Liquid-Phase Adsorption Behavior of β-D-Glucooligosaccharides When Using Activated Carbon for Separation, and the Antioxidant Stress Activity of Purified Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Batch-Mode Adsorption Studies
2.3. Theoretical Study of the Adsorption of β-Glucooligosaccharides by Activated Carbon
2.3.1. Adsorption Kinetics Analysis
2.3.2. Adsorption Equilibrium Isotherm
2.3.3. Thermodynamic Study
2.4. Desorption Separation of GLPWA
2.5. Sugar Content, Polymerization Analysis, and Structural Identification of Glucooligosaccharides
2.6. Assays of Antioxidative Stress Activity In Vitro
2.6.1. Establishment of Cell Model In Vitro and Protective Experiments’ Design
2.6.2. Cell Viability Assay
2.6.3. Detection of Intracellular Reactive Oxygen Species
2.6.4. Determination of SOD and GSH In Vitro
2.7. Statistics
3. Results
3.1. Effect of Adsorption Conditions on Adsorption
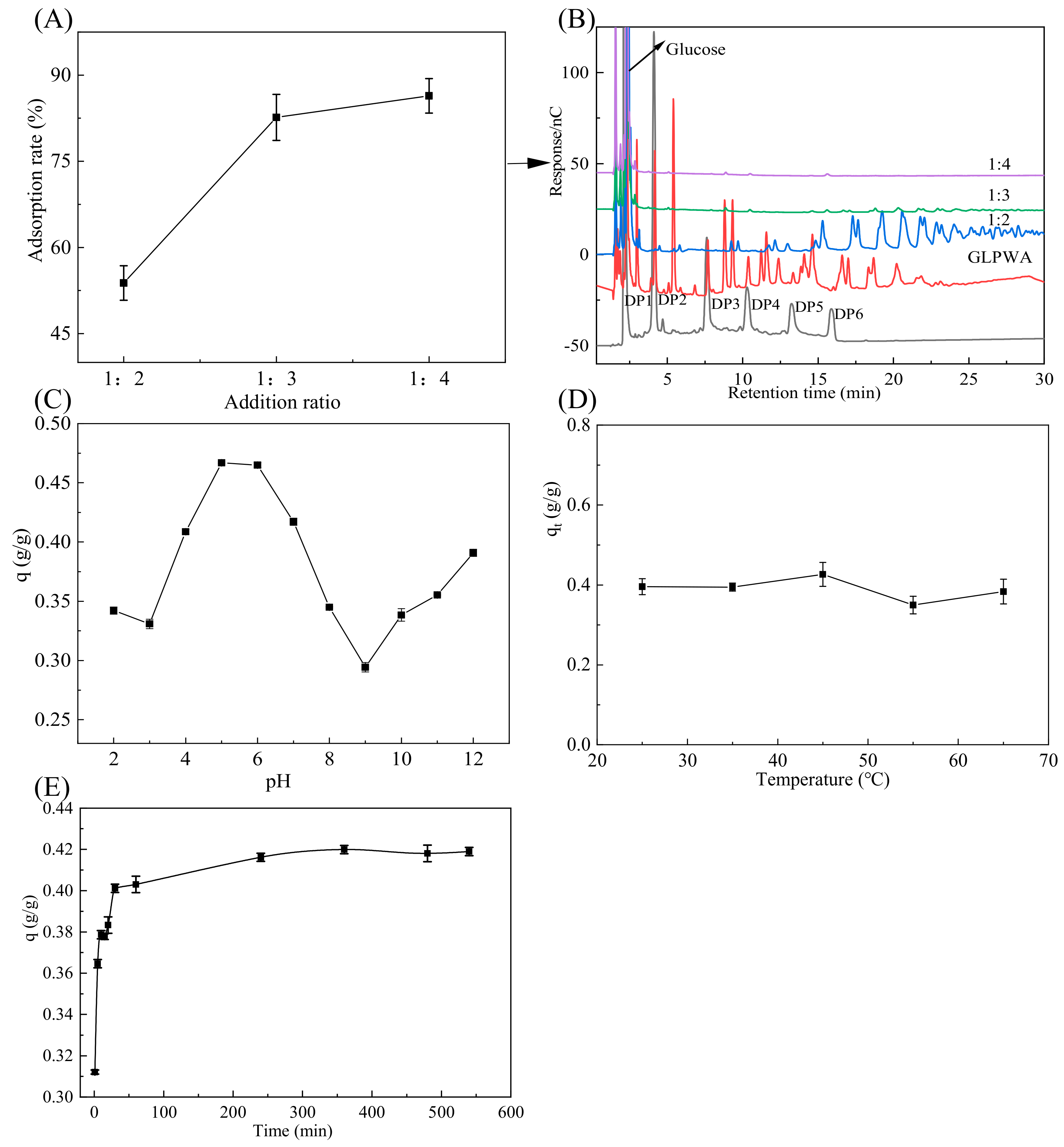
3.1.1. Effect of Activated Carbon Addition Ratio on Activated Carbon Adsorption of GLPWA
3.1.2. Effect of pH on Activated Carbon Adsorption of GLPWA
3.1.3. Effect of Temperature on Activated Carbon Adsorption of GLPWA
3.1.4. Effect of Time on Activated Carbon Adsorption of GLPWA
3.2. Theoretical Study on the Adsorption of β-Glucooligosaccharides by Activated Carbon
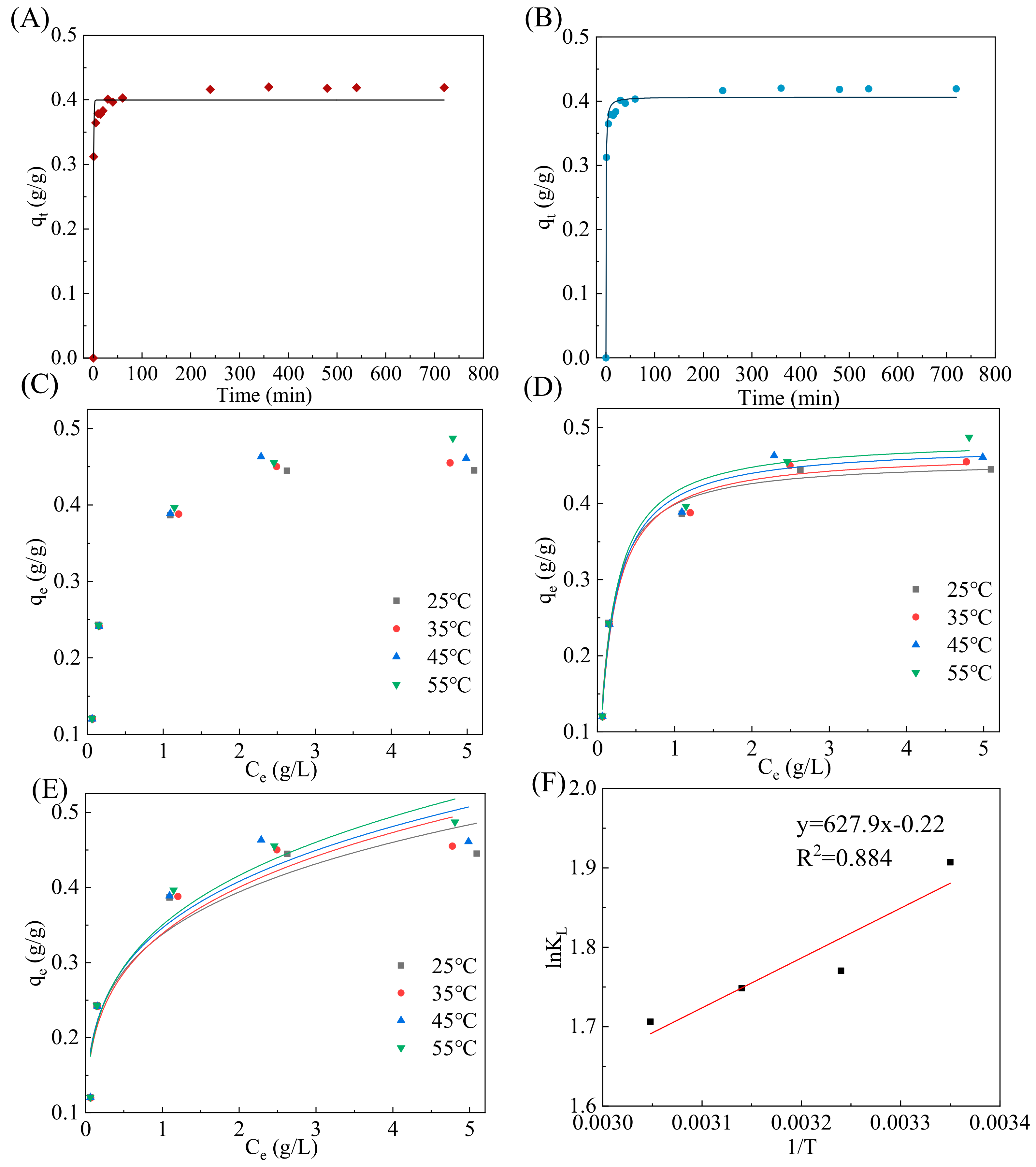
3.2.1. Adsorption Kinetics Analysis
3.2.2. Adsorption Isotherms’ Analysis
3.2.3. Thermodynamic Studies
3.3. Desorption Separation and Characterization Analysis of GLPWA
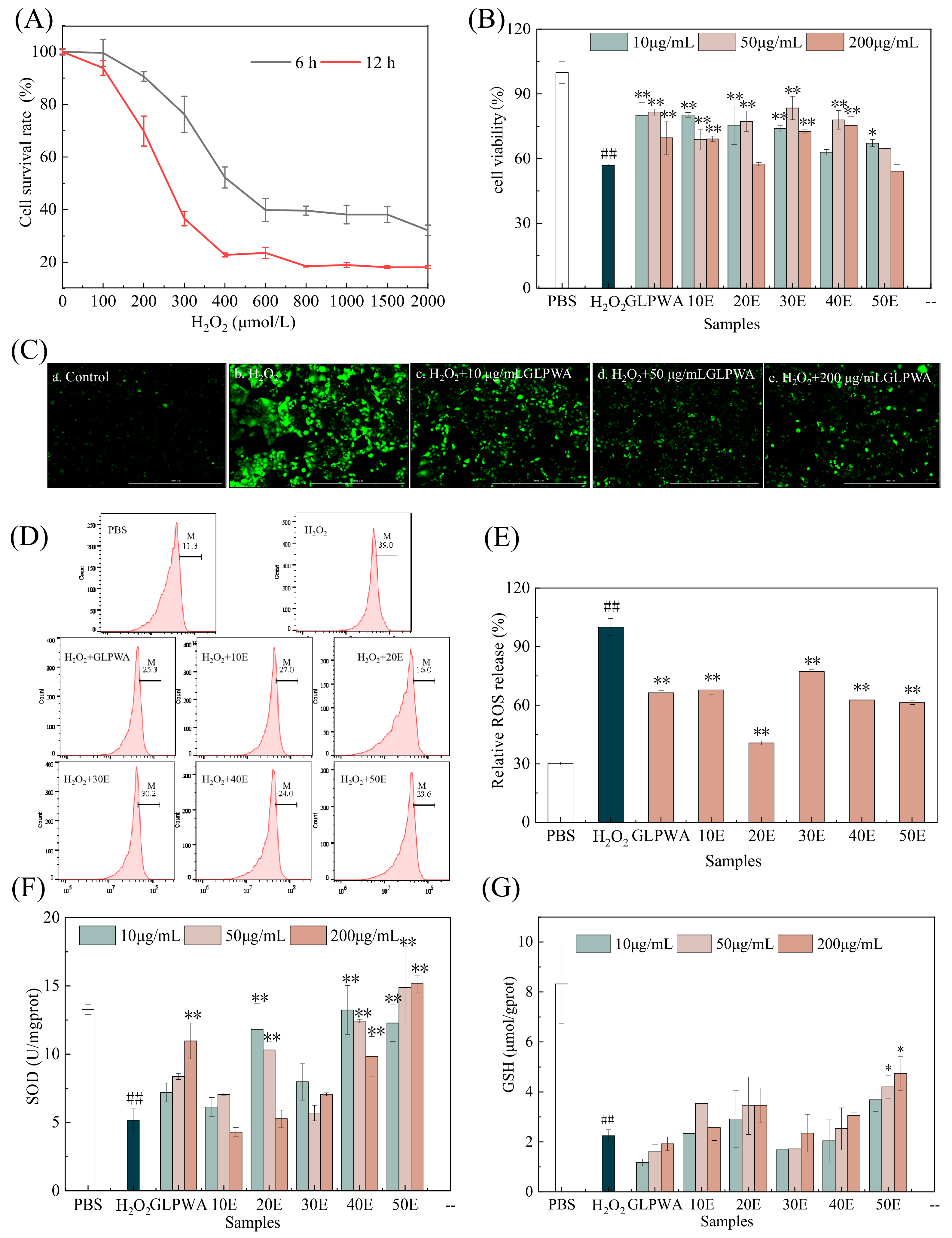
3.4. Construction of Oxidative Stress Model and Activity Evaluation
3.4.1. Oxidative Stress Model and Cell Damage Repair Ability
3.4.2. Measurement of Cellular Levels of ROS, SOD, and GSH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, Z.; Tang, Q.J.; Zhang, J.S.; Yang, Y.; Jia, W.; Pan, Y.J. Immunomodulation of RAW264.7 macrophages by GLIS, a proteopolysaccharide from Ganoderma lucidum. J. Ethnopharmacol. 2007, 112, 445–450. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.Q.; Wang, R.; Wang, S.Z.; Chen, C.M. