional Juice: The Impact of Physicochemical, Toxicological, Sensory, and Bioactive Value. Foods 2022, 11, 640. [Google Scholar] [CrossRef]
- Rong, L.; Chen, X.; Shen, M.; Yang, J.; Qi, X.; Li, Y.; Xie, J. The application of 3D printing technology on starch-based product: A review. Trends Food Sci. Technol. 2023, 134, 149–161. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Cai, Y.; Ahmad, I.; Zhang, A.; Ding, Y.; Qiu, Y.; Zhang, G.; Tang, W.; Lyu, F. Hot extrusion 3D printing technologies based on starchy food: A review. Carbohydr. Polym. 2022, 294, 119763. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.V.; Kusumawardani, S.; Kunyanee, K.; Luangsakul, N. Polyphenol-modified starches and their applications in the food industry: Recent updates and future directions. Foods 2022, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016, 192, 328–335. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, M.; Bhandari, B. Controlling the three-dimensional printing mechanical properties of Nostoc Sphaeroides system. Food Biophys. 2019, 15, 240–248. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of sweet potato starch as a structural enhancer for three-dimensional printing of Scomberomorus niphonius surimi. J. Texture Stud. 2019, 50, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT 2018, 87, 67–76. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B. Recent development in 3D food printing. Crit. Rev. Food Sci. Nutr. 2015, 57, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Geppert, J.; Funken, E.; Stamminger, R. Consumers perceptions and preference for strawberries—A case study from Germany. Int. J. Fruit Sci. 2015, 15, 405–424. [Google Scholar] [CrossRef]
- Buvé, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Chen, X.D. Food drying fundamentals. In Drying Technologies in Food Processing; Chen, X.D., Mujumdar, A.S., Eds.; Blackwell Publishing: Singapore, 2008; pp. 1–55. [Google Scholar]
