Literature Review of Proteomics Approach Associated with Coffee
Abstract
:1. Introduction

2. Integration of Proteomic Technology
3. Aim of the Current Article
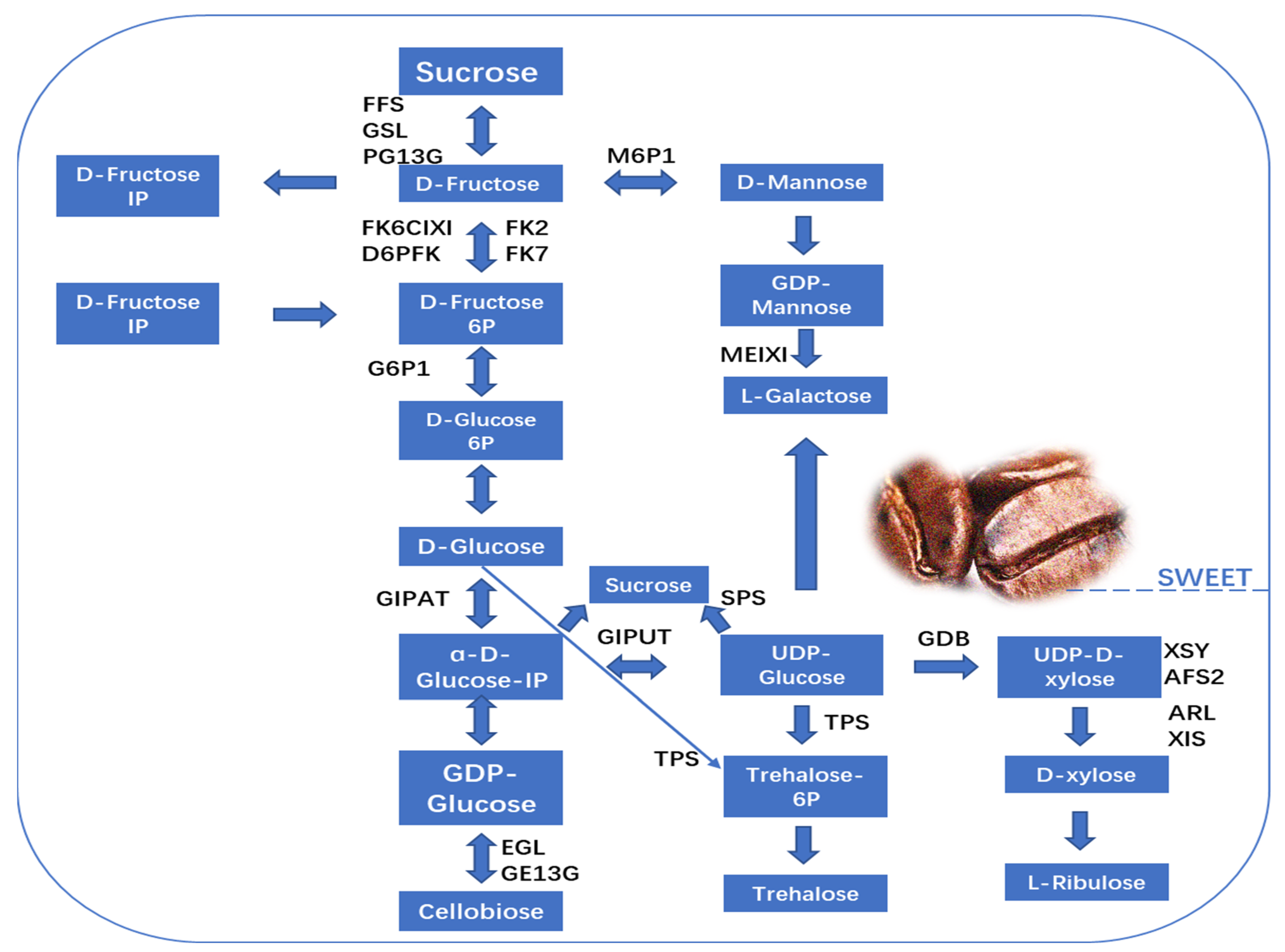
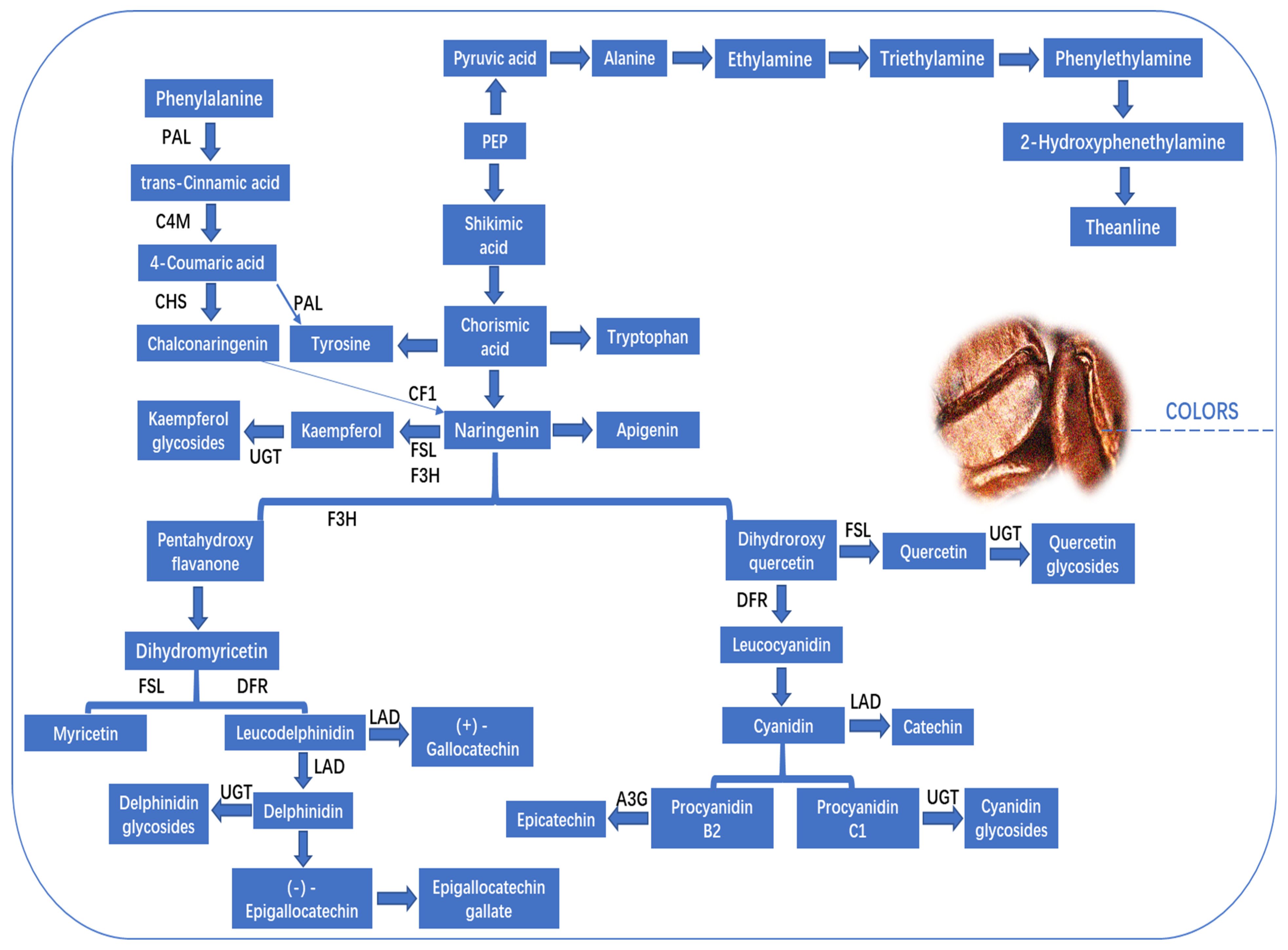
4. Proteomics Approach Associated with Flavor of Arabica Coffee
5. Proteomics Linked to Altitude and Different Environmental Adaptations of Arabica Coffee
6. Proteomics Linked to Antioxidative and Drought Responses in Arabica Coffee
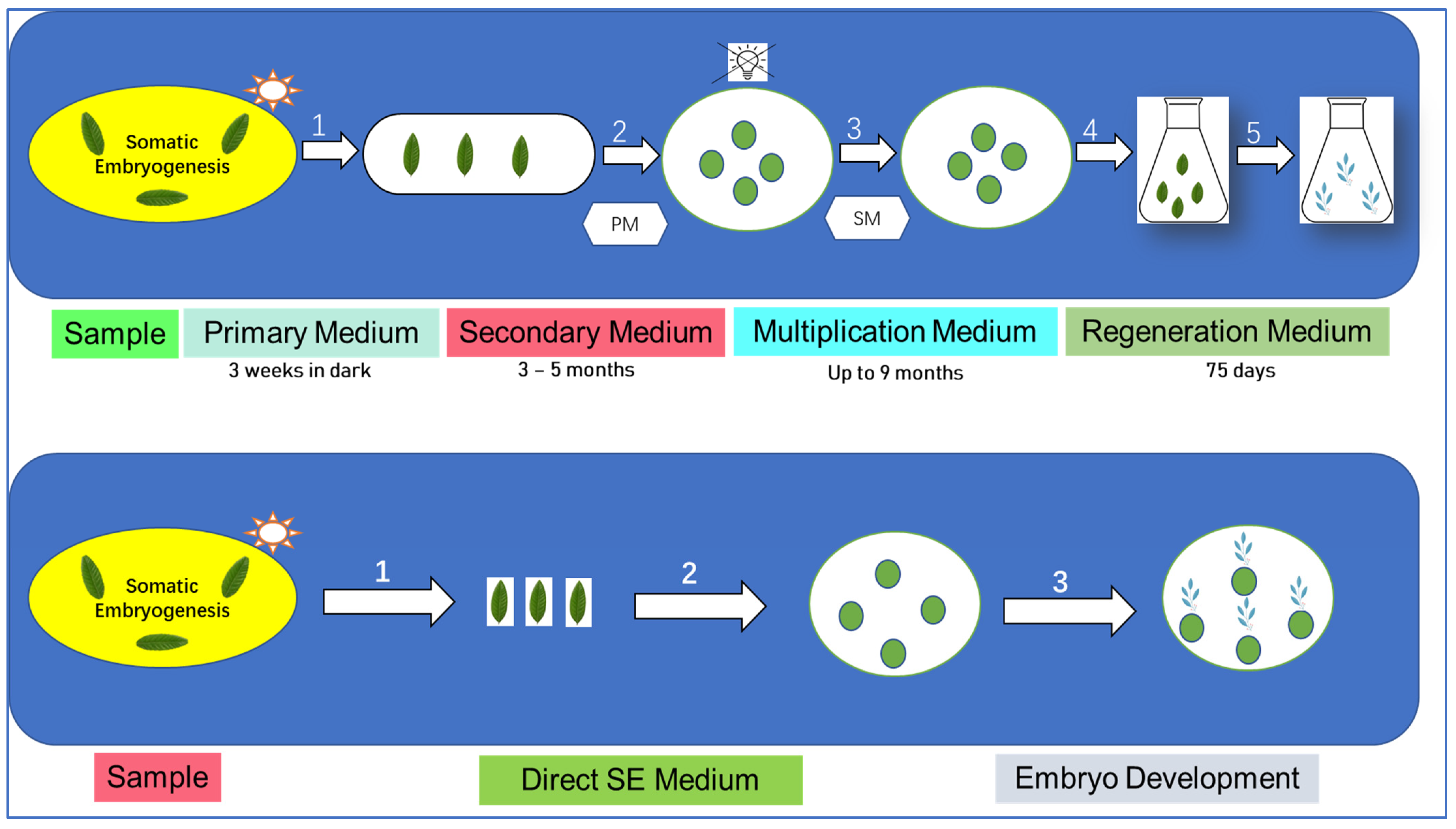
7. Proteomics Linked to Somatic Embryogenesis of Arabica Coffee
8. Lack of Proteomic Studies and Future Direction Associated with Coffee
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Xiao, Y.; Tang, W.; Jiang, F.; Zhu, J.; Zhou, Y.; Ye, L. Evaluation of Physicochemical Characteristics and Sensory Properties of Cold Brew Coffees Prepared Using Ultrahigh Pressure under Different Extraction Conditions. Foods 2023, 12, 3857. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.J.P.A.; Marques, M.B.F.; Franca, A.S.; Oliveira, L.S. Development of Films from Spent Coffee Grounds’ Polysaccharides Crosslinked with Calcium Ions and 1,4-Phenylenediboronic Acid: A Comparative Analysis of Film Properties and Biodegradability. Foods 2023, 12, 2520. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.A.R.; da Cruz, M.A.D.; Silva, L.C.F.; Costa, G.X.R.; Amaral, L.R.; Bertarini, P.L.L.; Gomes, M.S.; Santos, L.D. Evaluation of Arabica Coffee Fermentation Using Machine Learning. Foods 2024, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Poláková, K.; Bobková, A.; Demianová, A.; Bobko, M.; Lidiková, J.; Jurčaga, L.; Belej, Ľ.; Mesárošová, A.; Korčok, M.; Tóth, T. Quality Attributes and Sensory Acceptance of Different Botanical Coffee Co-Products. Foods 2023, 12, 2675. [Google Scholar] [CrossRef] [PubMed]
- van der Vossen, H.; Bertrand, B.; Charrier, A. Next Generation Variety Development for Sustainable Production of Arabica Coffee (Coffea arabica L.): A Revie. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Silva, L.C.F.; Pereira, P.V.R.; da Cruz, M.A.D.; Costa, G.X.R.; Rocha, R.A.R.; Bertarini, P.L.L.; do Amaral, L.R.; Gomes, M.S.; Santos, L.D. Enhancing Sensory Quality of Coffee: The Impact of Fermentation Techniques on Coffea arabica Cv. Catiguá MG2. Foods 2024, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.G.; Bertarini, P.L.L.; Gomes, M.S.; Amaral, L.R.; Zotarelli, M.F.; Santos, L.D.; Santana, R.C. Physicochemical