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Zhou, L.; Zou, H.; Luo, X.; Sun, B.; Zhuang, X. Polysaccharides from Ganoderma Sinense-rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complement. Med. Ther. 2021, 21, 169. [Google Scholar] [CrossRef]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The ameliorations of Ganoderma applanatum residue polysaccharides against CCl4 induced liver injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.P.; Cai, W.; Tan, J.B.; Yang, X.B.; Zhang, J.M. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch. Pharmacal Res. 2012, 35, 1793–1801. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, J.S.; Tang, Q.J.; Yang, Y.; Guo, Q.; Wang, Q.; Wu, D.; Cui, S.W. Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr. Polym. 2014, 101, 968–974. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, J.S.; Tang, Q.J.; Yang, Y.; Xia, Y.M.; Zhou, S.; Wu, D.; Zhang, Z.; Dong, L.L.; Cui, S.W. Rheological properties of β-D-glucan from the fruiting bodies of Ganoderma lucidum. Food Hydrocoll. 2016, 58, 120–125. [Google Scholar] [CrossRef]
- Fu, Y.B.; Wang, M.Y.; Wang, W.X.; Tuo, Y.Q.; Guo, Z.M.; Du, Y.G.; Yin, H. (1→3)-β-d-Glucan oligosaccharides monomers purification and its H2O2 induction effect study. Int. J. Biol. Macromol. 2015, 81, 1069–1073. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Lu, G.X.; Zhan, X.B.; Lin, C.C.; Zheng, Z.Y. Curdlan β-1,3-glucooligosaccharides induce the defense responses against Phytophthora infestans infection of potato (Solanum tuberosum L. cv. McCain G1) leaf cells. PLoS ONE 2014, 9, e97197. [Google Scholar] [CrossRef]
- Liu, L.P.; Feng, J.; Jiang, S.Q.; Zhou, S.; Yan, M.Q.; Zhang, Z.; Wang, W.H.; Liu, Y.F.; Zhang, J.S. Anti-inflammatory and intestinal microbiota modulation properties of Ganoderma lucidum β-d-glucans with different molecular weight in an ulcerative colitis model. Int. J. Biol. Macromol. 2023, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ma, G.H.; Liu, L.P.; Feng, J.; Zhou, S.; Han, W.; Zhou, J.; Liu, Y.F.; Zhang, J.S. Microwave-assisted degradation of β-D-glucan from Ganoderma lucidum and the structural and immunoregulatory properties of oligosaccharide fractions. Int. J. Biol. Macromol. 2022, 220, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Fu, X.T.; Huang, L.Q.; Xu, J.C.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef] [PubMed]