- Krebs, G.; Müller, M.; Becker, T.; Gastl, M. Characterization of the macromolecular and sensory profile of non-alcoholic beers produced with various methods. Food Res. Int. 2019, 116, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Christofides, S.R.; Sirangelo, T.M.; Bartella, L.; Muller, C.; Di Donna, L.; Muzzalupo, I.; Bruno, L.; Ferrante, A.; Chiappetta, A.A.C.; et al. Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars. Foods 2022, 11, 2554. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.F.d.O.; Bolini, H.M.A. Passion fruit juice with different sweeteners: Sensory profile by descriptive analysis and acceptance. Food Sci. Nutr. 2015, 3, 129–139. [Google Scholar] [CrossRef] [PubMed]
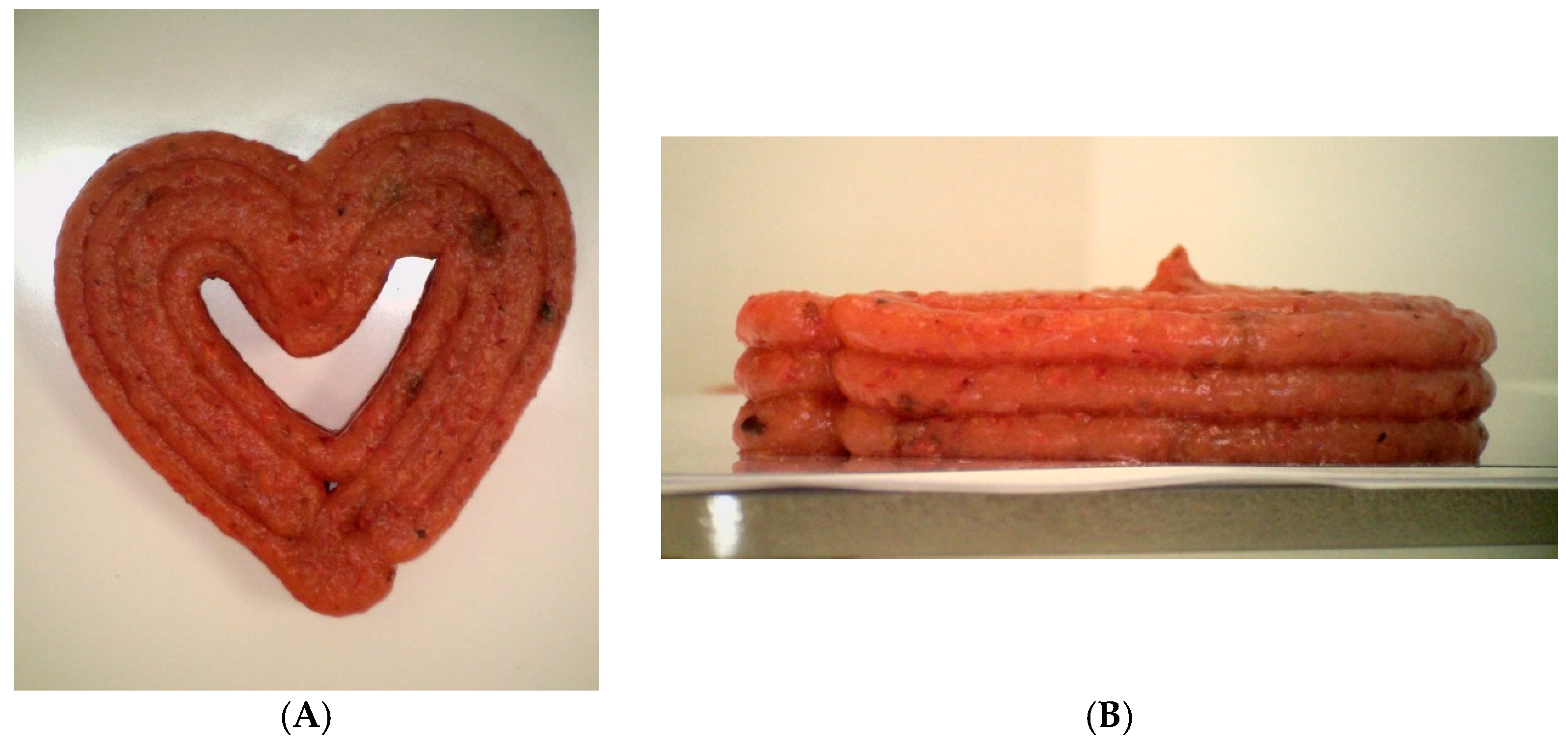
| Sample ID | Starch Content (%) | 3D Program |
|---|---|---|
| 1 | 0 | P1 |
| 2 | 0 | P2 |
| 3 | 6 | P1 |
| 4 | 6 | P2 |
| 5 | 8 | P1 |
| 6 | 8 | P2 |
| 7 | 10 | P1 |
| 8 | 10 | P2 |
| Simpe ID | Sweeteners | Sweetener Content (%) |
|---|---|---|
| A | Control sample | Without sweetener |
| B1 | Saccharose | 6.1 |
| B2 | Saccharose | 9.1 |
| C1 | Fructose | 7.1 |
| C2 | Fructose | 8.9 |
| D1 | Birch sugar (xylitol) | 5.6 |
| D2 | Birch sugar (xylitol) | 8.5 |
| E1 | Erythritol | 3.2 |
| E2 | Erythritol | 4.7 |
| F1 | Maple syrup | 5.5 |
| F2 | Maple syrup | 8.7 |
| G1 | Date syrup | 5.2 |
| G2 | Date syrup | 7.1 |
| H1 | Agave syrup | 6.7 |
| H2 | Agave syrup | 10.2 |
| I1 | Stevia and erythritol | 2.5 |
| I2 | Stevia and erythritol | 3.9 |
| Variable | n | TPC | HCAs | FLs | TFs | CTs |
|---|---|---|---|---|---|---|
| Starch level | † | † | † | † | † | |
| 0% | 4 | 429.50 ± 5.87 a | 84.99 ± 0.73 a | 55.41 ± 0.54 a | 10.66 ± 0.22 a | 171.33 ± 1.66 a |
| 6% | 4 | 392.96 ± 5.87 b | 78.16 ± 0.73 b | 52.55 ± 0.54 b | 9.65 ± 0.22 b | 141.87 ± 1.66 b |
| 8% | 4 | 353.60 ± 5.87 c | 71.11 ± 0.73 c | 49.25 ± 0.54 c | 9.90 ± 0.22 a,b | 141.41 ± 1.66 b |
| 10% | 4 | 344.21 ± 5.87 c | 64.89 ± 0.73 d | 42.16 ± 0.54 d | 9.22 ± 0.22 b | 147.59 ± 1.66 b |
| 3DP Program | ‡ | † | ‡ | ‡ | ‡ | |
| Program 1 | 8 | 382.48 ± 4.15 a | 76.45 ± 0.52 a | 50.47 ± 0.38 a | 9.69 ± 0.15 a | 149.71 ± 1.17 a |
| Program 2 | 8 | 377.65 ± 4.15 a | 73.12 ± 0.52 b | 49.22 ± 0.38 a | 10.02 ± 0.15 a | 151.39 ± 1.17 a |
