Enhancement of Colorimetric pH-Sensitive Film Incorporating Amomum tsao-ko Essential Oil as Antibacterial for Mantis Shrimp Spoilage Tracking and Fresh-Keeping
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AEO Extraction and GC-MS Analysis
2.3. Extraction and Characterization of PPA
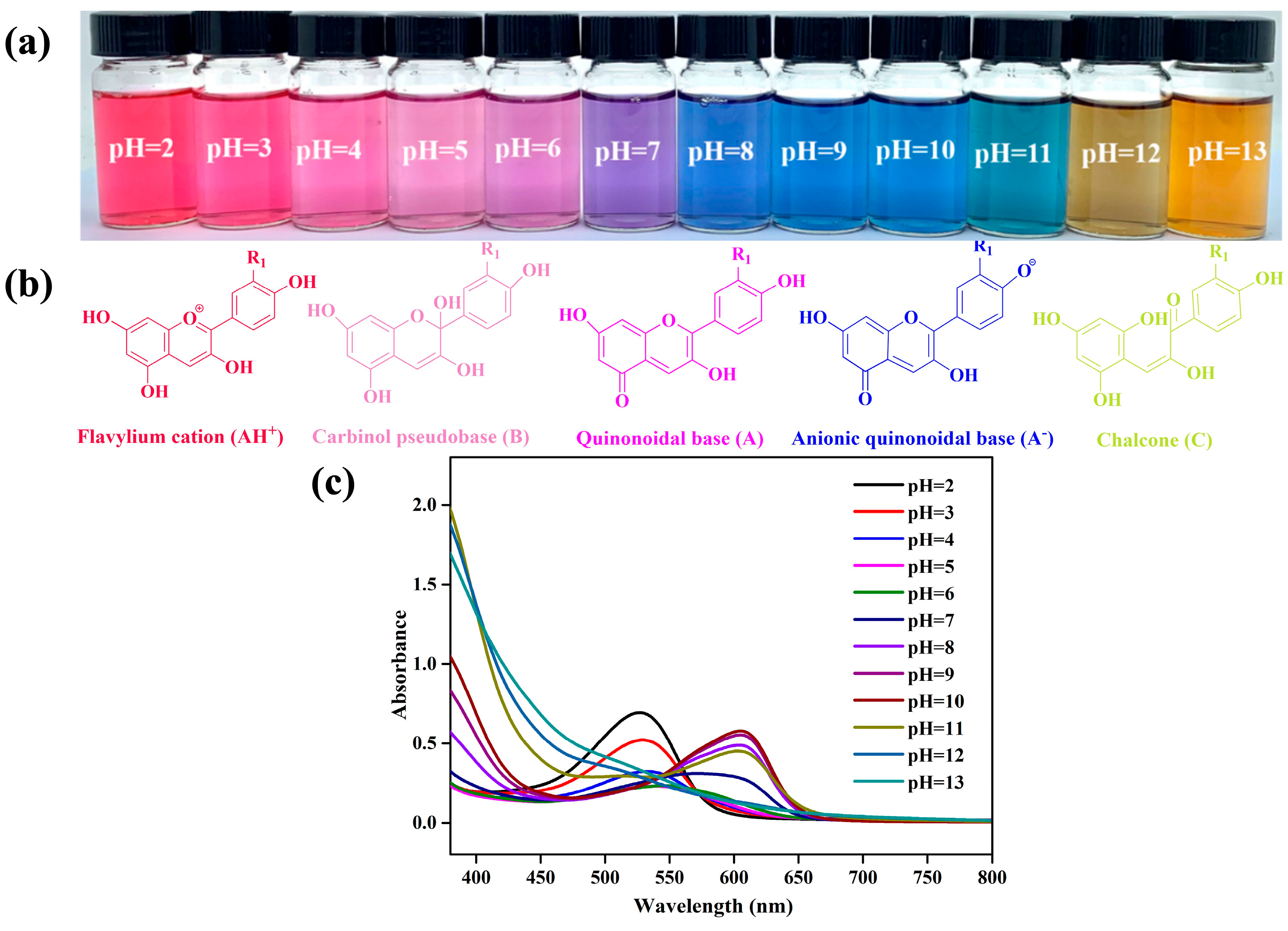
2.4. pH-Sensitivity of PPA
2.5. Fabrication of Smart Films
2.6. Characterization of Smart Films
2.6.1. Structure Characterization
2.6.2. Light Transmittance and Color Evaluation
2.6.3. Thickness and Barrier Properties
2.6.4. Mechanical Properties
2.6.5. Antibacterial Properties
2.6.6. Antioxidant Capacity
2.6.7. pH/NH3 Responsiveness of Smart Films
2.7. Application of Smart Film for Mantis Shrimp Preservation and Freshness Monitoring
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the AEO
3.2. Characterization of PPA
3.3. Characterization of Smart Films
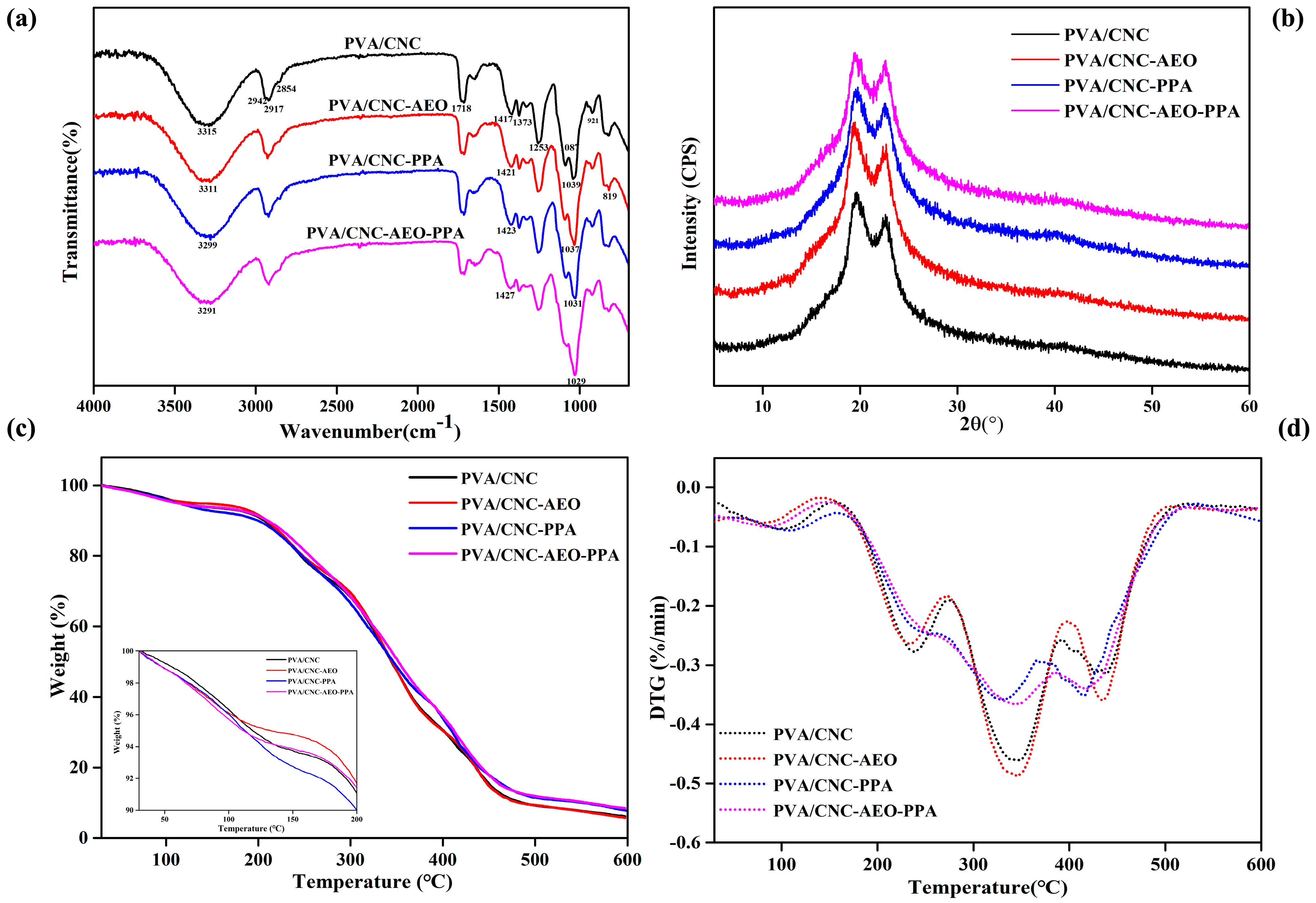
3.3.1. FT-IR
3.3.2. XRD
3.3.3. TGA
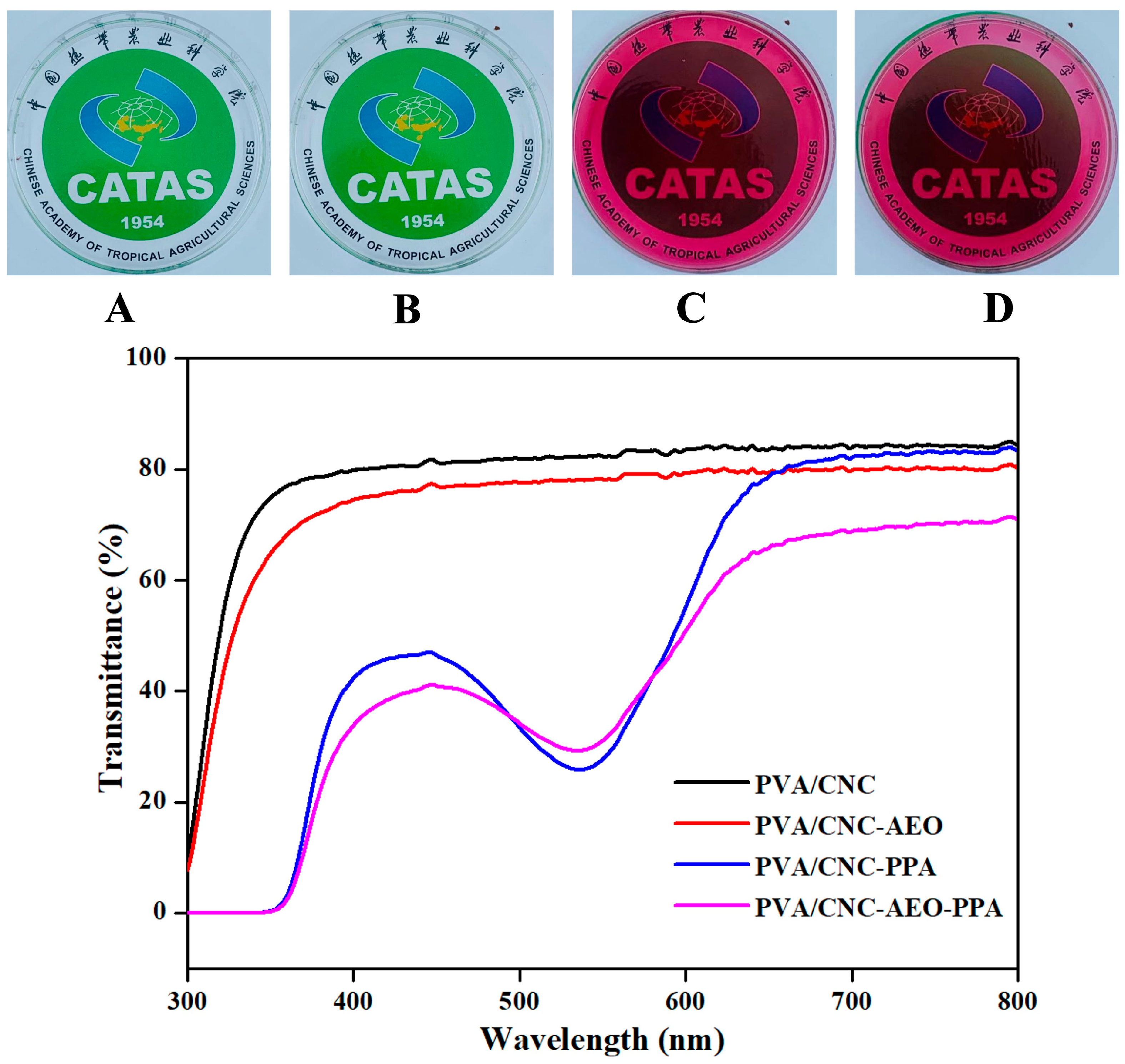
3.3.4. Colorimetric and Optical Properties
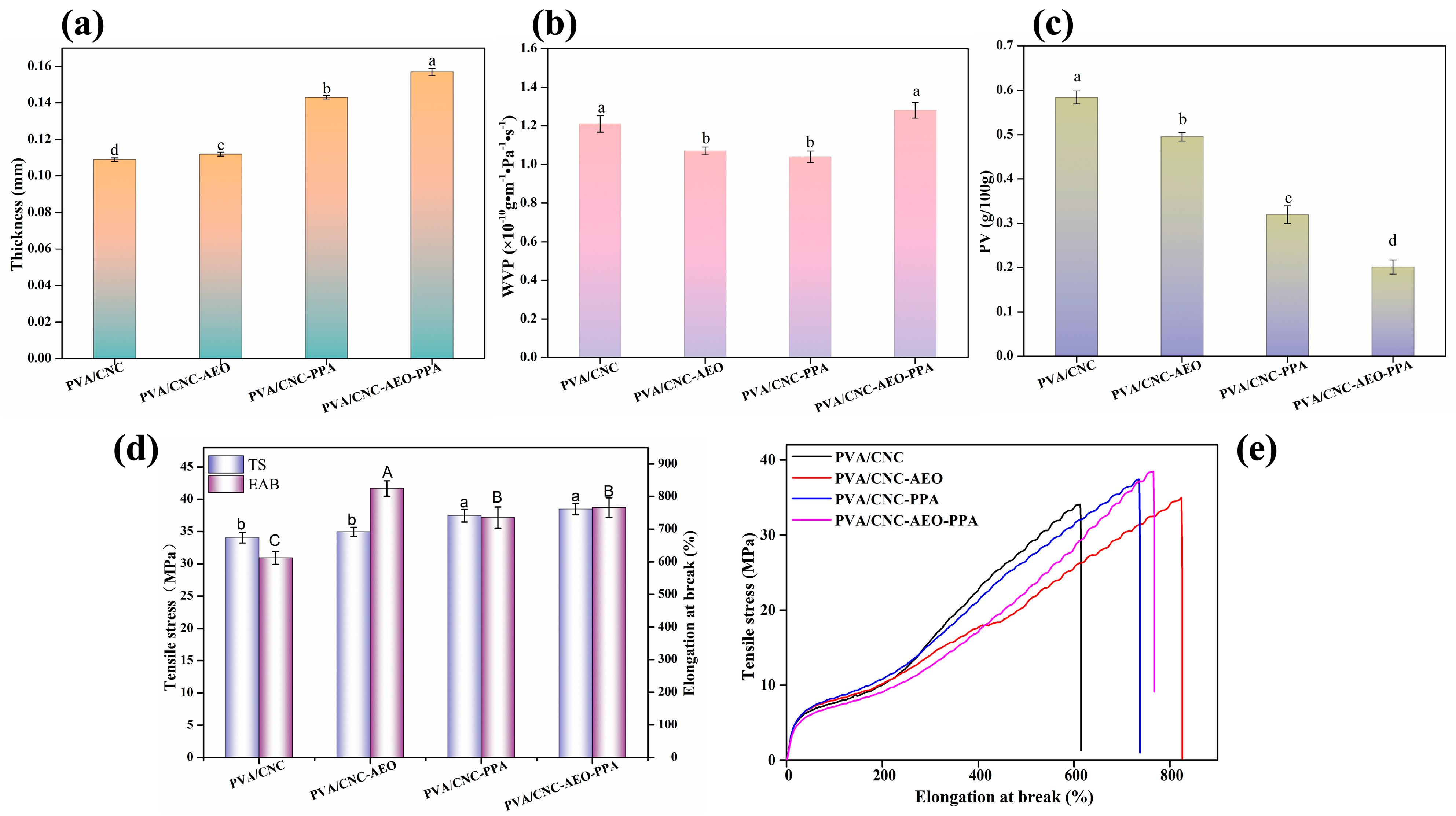
3.3.5. Thickness
3.3.6. WVP
3.3.7. Oxygen Permeability (OP)
3.3.8. Mechanical Properties
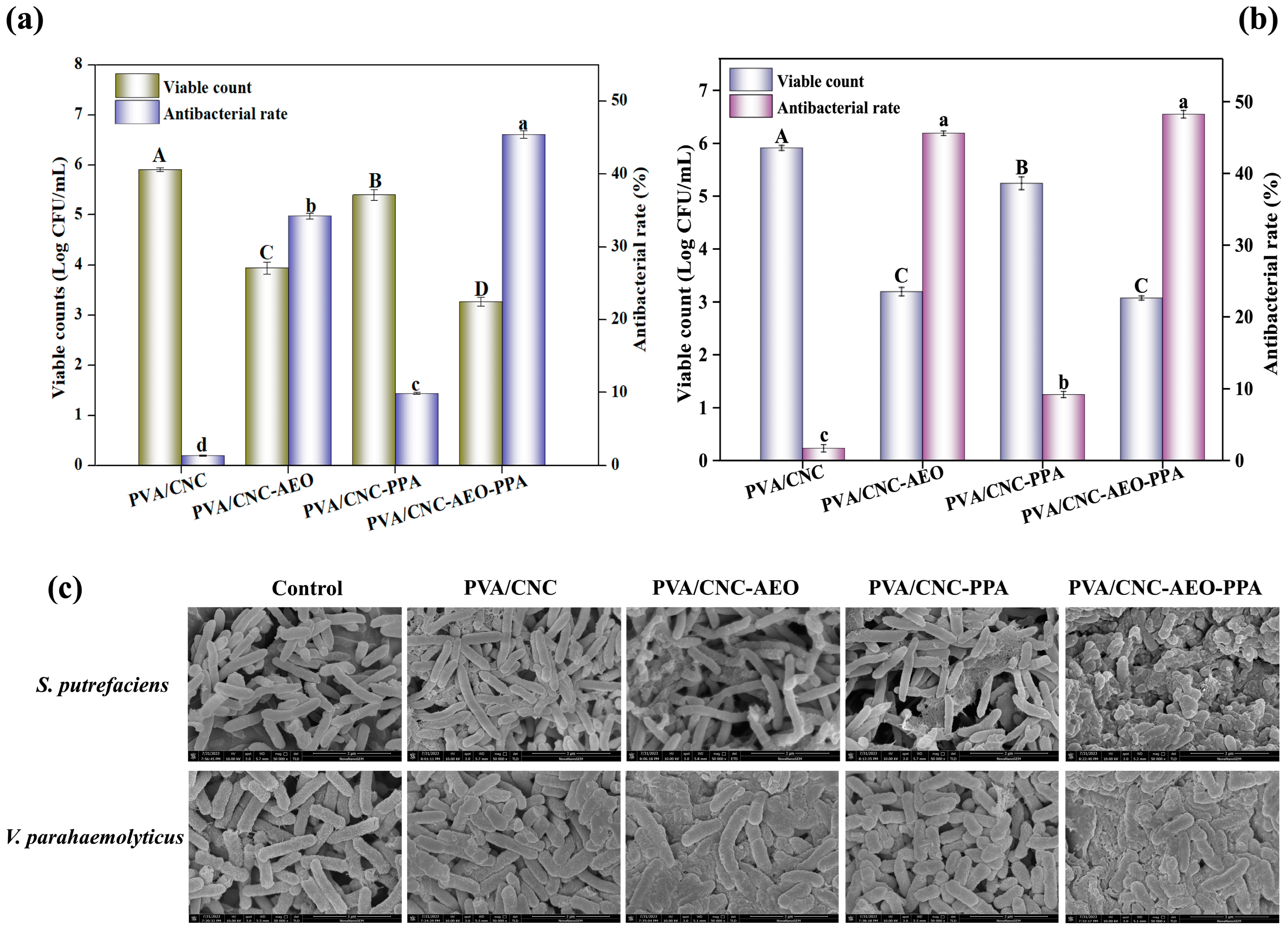
3.3.9. Antibacterial Activity
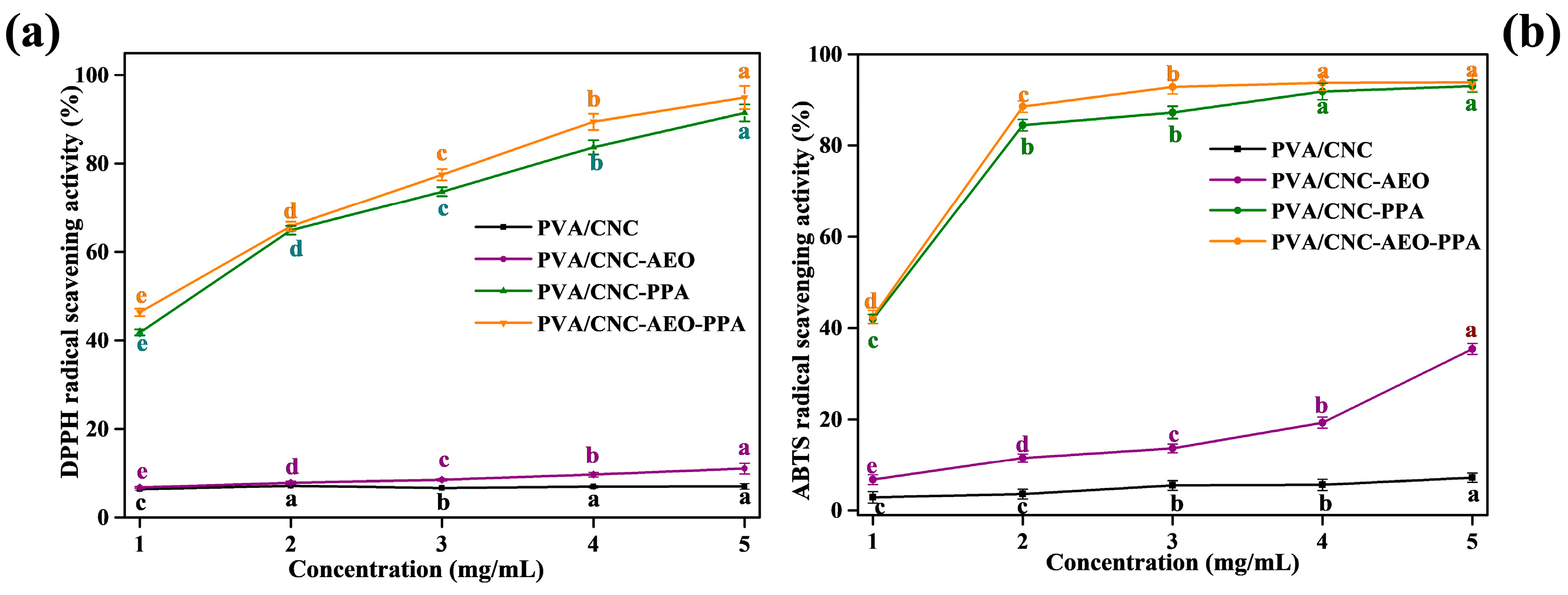
3.3.10. Antioxidant Activity
3.3.11. pH-Sensitivity Properties
3.3.12. Volatile Ammonia Response
3.4. Application of Smart Film for Mantis Shrimp Preservation and Freshness Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, B.; Chen, J.; Yang, G.; Bi, J.; Guo, J.; Shang, K.; Wang, S.; Wu, Z.; Zhang, K. Improvement of solubility, gelation and emulsifying properties of myofibrillar protein from mantis shrimp (Oratosquilla oratoria) by phosphorylation modification under low ionic strength of KCl. Food Chem. 2023, 403, 134497. [Google Scholar] [CrossRef]