and Sensory Properties of Arabica Coffee Beans of Arara Cv. Dried Using Different Methods. Foods 2024, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; da Silva, C.A.; Silva, L.O.E.; Espindula, M.C.; Rodrigues, W.P.; Vieira, H.D.; Tomaz, M.A.; Partelli, F.L. Accumulation of Nutrients and the Relation between Fruit, Grain, and Husk of Coffee Robusta Cultivated in Brazilian Amazon. Plants 2023, 12, 3476. [Google Scholar] [CrossRef]
- Min, C.; Biyi, M.; Jianneng, L.; Yimin, L.; Yijun, L.; Long, C. Characterization of the Volatile Organic Compounds Produced from Green Coffee in Different Years by Gas Chromatography Ion Mobility Spectrometry. RSC Adv. 2022, 12, 15534–15542. [Google Scholar] [CrossRef]
- Zhai, H.; Dong, W.; Tang, Y.; Hu, R.; Yu, X.; Chen, X. Characterization of the Volatile Flavour Compounds in Yunnan Arabica Coffee Prepared by Different Primary Processing Methods Using HS-SPME/GC-MS and HS-GC-IMS. LWT 2024, 192, 115717. [Google Scholar] [CrossRef]
- Caixeta, E. Selective Efficiency of Genome-Wide Selection in Coffea canephora Breeding. Tree Genet. Genomes 2020, 16, 41. [Google Scholar]