| Dataset average | 16 | 380.07 ± 9.09 | 74.79 ± 2.03 | 49.84 ± 1.31 | 9.86 ± 0.16 | 150.56 ± 3.31 |
| Variable | n | ANT | CAR | CHLA | CHLB | DPPH | FRAP |
|---|---|---|---|---|---|---|---|
| Starch level | † | † | † | † | ‡ | † | |
| 0% | 4 | 9.65 ± 0.16 a | 0.58 ± 0.002 b | 0.24 ± 0.01 a | 0.40 ± 0.01 a | 2.90 ± 0.07 a | 290.38 ± 0.35 a,b |
| 6% | 4 | 7.75 ± 0.16 b | 0.62 ± 0.002 a | 0.11 ± 0.01 b | 0.18 ± 0.01 b | 3.00 ± 0.07 a | 289.95 ± 0.35 a,b |
| 8% | 4 | 7.73 ± 0.16 b | 0.52 ± 0.002 c | 0.11 ± 0.01 b | 0.17 ± 0.01 b | 2.74 ± 0.07 a | 290.82 ± 0.35 a |
| 10% | 4 | 8.09 ± 0.16 b | 0.48 ± 0.002 d | 0.12 ± 0.01 b | 0.19 ± 0.01 b | 2.73 ± 0.07 a | 288.94 ± 0.35 b |
| 3DP Program | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ | |
| Program 1 | 8 | 8.35 ± 0.11 a | 0.55 ± 0.001 a | 0.15 ± 0.004 a | 0.25 ± 0.01 a | 2.90 ± 0.05 a | 289.96 ± 0.25 a |
| Program 2 | 8 | 8.27 ± 0.11 a | 0.55 ± 0.001 a | 0.14 ± 0.004 a | 0.23 ± 0.01 a | 2.78 ± 0.05 a | 290.08 ± 0.25 a |
| Dataset average | 16 | 8.31 ± 0.22 | 0.55 ± 0.01 | 0.14 ± 0.01 | 0.24 ± 0.03 | 2.84 ± 0.05 | 290.02 ± 0.26 |
| TPC | HCA | FL | TF | ANT | CT | CAR | CHL A | CHL B | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 0.92 * | 0.84 * | 0.56 * | 0.71 * | 0.69 * | 0.75 * | 0.77 * | 0.77 * | 0.51 * | 0.32 | |
| HCA | 0.92 * | 0.91 * | 0.66 * | 0.63 * | 0.61 * | 0.78 * | 0.69 * | 0.69 * | 0.50 * | 0.41 | |
| FL | 0.84 * | 0.91 * | 0.63 * | 0.44 | 0.42 | 0.86 * | 0.54 * | 0.55 * | 0.55 * | 0.44 | |
| TF | 0.56 * | 0.66 * | 0.63 * | 0.63 * | 0.64 * | 0.43 | 0.64 * | 0.65 * | 0.13 | 0.28 | |
| ANT | 0.71 * | 0.63 * | 0.44 | 0.63 * | 0.90 * | 0.22 | 0.93 * | 0.92 * | 0.25 | 0.09 | |
| CT | 0.69 * | 0.61 * | 0.42 | 0.64 * | 0.90 * | 0.19 | 0.81 * | 0.80 * | 0.07 | 0.05 | |
| CAR | 0.75 * | 0.78 * | 0.86 * | 0.43 | 0.22 | 0.19 | 0.33 | 0.35 | 0.68 * | 0.25 | |
| CHL A | 0.77 * | 0.69 * | 0.54 * | 0.64 * | 0.93 * | 0.81 * | 0.33 | 1.00 * | 0.26 | 0.20 | |
| CHL B | 0.77 * | 0.69 * | 0.55 * | 0.65 * | 0.92 * | 0.80 * | 0.35 | 1.00 * | 0.27 | 0.20 | |
| DPPH | 0.51 * | 0.50 * | 0.55 * | 0.13 | 0.25 | 0.07 | 0.68 * | 0.26 | 0.27 | −0.03 | |