- Meng, Y.; Luo, H.; Dong, C.; Zhang, C.; He, Z.; Long, Z.; Cha, R. Hydroxypropyl Guar/Cellulose Nanocrystal Film with Ionic Liquid and Anthocyanin for Real-Time and Visual Detection of NH3. ACS Sustain. Chem. Eng. 2020, 8, 9731–9741. [Google Scholar] [CrossRef]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart colorimetric sensing films with high mechanical strength and hydrophobic properties for visual monitoring of shrimp and pork freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; Zheng, M.; Sun, J.; Chen, Z.; Wang, J.; Ma, Q. Nanocellulose-enhanced smart film for the accurate monitoring of shrimp freshness via anthocyanin-induced color changes. Carbohydr. Polym. 2023, 301, 120352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, K.; Wang, J.; Wang, Y.; Tang, Y.; Gao, X.; Zhu, L.; Li, X.; Li, J. An on-package colorimetric sensing label based on a sol-gel matrix for fish freshness monitoring. Food Chem. 2020, 307, 125580. [Google Scholar] [CrossRef] [PubMed]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Corte-Tarazón, J.A.; Rochín-Wong, S.; Fernández-Quiroz, J.D.; Garzón-García, A.M.; Santos-Sauceda, I.; Plascencia-Martínez, D.F.; Chan-Chan, L.H.; Vásquez-López, C.; Barreras-Urbina, C.G.; et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. J. Food Eng. 2022, 334, 111153. [Google Scholar] [CrossRef]
- Yong, Y.; Gu, Y.; NabeelAhmad, H.; Wang, L.; Wang, R.; Zhu, J. Design and characterization of tannic acid/ε-polylysine biocomposite packaging films with excellent antibacterial and antioxidant properties for beef preservation. Food Chem. 2024, 439, 138155. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Jiang, C.; Munir, S.; Liu, R.; Yin, T.; You, J.; Rong, J.; Xiong, S.; Hu, Y. Synthesis and application of gelatin-based controlled-release antibacterial films containing oregano essential oil/β-cyclodextrin microcapsules for chilling preservation of grass carp fillets. Food Chem. 2024, 451, 139456. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Liu, Y.; Zhao, W.; Zhao, H.; Xue, X. Chemical markers of a rare honey from the traditional spice plant Amomum tsao-ko Crevost et Lemarié, via integrated GC-MS and LC-MS approaches. Food Res. Int. 2023, 172, 113234. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zang, Y.; Cui, Q.; Gai, Q.; Jiao, J.; Wang, W.; Zu, Y.; Fu, Y. The preservative potential of Amomum tsaoko essential oil against E. coil, its antibacterial property and mode of action. Food Control 2017, 75, 236–245. [Google Scholar] [CrossRef]
- Liu, J.; Lyu, H.; Fu, Y.; Cui, Q. Amomum tsao-ko essential oil, a novel anti-COVID-19 Omicron spike protein natural products: A computational study. Arab. J. Chem. 2022, 15, 103916. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, L.; Lin, Y.; Li, R.; Yuan, Y.; Lu, X.; Zou, Y.; Zhou, W.; Wang, Z.; Li, J. Intelligent pH-sensing film based on polyvinyl alcohol/cellulose nanocrystal with purple cabbage anthocyanins for visually monitoring shrimp freshness. Int. J. Biol. Macromol. 2022, 218, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.N.; Tola, A.J.; Nwabor, O.F.; Jayeoye, T.J. Centella asiatica phenolic extract-mediated biofabrication of silver nanoparticles: Characterization, reduction of industrially relevant dyes in water and antimicrobial activities against foodborne pathogens. RSC Adv. 2019, 9, 37957–37970. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Zhao, K.; Ma, Y.; Cheng, S.; Zhou, J.; Wu, Z. Producing a novel edible film from mushrooms (L. edodes and F. velutipes) byproducts with a two-stage treatment namely grinding and bleaching. J. Food Eng. 2019, 275, 109862. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, E.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Hossen, M.A.; Dai, J.; Qin, W.; Liu, Y. Facile fabrication of sandwich-like anthocyanin/chitosan/lemongrass essential oil films via 3D printing for intelligent evaluation of pork freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef] [PubMed]