- Kazlowski, B.; Pan, C.L.; Ko, Y.T. Monitoring and preparation of neoagaroand agaro-oligosaccharide products by high performance anion exchange chromatography systems. Carbohydr. Polym. 2015, 122, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.N.; Wang, X.; Wang, C.; Yan, Y.; Zhang, M.S.; Mayo, K.H.; Sun, L.; Zhou, Y.F. An efficient protocol for preparing linear β-manno-oligosaccharides. Carbohydr. Res. 2023, 532, 108895. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Han, F.; Lu, X.Z.; Fu, X.Y.; Ma, C.P.; Chu, Y.; Yu, W.G. A simple method of preparing diverse neoagaro-oligosaccharides with beta-agarase. Carbohydr. Res. 2007, 342, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Lee, B.H.; Chang, P.S.; Lee, H.G.; Yoo, S.H. Improved quantitative analysis of oligosaccharides from lichenase-hydrolyzed water-soluble barley beta-glucans by high-performance anion-exchange chromatography. J. Agric. Food. Chem. 2007, 55, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Erdinc, N.; Göktürk, S.; Tunçay, M. A study on the adsorption characteristics of an amphiphilic phenothiazine drug on activated charcoal in the presence of surfactants. Colloids Surf. B 2010, 75, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, R. Adsorption behavior of ruthenium ions on activated charcoal from nirtic acid medium. Colloids Surf. A 2007, 293, 217–223. [Google Scholar] [CrossRef]
- Aksu, Z.; Yener, J. A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Manag. 2001, 21, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.C.; Mazutti, M.A.; Albertini, L.B.; Filho, F.M. Evaluation of fructooligosaccharides separation using a fixed-bed column packed with activated charcoal. New Biotechnol. 2014, 31, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hernández, O.; Ruiz-Matute, A.; Olano, A.; Moreno, F.J.; Sanz, M.L. Comparison of fractionation techniques to obtain prebiotic galactooligosaccharides. Int. Dairy J. 2009, 19, 531–536. [Google Scholar] [CrossRef]