| FRAP | 0.32 | 0.41 | 0.44 | 0.28 | 0.09 | 0.05 | 0.25 | 0.20 | 0.20 | −0.03 |
| Sample | 3DP Program | Starch Content (%) | F (N) | W (Nmm) | Fp (N) | Wp (Nmm) |
|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 16.89 | 168.76 | 0.01 | 0.01 |
| 2 | 2 | 0 | 7.58 | 75.79 | 0.01 | 0.01 |
| 3 | 1 | 6 | 454.73 | 4545.01 | 0.07 | 0.06 |
| 4 | 2 | 6 | 54.08 | 540.43 | 0.05 | 0.05 |
| 5 | 1 | 8 | 112.82 | 1127.64 | 0.05 | 0.06 |
| 6 | 2 | 8 | 66.77 | 667.37 | 0.05 | 0.06 |
| 7 | 1 | 10 | 75.69 | 756.59 | 0.07 | 0.07 |
| 8 | 2 | 10 | 1003.08 | 10,025.82 | 0.06 | 0.04 |
| Parameter | 3DP Program | Starch Content (%) |
|---|---|---|
| F | 0.725588 | 0.695556 |
| W | 0.725577 | 0.695550 |
| Fp | 0.178047 | 0.158750 |
| Wp | 0.236516 | 0.876531 |
| D [3.2] | 0.949818 | 0.003742 * |
| D [4.3] | 0.660607 | 0.024213 * |
| d [0.1] | 0.730548 | 0.029678 * |
| d [0.5] | 0.616439 | 0.004638 * |
| d [0.9] | 0.603835 | 0.037802 * |
| Length | 0.147469 | 0.754530 |
| Width | 0.659869 | 0.795063 |
| Height | 0.122649 | 0.334880 |
| Sample | 3DP Program | Starch Content (%) | Length (mm) | Width (mm) | Height (mm) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 58.88 ± 0.11 | 58.69 ± 0.22 | 9.40± 0.09 |
| 2 | 2 | 0 | 62.01± 0.17 | 59.94 ± 0.19 | 9.39± 0.12 |
| 3 | 1 | 6 | 54.85 ± 0.21 | 53.33 ± 0.53 | 12.55 ± 0.14 |
| 4 | 2 | 6 | 55.70 ± 0.15 | 53.57 ± 0.31 | 12.12 ± 0.15 |
| 5 | 1 | 8 | 55.06 ± 0.07 | 51.57 ± 0.23 | 13.47 ± 0.52 |
| 6 | 2 | 8 | 55.60 ± 0.32 | 53.99 ± 0.44 | 12.83 ± 0.32 |
| 7 | 1 | 10 | 53.73 ± 0.56 | 54.18 ± 0.09 | 13.50 ± 0.17 |
| 8 | 2 | 10 | 55.98 ± 0.37 | 53.09 ± 0.21 | 11.98 ± 0.26 |
| Sample | 3DP Program | Starch Content (%) | D [3.2] (μm) | D [4.3] (μm) | d [0.1] (μm) | d [0.5] (μm) | d [0.9] (μm) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 120.24 | 429.11 | 62.18 | 297.58 | 1012.46 |
| 2 | 2 | 0 | 117.87 | 418.75 | 61.57 | 290.21 | 985.83 |
| 3 | 1 | 6 | 54.63 | 234.50 | 24.05 | 92.45 | 682.84 |
| 4 | 2 | 6 | 54.02 | 216.56 | 24.23 | 88.52 | 622.87 |
| 5 | 1 | 8 | 40.07 | 167.06 | 20.05 | 59.94 | 501.01 |
| 6 | 2 | 8 | 39.41 | 174.85 | 19.28 | 59.94 | 530.68 |