- Baysal, G.; Olcay, H.S.; Günneç, Ç. Encapsulation and antibacterial studies of goji berry and garlic extract in the biodegradable chitosan. J. Bioact. Compat. Polym. 2023, 38, 209–219. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, C.; Rong, X.; Sameen, D.; Guo, L.; Zhang, J.; Chu, X.; Chen, M.; Liu, Y.; Qin, W. Development of curcumin-containing polyvinyl alcohol/chitosan active/intelligent films for preservation and monitoring of Schizothorax prenanti fillets freshness. Int. J. Biol. Macromol. 2023, 253, 127343. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.228-2016; National Food Safety Standard. Determination of Volatile Salt Nitrogen in Foods. China Standard Publishing House: Beijing, China, 2016.
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int. J. Biol. Macromol. 2018, 107, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, H.; Qi, D.; Xia, L.; Li, L.; Li, X.; Jiang, S. Multifunctional colorimetric cellulose acetate membrane incorporated with Perilla frutescens (L.) Britt. anthocyanins and chamomile essential oil. Carbohydr. Polym. 2022, 278, 118914. [Google Scholar] [CrossRef] [PubMed]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Córdoba, L.J.; Norton, I.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J.A. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, H.; Zhao, J.; Humayun, M.; Wu, S.; Wang, C.; Zhi, Z.; Pang, J. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness. Food Hydrocoll. 2022, 133, 107998. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and UV blocking properties of composite chitosan films with curcumin grafted cellulose nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Cao, J.; Jiang, W. Effect of purple sugarcane peel extracts on properties of films based on lemon peel waste pectin and the application in the visible detection of food freshness. Food Hydrocoll. 2022, 133, 107982. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, L. An active and pH-responsive film developed by sodium carboxymethyl cellulose/polyvinyl alcohol doped with rose anthocyanin extracts. Food Chem. 2022, 373, 131367. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Chavez-Marquez, E.; Bustamante Bernedo, M.S.; de Jara, E.M.; Quequezana-Bedregal, M.J.; Gutierrez-Oppe, E.E.; Pessôa Filho, P.A. Development of intelligent and active potato starch films based on purple corn cob extract and molle essential oil. Int. J. Biol. Macromol. 2023, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yan, X.; Zhang, J.; Wu, X.; Luan, M.; Zhang, Q. Preparation and characterization of pH-sensitive intelligent packaging films based on cassava starch/polyvinyl alcohol matrices containing Aronia melanocarpa anthocyanins. LWT-Food Sci. Technol. 2024, 194, 115818. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Hu, H.; Yun, D.; Liu, J. Development and comparison of different polysaccharide/PVA-based active/intelligent packaging films containing red pitaya betacyanins. Food Hydrocoll. 2022, 124, 107305. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohyd. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Rhim, J.W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2019, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, H.; Chen, B.; Huang, J.; Li, Y.; Zheng, H.; Liu, H.; Zhao, Y.; Wang, J. Dual-species biofilms formation of Vibrio parahaemolyticus and Shewanella putrefaciens and their tolerance to photodynamic inactivation. Food Control 2021, 125, 107983. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Fabrication of cellulose nanofiber-based functional color indicator film incorporated with shikonin extracted from Lithospermum erythrorhizon root. Food Hydrocoll. 2021, 114, 106566. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of intelligent pH-sensing films with antioxidant potential for monitoring shrimp freshness via the fortification of chitosan matrix with broken Riceberry phenolic extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Wahab, N.S.A.; Soo, J. Water soluble phenolics, flavonoids and anthocyanins extracted from jaboticaba berries using maceration with ultrasonic pretreatment. Food Chem. Adv. 2023, 3, 100387. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Dias, A.R.G.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT-Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]


| Sample | PVA (% w/v) | CNC (% w/v) | AEO (% w/v) | Tween-80 (% w/v) | PPA (% w/v) | Glycerol (% w/v) |
|---|---|---|---|---|---|---|
| PVA/CNC | 4.5 | 0.5 | - | - | - | 1.0 |
| PVA/CNC-AEO | 4.5 | 0.5 | 0.4 | 0.1 | - | 1.0 |
| PVA/CNC-PPA | 4.5 | 0.5 | - | - | 0.6 | 1.0 |
| PVA/CNC-AEO-PPA | 4.5 | 0.5 | 0.4 | 0.1 | 0.6 | 1.0 |
| No. | Constituents | RT | Molecular Formula | RI | % Relative Peak Area | |
|---|---|---|---|---|---|---|
| RI(C) | RI(Lit) | |||||
| 1 | α-Pinene | 5.415 | C10H16 | 931 | 933 | 2.78 |
| 2 | Sabinene | 6.362 | C10H16 | 974 | 974 | 1.56 |
| 3 | β-Pinene | 6.449 | C10H16 | 977 | 978 | 1.63 |
| 4 | Octanal | 6.951 | C8H16O | 1000 | 1001 | 2.49 |
| 5 | α-Phellandrene | 7.040 | C10H16 | 1005 | 1005 | 3.61 |
| 6 | ρ-Cymene | 7.490 | C10H14 | 1024 | 1025 | 2.08 |
| 7 | D-Limonene | 7.590 | C10H16 | 1028 | 1030 | 2.23 |
| 8 | 1,8-Cineole | 7.759 | C10H18O | 1031 | 1032 | 19.03 |
| 9 | β-Ocimene | 8.000 | C10H16 | 1039 | 1040 | 1.44 |
| 10 | 2-Octenal | 8.249 | C8H14O | 1055 | 1056 | 2.84 |
| 11 | Isoborneol | 10.939 | C10H18O | 1161 | 1160 | 0.62 |
| 12 | Terpinen-4-ol | 11.208 | C10H18O | 1180 | 1180 | 0.82 |
| 13 | α-Terpineol | 11.538 | C10H18O | 1193 | 1195 | 2.55 |
| 14 | Decanal | 11.810 | C10H18O | 1199 | 1200 | 0.69 |
| 15 | Citral | 12.784 | C10H16O | 1213 | 1216 | 7.55 |
| 16 | Geraniol | 13.116 | C10H18O | 1246 | 1247 | 2.01 |
| 17 | tran-2-Decenal | 13.292 | C10H18O | 1260 | 1260 | 10.00 |
| 18 | Geranial | 13.559 | C10H16O | 1278 | 1279 | 9.96 |
| 19 | 4-Propylbenzaldehyde | 14.055 | C10H12O | 1293 | 1294 | 10.86 |
| 20 | Citral dimethyl acetal | 14.505 | C12H22O2 | 1320 | 1322 | 1.19 |