- Huded, A.K.C.; Jingade, P.; Bychappa, M.; Mishra, M.K. Genetic Diversity and Population Structure Analysis of Coffee (Coffea canephora) Germplasm Collections in Indian Gene Bank Employing SRAP and SCoT Markers. Int. J. Fruit Sci. 2020, 20, S757–S784. [Google Scholar] [CrossRef]
- Berni, R.; Charton, S.; Planchon, S.; Legay, S.; Romi, M.; Cantini, C.; Cai, G.; Hausman, J.-F.; Renaut, J.; Guerriero, G. Molecular Investigation of Tuscan Sweet Cherries Sampled over Three Years: Gene Expression Analysis Coupled to Metabolomics and Proteomics. Hortic. Res. 2021, 8, 12. [Google Scholar] [CrossRef]
- Montavon, P.; Mauron, A.-F.; Duruz, E. Changes in Green Coffee Protein Profiles during Roasting. J Agric Food Chem 2003, 51, 2335–2343. [Google Scholar] [CrossRef]
- Anthony, F.; Clifford, M.N.; Noirot, M. Biochemical Diversity in the Genus Coffea L.: Chlorogenic Acids, Caffeine and Mozambioside Contents. Genet. Resour. Crop Evol. 1993, 40, 61–70. [Google Scholar] [CrossRef]
- Coffee: Botany, Biochemistry and Production of Beans and Beverage [1 ed.] 9781461566571, 1461566576. Available online: https://ebin.pub/coffee-botany-biochemistry-and-production-of-beans-and-beverage-1nbsped-9781461566571-1461566576.html (accessed on 13 May 2024).