| 7 | 1 | 10 | 35.30 | 145.02 | 17.82 | 53.20 | 437.02 |
| 8 | 2 | 10 | 36.72 | 143.65 | 19.13 | 54.41 | 419.94 |
| Variable | n | L* | a* | b* | C* | H* | ΔE |
|---|---|---|---|---|---|---|---|
| Starch level | † | ‡ | † | † | † | † | |
| 0% | 4 | 41.43 ± 0.19 c | 22.92 ± 0.39 a | 14.44 ± 0.30 b | 27.09 ± 0.46 b | 32.23 ± 0.37 b | 0.00 ± 0.41 c |
| 6% | 4 | 46.74 ± 0.19 a | 24.48 ± 0.39 a | 16.98 ± 0.30 a | 29.80 ± 0.46 a | 34.74 ± 0.37 a | 6.17 ± 0.41 a |
| 8% | 4 | 44.79 ± 0.19 b | 23.45 ± 0.39 a | 15.70 ± 0.30 a,b | 28.22 ± 0.46 a,b | 33.80 ± 0.37 a,b | 3.89 ± 0.41 b |
| 10% | 4 | 44.19 ± 0.19 b | 22.77 ± 0.39 a | 14.82 ± 0.30 b | 27.17 ± 0.46 b | 33.06 ± 0.37 a,b | 3.00 ± 0.41 b |
| 3DP Program | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ | |
| Program 1 | 8 | 44.18 ± 0.13 a | 23.48 ± 0.27 a | 15.34 ± 0.21 a | 28.05 ± 0.32 a | 33.13 ± 0.26 a | 3.59 ± 0.29 a |
| Program 2 | 8 | 44.39 ± 0.13 a | 23.33 ± 0.27 a | 15.64 ± 0.21 a | 28.09 ± 0.32 a | 33.79 ± 0.26 a | 2.94 ± 0.29 a |
| Dataset average | 16 | 44.29 ± 0.50 | 23.40 ± 0.25 | 15.49 ± 0.29 | 28.07 ± 0.35 | 33.46 ± 0.31 | 3.26 ± 0.60 |
| Variable | n | Intensity of Orange Color | Strawberry Odor | Off-Odor | Strawberry Flavor | Strawberry Tree Fruit Flavor | Off-Flavor | Sweet Taste | Sour Taste | Harmony Taste | Off-Taste | Homogeneity | Glossy Appearance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ | † | ‡ | † | ‡ | ‡ | ‡ | |
| A | 13 | 5.85 ± 0.32 a | 5.38 ± 0.37 a | 1.23 ± 0.19 a | 4.46 ± 0.38 a | 4.15 ± 0.45 a | 1.15 ± 0.18 a | 2.46 ± 0.36 d | 4.69 ± 0.38 a | 3.69 ± 0.34 a,b | 1.15 ± 0.18 a | 5.15 ± 0.41 a | 5.77 ± 0.35 a |
| B1 | 13 | 5.92 ± 0.32 a | 5.08 ± 0.37 a | 1.08 ± 0.19 a | 4.92 ± 0.38 a | 3.54 ± 0.45 a | 1.08 ± 0.18 a | 4.46 ± 0.36 a,b | 3.23 ± 0.38 a | 4.85 ± 0.34 a,b | 1.08 ± 0.18 a | 4.77 ± 0.41 a | 5.77 ± 0.35 a |
| B2 | 13 | 6.08 ± 0.32 a | 5.00 ± 0.37 a | 1.23 ± 0.19 a | 4.85 ± 0.38 a | 3.46 ± 0.45 a | 1.08 ± 0.18 a | 5.00 ± 0.36 a,b | 2.92 ± 0.38 a | 4.85 ± 0.34 a,b | 1.08 ± 0.18 a | 5.15 ± 0.41 a | 6.08 ± 0.35 a |