| 21 | Geranyl acetate | 16.020 | C12H20O2 | 1385 | 1386 | 1.31 |
| 22 | 2-Dodecenal | 17.849 | C12H22O | 1446 | 1449 | 4.76 |
| 23 | trans-Nerolidol | 19.720 | C15H26O | 1565 | 1565 | 2.53 |
| Total | 94.54 | |||||
| Films | L* | a* | b* | ΔE | Opacity |
|---|---|---|---|---|---|
| PVA/CNC | 90.60 ± 0.30 a | −0.68 ± 0.15 c | 2.27 ± 0.24 b | 4.41 ± 0.27 c | 0.04 ± 0.01 d |
| PVA/CNC-AEO | 91.28 ± 0.21 a | −0.72 ± 0.04 c | 2.90 ± 0.01 a | 4.01 ± 0.19 c | 0.23 ± 0.03 c |
| PVA/CNC-PPA | 44.62 ± 0.08 c | 48.24 ± 0.13 a | −2.63 ± 0.04 c | 68.46 ± 0.04 a | 1.32 ± 0.02 b |
| PVA/CNC-AEO-PPA | 48.69 ± 0.06 b | 45.22 ± 0.23 b | −2.08 ± 0.06 c | 63.40 ± 0.20 b | 1.67 ± 0.10 a |
| Samples | pH | L* | a* | b* | ΔE | Photograph |
|---|---|---|---|---|---|---|
| PVA/CNC- AEO-PPA | 2 | 45.74 ± 1.46 cd | 45.48 ± 0.43 a | 8.53 ± 0.29 b | 11.01 ± 0.75 a |  |
| 3 | 41.46 ± 2.19 e | 35.86 ± 0.54 b | 3.58 ± 0.17 d | 13.11 ± 1.52 b |  | |
| 4 | 40.04 ± 0.57 e | 34.69 ± 0.28 c | 3.45 ± 0.02 d | 14.71 ± 0.33 c |  | |
| 5 | 48.66 ± 0.4 bc | 30.10 ± 0.10 d | 1.83 ± 0.18 e | 15.62 ± 0.28 c |  | |
| 6 | 55.92 ± 1.96 a | 20.48 ± 0.30 e | 2.00 ± 0.05 e | 26.10 ± 0.61 e |  | |
| 7 | 48.47 ± 2.43 bc | 20.64 ± 0.88 e | 0.63 ± 0.15 f | 24.73 ± 0.89 d |  | |
| 8 | 51.33 ± 0.20 b | 11.24 ± 0.06 g | −1.93 ± 0.01 g | 34.08 ± 0.18 g |  | |
| 9 | 50.17 ± 1.40 b | 11.39 ± 0.58 g | −2.20 ± 0.05 g | 33.86 ± 0.32 g |  | |
| 10 | 45.16 ± 1.39 d | 13.23 ± 0.24 f | −1.72 ± 0.03 g | 32.19 ± 0.28 f |  | |
| 11 | 39.53 ± 0.59 e | 6.95 ± 0.30 h | −5.32 ± 0.02 h | 39.48 ± 0.54 h |  | |
| 12 | 29.55 ± 0.62 f | 2.86 ± 0.06 i | 4.49 ± 0.13 c | 46.95 ± 0.27 i |  | |
| 13 | 50.88 ± 1.82 b | 20.73 ± 0.28 e | 38.47 ± 0.76 a | 47.42 ± 0.16 i |  |
| Samples | Time/min | L* | a* | b* | ΔE | Photograph |
|---|---|---|---|---|---|---|
| PVA/CNC-AEO-PPA | 0 | 48.69 ± 0.06 a | 45.22 ± 0.23 a | −2.08 ± 0.06 a | - |  |
| 10 | 44.57 ± 0.40 d | 33.05 ± 0.28 b | −4.57 ± 0.62 b | 13.09 ± 0.54 a |  | |