- Mazzafera, P.; Schimpl, F.; Kiyota, E. Proteins of Coffee Beans: Recent Advances; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Livramento, K.; Borém, F.; Torres, L.; Silva, F.; Livramento, D.; Paiva, L. Proteomic Analysis of Natural and Demucilaged Coffee Beans from Plantations at Different Altitudes in the Mantiqueira Mountains. J. Exp. Agric. Int. 2017, 19, 1–15. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef]
- Fenrich, C.; Lauman, P.; Wickramasinghe, P. Proteomic Analysis of Higher & Lower Altitude Cultivars of Coffea arabica Reveals Differences Related to Environmental Adaptations and Coffee Bean Flavour. Eureka 2023, 8. [Google Scholar] [CrossRef]
- Hendre, P.S.; Aggarwal, R.K. Development of Genic and Genomic SSR Markers of Robusta Coffee (Coffea canephora Pierre Ex A. Froehner). PLoS ONE 2014, 9, e113661. [Google Scholar] [CrossRef]
- ME THAI|Bangkok. Available online: https://methaicoffee.com/lander (accessed on 23 May 2024).
- Martínez-Esteso, M.J.; Martínez-Márquez, A.; Sellés-Marchart, S.; Morante-Carriel, J.A.; Bru-Martínez, R. The Role of Proteomics in Progressing Insights into Plant Secondary Metabolism. Front. Plant Sci. 2015, 6, 504. [Google Scholar] [CrossRef]
- Mathivanan, S. Abiotic Stress-Induced Molecular and Physiological Changes and Adaptive Mechanisms in Plants. In Abiotic Stress in Plants; IntechOpen: London, UK, 2021; ISBN 978-1-83881-062-7. [Google Scholar]
- Yadav, B.G.; Aakanksha; Kumar, R.; Yadava, S.K.; Kumar, A.; Ramchiary, N. Understanding the Proteomes of Plant Development and Stress Responses in Brassica Crops. J. Proteome Res. 2023, 22, 660–680. [Google Scholar] [CrossRef]
- Vanderschuren, H.; Lentz, E.; Zainuddin, I.; Gruissem, W. Proteomics of Model and Crop Plant Species: Status, Current Limitations and Strategic Advances for Crop Improvement. J. Proteom. 2013, 93, 5–19. [Google Scholar] [CrossRef]
- Montavon, P.; Duruz, E.; Rumo, G.; Pratz, G. Evolution of Green Coffee Protein Profiles with Maturation and Relationship to Coffee Cup Quality. J. Agric. Food Chem. 2003, 51, 2328–2334. [Google Scholar] [CrossRef]
- Kerler, J.; Winkel, C.; Davidek, T.; Blank, I. Basic Chemistry and Process Conditions for Reaction Flavours with Particular Focus on Maillard-Type Reactions. In Food Flavour Technology; Taylor, A.J., Linforth, R.S.T., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 51–88. ISBN 978-1-4051-8543-1. [Google Scholar]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea Robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Al-Ali, H.; Johnson, S.K. Extraction, Isolation and Nutritional Quality of Coffee Protein. Foods 2022, 11, 3244. [Google Scholar] [CrossRef]