- Pöhnl, T.; Böttcher, C.; Schulz, H.; Stürtz, M.; Widder, S.; Carle, R.; Schweiggert, R.M. Comparison of high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and ultra-high performance liquid chromatography with evaporative light scattering (UHPLC-ELSD) for the analyses of fructooligosaccharides in onion (Allium cepa L.). J. Food Compos. Anal. 2017, 63, 148–156. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dad, F.P.; Khan, W.D.; Sharif, F.; Nizami, A.S. Adsorption of trace heavy metals through organic compounds enriched biochar using isotherm adsorption and kinetic models. Environ. Res. 2024, 241, 117702. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tellinghuisen, J. Van’t Hoff analysis of K degrees (T): How good or bad? Biophys. Chem. 2006, 120, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.X.; Ma, Q.; Zhang, Z.; Li, W.; Chen, W.C.; Liu, P.; Wu, D.; Yang, Y. Semi-solid enzymolysis enhanced the protective effects of fruiting body powders and polysaccharides of Herinaceus erinaceus on gastric mucosal injury. Int. J. Biol. Macromol. 2023, 251, 126388. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.T.; Lo, H.H.; Wang, L.K.; Taricska, J.R.; Li, K.H. Granular Activated Carbon Adsorption. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2005; Volume 3, pp. 573–633. [Google Scholar] [CrossRef]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. Fructo-oligosaccharides purification from a fermentative broth using an activated charcoal column. New Biotechnol. 2012, 29, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Wang, H.; Wang, F.Y.; Huang, Z.H. Adsorption of 2,4-dichlorophenol from aqueous solution by a new low-cost adsorbent-Activated bamboo charcoal. Sep. Sci. Technol. 2010, 45, 2329–2336. [Google Scholar] [CrossRef]
- Qadeer, R. Concentration effects associated with the kinetics of ruthenium ions adsorption on activated charcoal. J. Radioanal. Nucl. Chem. 2013, 295, 1649–1653. [Google Scholar] [CrossRef]
- Li, K.C.; Liu, S.; Xing, R.G.; Yu, H.; Qin, Y.K.; Li, P.C. Liquid phase adsorption behavior of inulin-type fructan onto activated charcoal. Carbohydr. Polym. 2015, 122, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, K. Adsorption characteristics of chitooligosaccharides onto activated charcoal in aqueous solutions. J. Oceanol. Limnol. 2019, 38, 342–350. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Correlative biosorption equilibria model for a binary batch system. Chem. Eng. Sci. 2000, 55, 817–825. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1917, 38, 2221–2295. [Google Scholar] [CrossRef]