| C1 | 13 | 6.15 ± 0.32 a | 4.85 ± 0.37 a | 1.31 ± 0.19 a | 4.31 ± 0.38 a | 3.46 ± 0.45 a | 1.23 ± 0.18 a | 3.92 ± 0.36 a,b,c,d | 3.31 ± 0.38 a | 4.54 ± 0.34 a,b | 1.23 ± 0.18 a | 5.38 ± 0.41 a | 5.92 ± 0.35 a |
| C2 | 13 | 5.92 ± 0.32 a | 4.77 ± 0.37 a | 1.23 ± 0.19 a | 4.31 ± 0.38 a | 3.23 ± 0.45 a | 1.23 ± 0.18 a | 4.77 ± 0.36 a,b | 2.92 ± 0.38 a | 4.85 ± 0.34 a,b | 1.08 ± 0.18 a | 5.31 ± 0.41 a | 6.15 ± 0.35 a |
| D1 | 13 | 6.08 ± 0.32 a | 4.69 ± 0.37 a | 1.38 ± 0.19 a | 3.92 ± 0.38 a | 3.46 ± 0.45 a | 1.38 ± 0.18 a | 3.38 ± 0.36 b,c,d | 3.92 ± 0.38 a | 3.54 ± 0.34 b | 1.38 ± 0.18 a | 5.54 ± 0.41 a | 5.38 ± 0.35 a |
| D2 | 13 | 6.23 ± 0.32 a | 4.92 ± 0.37 a | 1.08 ± 0.19 a | 4.15 ± 0.38 a | 3.46 ± 0.45 a | 1.08 ± 0.18 a | 4.00 ± 0.36 a,b,c,d | 3.54 ± 0.38 a | 4.31 ± 0.34 a,b | 1.23 ± 0.18 a | 5.54 ± 0.41 a | 5.46 ± 0.35 a |
| E1 | 13 | 6.08 ± 0.32 a | 4.23 ± 0.37 a | 1.31 ± 0.19 a | 3.54 ± 0.38 a | 3.69 ± 0.45 a | 1.23 ± 0.18 a | 2.69 ± 0.36 c,d | 4.15 ± 0.38 a | 3.23 ± 0.34 b | 1.46 ± 0.18 a | 5.69 ± 0.41 a | 4.69 ± 0.35 a |
| E2 | 13 | 6.15 ± 0.32 a | 4.15 ± 0.37 a | 1.23 ± 0.19 a | 3.46 ± 0.38 a | 3.54 ± 0.45 a | 1.31 ± 0.18 a | 3.54 ± 0.36 a,b,c,d | 4.08 ± 0.38 a | 3.85 ± 0.34 a,b | 1.23 ± 0.18 a | 5.46 ± 0.41 a | 4.62 ± 0.35 a |
| F1 | 13 | 5.54 ± 0.32 a | 5.15 ± 0.37 a | 1.31 ± 0.19 a | 4.62 ± 0.38 a | 3.38 ± 0.45 a | 1.15 ± 0.18 a | 4.15 ± 0.36 a,b,c,d | 3.62 ± 0.38 a | 4.38 ± 0.34 a,b | 1.08 ± 0.18 a | 5.00 ± 0.41 a | 5.77 ± 0.35 a |
| F2 | 13 | 5.38 ± 0.32 a | 4.92 ± 0.37 a | 1.15 ± 0.19 a | 5.15 ± 0.38 a | 3.38 ± 0.45 a | 1.15 ± 0.18 a | 4.62 ± 0.36 a,b | 3.31 ± 0.38 a | 4.69 ± 0.34 a,b | 1.23 ± 0.18 a | 5.38 ± 0.41 a | 5.69 ± 0.35 a |
| G1 | 13 | 5.62 ± 0.32 a | 4.69 ± 0.37 a | 1.31 ± 0.19 a | 4.00 ± 0.38 a | 3.62 ± 0.45 a | 1.54 ± 0.18 a | 3.62 ± 0.36 a,b,c,d | 3.92 ± 0.38 a | 3.92 ± 0.34 a,b | 1.62 ± 0.18 a | 5.54 ± 0.41 a | 5.31 ± 0.35 a |
| G2 | 13 | 5.62 ± 0.32 a | 5.00 ± 0.37 a | 1.38 ± 0.19 a | 4.46 ± 0.38 a | 3.38 ± 0.45 a | 1.38 ± 0.18 a | 4.00 ± 0.36 a,b,c,d | 3.54 ± 0.38 a | 4.31 ± 0.34 a,b | 1.38 ± 0.18 a | 5.69 ± 0.41 a | 5.54 ± 0.35 a |