- Fitri; Laga, A.; Dwyana, Z.; Tawali, A.B. Composition of Amino Acids and Fatty Acids on Luwak Coffee Processing. Food Res. 2021, 5, 60–64. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, B.; Zheng, T.; Zhao, C.; Shen, X.; Wang, X.; Qiu, M.; Fan, J. Integrating Metabolomics and Proteomics Technologies Provides Insights into the Flavor Precursor Changes at Different Maturity Stages of Arabica Coffee Cherries. Foods 2023, 12, 1432. [Google Scholar] [CrossRef]
- Marques, I.; Gouveia, D.; Gaillard, J.-C.; Martins, S.; Semedo, M.C.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Armengaud, J.; Ramalho, J.C. Next-Generation Proteomics Reveals a Greater Antioxidative Response to Drought in Coffea arabica Than in Coffea canephora. Agronomy 2022, 12, 148. [Google Scholar] [CrossRef]
- Takahashi, S.; Saito, K.; Li, X.; Jia, H.; Kato, H. iTRAQ-Based Quantitative Proteomics Reveals the Energy Metabolism Alterations Induced by Chlorogenic Acid in HepG2 Cells. Nutrients 2022, 14, 1676. [Google Scholar] [CrossRef]
- Campos, N.A.; Paiva, L.V.; Panis, B.; Carpentier, S.C. The Proteome Profile of Embryogenic Cell Suspensions of Coffea arabica L. Proteomics 2016, 16, 1001–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Hu, G.; Hong, D.; Bai, X.; Guo, T.; Zhou, H.; Li, J.; Qiu, M. Chemical Ingredients Characterization Basing on 1H NMR and SHS-GC/MS in Twelve Cultivars of Coffea arabica Roasted Beans. Food Res. Int. 2021, 147, 110544. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics Combined with Proteomics Provides a Novel Interpretation of the Compound Differences among Chinese Tea Cultivars (Camellia sinensis var. sinensis) with Different Manufacturing Suitabilities. Food Chem 2022, 377, 131976. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, L.; Yang, N.; Song, J.; Zhao, H.; Zhang, J.; Ma, F.; Li, M. Proteomics and Metabolomics Reveal the Regulatory Pathways of Ripening and Quality in Post-Harvest Kiwifruits. J. Agric. Food Chem. 2021, 69, 824–835. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Chen, Z.; Jackson, A. Transcriptomic, Proteomic and Metabolomic Analysis of Flavonoid Biosynthesis during Fruit Maturation in Rubus Chingii Hu. Front. Plant Sci. 2021, 12, 706667. [Google Scholar] [CrossRef]
- Haure, M.; Nguyen, T.K.C.; Cendres, A.; Perino, S.; Licandro, H.; Waché, Y. Glycosidically Bound Volatile Profiles of Green and Roasted Coffee Beans and Aromatic Potential of the Spent Coffee Ground. Eur. Food Res. Technol. 2022, 248, 2125–2134. [Google Scholar] [CrossRef]
- Subcellular Localisation Database for Arabidopsis Proteins Version 5. Available online: https://research-repository.uwa.edu.au/en/datasets/subcellular-localisation-database-for-arabidopsis-proteins-versio-3 (accessed on 17 April 2024).