| 20 | 46.44 ± 0.01 b | 20.76 ± 0.71 c | −4.89 ± 0.24 b | 24.72 ± 0.23 b |  | |
| 30 | 45.38 ± 0.13 c | 7.31 ± 0.52 d | −6.57 ± 0.13 c | 38.32 ± 0.05 c |  |
| Sample | Time (Days) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| PVA/CNC-AEO-PPA | 0 | 48.53 ± 0.07 b | 45.86 ± 0.10 a | −2.10 ± 0.05 e | - |
| 2 | 50.23 ± 0.06 a | 42.27 ± 0.04 b | −1.42 ± 0.06 d | 4.03 ± 0.12 d | |
| 4 | 47.21 ± 0.10 c | 35.21 ± 0.05 c | 1.90 ± 0.03 c | 11.45 ± 0.31 c | |
| 6 | 40.28 ± 0.09 d | 20.67 ± 0.06 d | 2.89 ± 0.06 b | 26.97 ± 0.48 b | |
| 8 | 38.46 ± 0.08 e | 16.26 ± 0.04 e | 3.08 ± 0.07 a | 31.69 ± 0.61 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Yuan, Y.; Gao, Y.; Chen, M.; Li, Y.; Zou, Y.; Liao, L.; Li, X.; Wang, Z.; Li, J.; et al. Enhancement of Colorimetric pH-Sensitive Film Incorporating Amomum tsao-ko Essential Oil as Antibacterial for Mantis Shrimp Spoilage Tracking and Fresh-Keeping. Foods 2024, 13, 1638. https://doi.org/10.3390/foods13111638
He Y, Yuan Y, Gao Y, Chen M, Li Y, Zou Y, Liao L, Li X, Wang Z, Li J, et al. Enhancement of Colorimetric pH-Sensitive Film Incorporating Amomum tsao-ko Essential Oil as Antibacterial for Mantis Shrimp Spoilage Tracking and Fresh-Keeping. Foods. 2024; 13(11):1638. https://doi.org/10.3390/foods13111638
Chicago/Turabian StyleHe, Yunxia, Yuay Yuan, Yuanyuan Gao, Mianhong Chen, Yingying Li, Ying Zou, Liangkun Liao, Xiaotong Li, Zhuo Wang, Jihua Li, and et al. 2024. "Enhancement of Colorimetric pH-Sensitive Film Incorporating Amomum tsao-ko Essential Oil as Antibacterial for Mantis Shrimp Spoilage Tracking and Fresh-Keeping" Foods 13, no. 11: 1638. https://doi.org/10.3390/foods13111638
APA StyleHe, Y., Yuan, Y., Gao, Y., Chen, M., Li, Y., Zou, Y., Liao, L., Li, X., Wang, Z., Li, J., & Zhou, W. (2024). Enhancement of Colorimetric pH-Sensitive Film Incorporating Amomum tsao-ko Essential Oil as Antibacterial for Mantis Shrimp Spoilage Tracking and Fresh-Keeping. Foods, 13(11), 1638. https://doi.org/10.3390/foods13111638