| H1 | 13 | 5.54 ± 0.32 a | 4.85 ± 0.37 a | 1.31 ± 0.19 a | 4.62 ± 0.38 a | 3.62 ± 0.45 a | 1.15 ± 0.18 a | 4.38 ± 0.36 a,b,c | 3.62 ± 0.38 a | 4.38 ± 0.34 a,b | 1.38 ± 0.18 a | 6.23 ± 0.41 a | 5.46 ± 0.35 a |
| H2 | 13 | 5.54 ± 0.32 a | 5.08 ± 0.37 a | 1.23 ± 0.19 a | 5.08 ± 0.38 a | 3.69 ± 0.45 a | 1.15 ± 0.18 a | 5.15 ± 0.36 a | 3.38 ± 0.38 a | 5.23 ± 0.34 a | 1.23 ± 0.18 a | 5.85 ± 0.41 a | 5.54 ± 0.35 a |
| I1 | 13 | 5.69 ± 0.32 a | 5.15 ± 0.37 a | 1.31 ± 0.19 a | 4.38 ± 0.38 a | 3.54 ± 0.45 a | 1.62 ± 0.18 a | 4.00 ± 0.36 a,b,c,d | 3.85 ± 0.38 a | 4.15 ± 0.34 a,b | 1.46 ± 0.18 a | 5.62 ± 0.41 a | 5.08 ± 0.35 a |
| I2 | 13 | 5.77 ± 0.32 a | 5.15 ± 0.37 a | 1.15 ± 0.19 a | 4.69 ± 0.38 a | 3.54 ± 0.45 a | 1.62 ± 0.18 a | 4.53 ± 0.36 a,b | 3.85 ± 0.38 a | 4.38 ± 0.34 a,b | 1.38 ± 0.18 a | 6.00 ± 0.41 a | 5.08 ± 0.35 a |
| Sample grouped | ‡ | ‡ | ‡ | † | ‡ | ‡ | † | † | † | ‡ | ‡ | † | |
| A | 13 | 5.85 ± 0.32 a | 5.38 ± 0.37 a | 1.23 ± 0.19 a | 4.46 ± 0.38 a,b | 4.15 ± 0.45 a | 1.15 ± 0.18 a | 2.46 ± 0.36 b | 4.69 ± 0.38 a | 3.69 ± 0.34 a,b | 1.15 ± 0.18 a | 5.15 ± 0.41 a | 5.77 ± 0.35 a,b |
| B | 26 | 6.00 ± 0.23 a | 5.04 ± 0.26 a | 1.15 ± 0.13 a | 4.88 ± 0.27 a | 3.50 ± 0.32 a | 1.08 ± 0.12 a | 4.73 ± 0.25 a | 3.08 ± 0.27 b | 4.85 ± 0.24 a | 1.08 ± 0.13 a | 4.96 ± 0.29 a | 5.92 ± 0.25 a |
| C | 26 | 6.04 ± 0.23 a | 4.81 ± 0.26 a | 1.27 ± 0.13 a | 4.31 ± 0.27 a,b | 3.35 ± 0.32 a | 1.23 ± 0.12 a | 4.35 ± 0.25 a | 3.12 ± 0.27 b | 4.69 ± 0.24 a | 1.15 ± 0.13 a | 5.35 ± 0.29 a | 6.03 ± 0.25 a |
| D | 26 | 6.15 ± 0.23 a | 4.81 ± 0.26 a | 1.23 ± 0.13 a | 4.04 ± 0.27 a,b | 3.46 ± 0.32 a | 1.23 ± 0.12 a | 3.69 ± 0.25 a,b | 3.73 ± 0.27 a,b | 3.92 ± 0.24 a,b | 1.31 ± 0.13 a | 5.54 ± 0.29 a | 5.42 ± 0.25 a,b |
| E | 26 | 6.12 ± 0.23 a | 4.19 ± 0.26 a | 1.27 ± 0.13 a | 3.50 ± 0.27 b | 3.62 ± 0.32 a | 1.27 ± 0.12 a | 3.12 ± 0.25 b | 4.12 ± 0.27 a,b | 3.54 ± 0.24 b | 1.35 ± 0.13 a | 5.58 ± 0.29 a | 4.65 ± 0.25 b |
| F | 26 | 5.46 ± 0.23 a | 5.04 ± 0.26 a | 1.23 ± 0.13 a | 4.88 ± 0.27 a | 3.38 ± 0.32 a | 1.15 ± 0.12 a | 4.38 ± 0.25 a | 3.46 ± 0.27 a,b | 4.54 ± 0.24 a,b | 1.15 ± 0.13 a | 5.19 ± 0.29 a | 5.73 ± 0.25 a,b |