- Wulfert, S.; Schilasky, S.; Krueger, S. Transcriptional and Biochemical Characterization of Cytosolic Pyruvate Kinases in Arabidopsis thaliana. Plants 2020, 9, 353. [Google Scholar] [CrossRef]
- Yildirim, S.; Castano, E.; Sobol, M.; Philimonenko, V.V.; Dzijak, R.; Venit, T.; Hozák, P. Involvement of Phosphatidylinositol 4,5-Bisphosphate in RNA Polymerase I Transcription. J. Cell Sci. 2013, 126, 2730–2739. [Google Scholar] [CrossRef]
- Sreerama, L.; Hedge, M.W.; Sladek, N.E. Identification of a Class 3 Aldehyde Dehydrogenase in Human Saliva and Increased Levels of This Enzyme, Glutathione S-Transferases, and DT-Diaphorase in the Saliva of Subjects Who Continually Ingest Large Quantities of Coffee or Broccoli. Clin. Cancer Res. 1995, 1, 1153–1163. [Google Scholar]
- Gonzalez, D.; Fraichard, S.; Grassein, P.; Delarue, P.; Senet, P.; Nicolaï, A.; Chavanne, E.; Mucher, E.; Artur, Y.; Ferveur, J.-F.; et al. Characterization of a Drosophila Glutathione Transferase Involved in Isothiocyanate Detoxification. Insect Biochem. Mol. Biol. 2018, 95, 33–43. [Google Scholar] [CrossRef]
- Schwartz, M.; Boichot, V.; Fraichard, S.; Muradova, M.; Senet, P.; Nicolai, A.; Lirussi, F.; Bas, M.; Canon, F.; Heydel, J.-M.; et al. Role of Insect and Mammal Glutathione Transferases in Chemoperception. Biomolecules 2023, 13, 322. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the Kill: How Plants Deploy Effector-Triggered Immunity to Combat Pathogens. [Corrected]. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, X.; Feng, B.; Sheen, J.; Shan, L.; He, P. Plant Immune Response to Pathogens Differs with Changing Temperatures. Nat. Commun. 2013, 4, 2530. [Google Scholar] [CrossRef]
- Bäckström, S.; Elfving, N.; Nilsson, R.; Wingsle, G.; Björklund, S. Purification of a Plant Mediator from Arabidopsis thaliana Identifies PFT1 as the Med25 Subunit. Mol. Cell 2007, 26, 717–729. [Google Scholar] [CrossRef]
- Hemsley, P.A.; Hurst, C.H.; Kaliyadasa, E.; Lamb, R.; Knight, M.R.; De Cothi, E.A.; Steele, J.F.; Knight, H. The Arabidopsis Mediator Complex Subunits MED16, MED14, and MED2 Regulate Mediator and RNA Polymerase II Recruitment to CBF-Responsive Cold-Regulated Genes. Plant Cell 2014, 26, 465–484. [Google Scholar] [CrossRef]
- Hasan, M.M.-U.; Ma, F.; Prodhan, Z.H.; Li, F.; Shen, H.; Chen, Y.; Wang, X. Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 2636. [Google Scholar] [CrossRef]
- dos Santos, C.S.; de Freitas, A.F.; da Silva, G.H.B.; Pennacchi, J.P.; Figueiredo de Carvalho, M.A.; Santos, M.d.O.; Junqueira de Moraes, T.S.; de Rezende Abrahão, J.C.; Pereira, A.A.; Carvalho, G.R.; et al. Phenotypic Plasticity Index as a Strategy for Selecting Water-Stress-Adapted Coffee Genotypes. Plants 2023, 12, 4029. [Google Scholar] [CrossRef]
- Ford, K.L.; Cassin, A.; Bacic, A. Quantitative Proteomic Analysis of Wheat Cultivars with Differing Drought Stress Tolerance. Front. Plant Sci. 2011, 2, 44. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Barták, P.; Valenta, T.; Dokládalová, K. Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews. Foods 2023, 12, 4125. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Jia, Y.; Wong, D.C.; Sweetman, C.; Bruning, J.B.; Ford, C.M. New Insights into the Evolutionary History of Plant Sorbitol Dehydrogenase. BMC Plant Biol. 2015, 15, 101. [Google Scholar] [CrossRef]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Srinivasan, T.; Kumar, K.R.R.; Kirti, P.B. Constitutive Expression of a Trypsin Protease Inhibitor Confers Multiple Stress Tolerance in Transgenic Tobacco. Plant Cell Physiol. 