- Fytianos, K.; Voudrias, E.; Kokkalis, E. Sorption-desorption behaviour of 2,4-dichlorophenol by marine sediments. Chemosphere 2000, 40, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Kittur, F.S.; Kumar, A.B.V.; Varadaraj, M.C.; Tharanathan, R.N. Chitooligosaccharides-preparation with the aid of pectinase isozyme from Aspergillus niger and their antibacterial activity. Carbohydr. Res. 2005, 340, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Hattori, T.; Uno, S.; Murata, T.; Usui, T. Enzymatic synthesis of gentiooligosaccharides by transglycosylation with β-glycosidases from Penicillium multicolor. Carbohydr. Res. 2009, 344, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Morales, V.; Sanz, M.L.; Olano, A.; Corzo, N. Rapid separation on activated charcoal of high oligosaccharides in honey. Chromatographia 2006, 64, 1–6. [Google Scholar] [CrossRef]
- Kaplan, H.; Hutkins, R.W. Fermentation of Fructooligosaccharides by Lactic Acid Bacteria and Bifidobacteria. Appl. Environ. Microbiol. 2000, 66, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols-induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. H2O2 inhibits the growth of human pulmonary fibroblast cells by inducing cell death, GSH depletion and G1 phase arrest. Mol. Med. Rep. 2013, 7, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Z.; Jiang, R.; Zhang, Q.Q.; Wang, R.F.; Yang, C.H.; Ma, J.; Du, H. Increased 8-hydroxy-2′-deoxyguanosine in leukocyte DNA from patients with type 2 diabetes and microangiopathy. J. Int. Med. Res. 2016, 44, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol.-Lung 2006, 290, L790–L796. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.C.; Jee, D.; Rho, C.R.; Kang, S. Melissa officinalis L. extracts protect human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Med. Sci. 2016, 13, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Rev. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Abe, M.; Kohda, T.; Fujimoto, Y. Hydrogen peroxide generated by xanthine/xanthine oxidase system represses the proliferation of colorectal cancer cell line Caco-2. J. Clin. Biochem. Nutr. 2015, 56, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Lin, Y.L.; Su, H.; Ye, D.Q. Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochem. Res. 2015, 40, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Ruan, H.H.; Li, H.T. Progress in the research of GSH in cells. Chin. Sci. Bull. 2011, 56, 7. [Google Scholar] [CrossRef]