| G | 26 | 5.62 ± 0.23 a | 4.85 ± 0.26 a | 1.35 ± 0.13 a | 4.23 ± 0.27 a,b | 3.50 ± 0.32 a | 1.46 ± 0.12 a | 3.81 ± 0.25 a,b | 3.73 ± 0.27 a,b | 4.12 ± 0.24 a,b | 1.50 ± 0.13 a | 5.62 ± 0.29 a | 5.42 ± 0.25 a,b |
| H | 26 | 5.54 ± 0.23 a | 4.96 ± 0.26 a | 1.27 ± 0.13 a | 4.85 ± 0.27 a | 3.65 ± 0.32 a | 1.15 ± 0.12 a | 4.77 ± 0.25 a | 3.50 ± 0.27 a,b | 4.81 ± 0.24 a | 1.31 ± 0.13 a | 6.04 ± 0.29 a | 5.50 ± 0.25 a,b |
| I | 26 | 5.73 ± 0.23 a | 5.15 ± 0.26 a | 1.23 ± 0.13 a | 4.54 ± 0.27 a,b | 3.54 ± 0.32 a | 1.62 ± 0.12 a | 4.27 ± 0.25 a | 3.85 ± 0.27 a,b | 4.27 ± 0.24 a,b | 1.42 ± 0.13 a | 5.81 ± 0.29 a | 5.08 ± 0.25 a,b |
| Dataset average | 221 | 5.83 ± 0.08 | 4.89 ± 0.09 | 1.25 ± 0.04 | 4.41 ± 0.10 | 3.54 ± 0.11 | 1.27 ± 0.04 | 4.04 ± 0.10 | 3.64 ± 0.09 | 4.30 ± 0.09 | 1.28 ± 0.04 | 5.49 ± 0.10 | 5.49 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Bosiljkov, T.; Janči, T.; Kostić, M.; Dedović, N.; Lučić, E.; Bavrka, K.; Pavlić, B.; Bursać Kovačević, D. Characterization of Antioxidant Bioactive Compounds and Rheological, Color and Sensory Properties in 3D-Printed Fruit Snacks. Foods 2024, 13, 1623. https://doi.org/10.3390/foods13111623
Bebek Markovinović A, Bosiljkov T, Janči T, Kostić M, Dedović N, Lučić E, Bavrka K, Pavlić B, Bursać Kovačević D. Characterization of Antioxidant Bioactive Compounds and Rheological, Color and Sensory Properties in 3D-Printed Fruit Snacks. Foods. 2024; 13(11):1623. https://doi.org/10.3390/foods13111623
Chicago/Turabian StyleBebek Markovinović, Anica, Tomislav Bosiljkov, Tibor Janči, Marko Kostić, Nebojša Dedović, Ela Lučić, Katarina Bavrka, Branimir Pavlić, and Danijela Bursać Kovačević. 2024. "Characterization of Antioxidant Bioactive Compounds and Rheological, Color and Sensory Properties in 3D-Printed Fruit Snacks" Foods 13, no. 11: 1623. https://doi.org/10.3390/foods13111623
APA StyleBebek Markovinović, A., Bosiljkov, T., Janči, T., Kostić, M., Dedović, N., Lučić, E., Bavrka, K., Pavlić, B., & Bursać Kovačević, D. (2024). Characterization of Antioxidant Bioactive Compounds and Rheological, Color and Sensory Properties in 3D-Printed Fruit Snacks. Foods, 13(11), 1623. https://doi.org/10.3390/foods13111623