| Sample | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| β-Glucooligosaccharides | k1 (min) | q1 (g/g) | R2 | k2 (g g−1 min−1) | q2 (g/g) | R2 |
| 1.512 | 0.3997 | 0.9729 | 7.047 | 0.4063 | 0.9871 | |
| Temperature (°C) | Langmuir Model | Freundlich Model | ∆H0 (kJ/mol) | ∆S0 (J mol−1 K−1) | ∆G0 (kJ/mol) | ||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (g/g) | KL (L/g) | R2 | Kf (L/g) | n | R2 | ||||
| 25 | 0.4581 | 6.7322 | 0.9889 | 0.3374 | 0.2236 | 0.8623 | −5.220 | −3.553 | −4.161 |
| 35 | 0.4679 | 5.8734 | 0.9847 | 0.3386 | 0.2415 | 0.9140 | −4.126 | ||
| 45 | 0.4783 | 5.7461 | 0.9866 | 0.3460 | 0.2380 | 0.8987 | −4.090 | ||
| 55 | 0.4865 | 5.5089 | 0.9856 | 0.3499 | 0.2494 | 0.9371 | −4.054 | ||
| Component | Water | 10E | 20E | 30E | 40E | 50E |
|---|---|---|---|---|---|---|
| Yield (%) | 19.47 | 13.80 | 17.51 | 15.46 | 8.49 | 9.80 |
| Sugar content (%) | 76.98 | 79.25 | 57.44 | 99.75 | 75.80 | 82.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.-H.; Jiang, S.-Q.; Liu, L.-P.; Feng, J.; Zhang, J.-S.; Li, E.-X.; Li, S.-H.; Liu, Y.-F. Liquid-Phase Adsorption Behavior of β-D-Glucooligosaccharides When Using Activated Carbon for Separation, and the Antioxidant Stress Activity of Purified Fractions. Foods 2024, 13, 1634. https://doi.org/10.3390/foods13111634
Ma G-H, Jiang S-Q, Liu L-P, Feng J, Zhang J-S, Li E-X, Li S-H, Liu Y-F. Liquid-Phase Adsorption Behavior of β-D-Glucooligosaccharides When Using Activated Carbon for Separation, and the Antioxidant Stress Activity of Purified Fractions. Foods. 2024; 13(11):1634. https://doi.org/10.3390/foods13111634
Chicago/Turabian StyleMa, Guan-Hua, Si-Qi Jiang, Li-Ping Liu, Jie Feng, Jing-Song Zhang, E-Xian Li, Shu-Hong Li, and Yan-Fang Liu. 2024. "Liquid-Phase Adsorption Behavior of β-D-Glucooligosaccharides When Using Activated Carbon for Separation, and the Antioxidant Stress Activity of Purified Fractions" Foods 13, no. 11: 1634. https://doi.org/10.3390/foods13111634