2009, 50, 541–553. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C.; Leitão, A.E.; Fortunato, A.S.; Pais, I.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; et al. Stress Cross-Response of the Antioxidative System Promoted by Superimposed Drought and Cold Conditions in Coffea spp. PLoS ONE 2018, 13, e0198694. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X. Regulation of Somatic Embryogenesis in Higher Plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Staritsky, G. EMBRYOID FORMATION IN CALLUS TISSUES OF COFFEE. Acta Bot. Neerl. 1970, 19, 509–514. [Google Scholar] [CrossRef]
- Santana-Buzzy, N.; Rojas-Herrera, R.; Galaz-Ávalos, R.M.; Ku-Cauich, J.R.; Mijangos-Cortés, J.; Gutiérrez-Pacheco, L.C.; Canto, A.; Quiroz-Figueroa, F.; Loyola-Vargas, V.M. Advances in Coffee Tissue Culture and Its Practical Applications. In Vitro Cell. Dev. Biol.-Plant 2007, 43, 507–520. [Google Scholar] [CrossRef]
- Campos, N.A.; Panis, B.; Carpentier, S.C. Somatic Embryogenesis in Coffee: The Evolution of Biotechnology and the Integration of Omics Technologies Offer Great Opportunities. Front. Plant Sci. 2017, 8, 1460. [Google Scholar] [CrossRef]
- Tonietto, Â.; Sato, J.H.; Teixeira, J.B.; De Souza, E.M.; Pedrosa, F.O.; Franco, O.L.; Mehta, A. Proteomic Analysis of Developing Somatic Embryos of Coffea arabica. Plant Mol. Biol. Rep. 2012, 30, 1393–1399. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B.; Dechamp, E.; Maurel, P.; Georget, F.; Guyot, R.; Breitler, J.C. Are genetics and epigenetic instabilities of plant embryogenic cells a fatality? The experience of coffee somatic embryogenesis. Hum. Genet. Embryol. 2016, 6, 136. Available online: https://publications.cirad.fr/une_notice.php?dk=580450 (accessed on 17 April 2024). [CrossRef]

| Serial Number | Amino Acids and Proteins in Coffee | References |
|---|---|---|
| Amino Acids in Coffee | ||
| 1 | Arginine | |
| 2 | Leucine | |
| 3 | Phenylalanine | |
| 4 | Threonine | |
| 5 | Methionine | |
| 6 | Lysine | |
| 7 | Histidine | |
| 8 | Isoleucine | |
| 9 | Aspartic acid | [29,30,31,32] |
| 10 | Valine | |
| 11 | Alanine | |
| 12 | Glycine | |
| 13 | Proline | |
| 14 | Glutamic acid | |
| 15 | Tyrosine | |
| 16 | Serine | |
| Proteins in Coffee | ||
| 17 | NB-ARC domain-containing protein | |
| 18 | Elongation factor1-gamma 2 | |
| 19 | Mediator of RNA polymerase II transcription subunit 36a-like | |
| 20 | Pyruvate kinase 1, cytosolic | |
| 21 | Epimerase domain-containing protein | |
| 22 | Auxin-binding protein ABP20 | |
| 23 | Oxygen-evolving enhancer protein 1 | |
| 24 | Photosystem I reaction center subunit I | [14,27,28,33,34,35] |
| 25 | Oxygen-evolving enhancer protein 2 | |
| 26 | Peptidyl-prolyl cis–trans isomerase | |
| 27 | Quinone oxidoreductase-like protein At1g23740 | |
| 28 | Uncharacterized protein At2g37660 | |
| 29 | Chlorophyll a–b binding protein CP26 | |
| 30 | Polyphenol oxidase I | |
| 31 | Phosphoglycerate kinase | |
| 32 | Glyceraldehyde-3-phosphate dehydrogenase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, S.; Shan, Z. Literature Review of Proteomics Approach Associated with Coffee. Foods 2024, 13, 1670. https://doi.org/10.3390/foods13111670
Zaman S, Shan Z. Literature Review of Proteomics Approach Associated with Coffee. Foods. 2024; 13(11):1670. https://doi.org/10.3390/foods13111670
Chicago/Turabian StyleZaman, Shah, and Zhiguo Shan. 2024. "Literature Review of Proteomics Approach Associated with Coffee" Foods 13, no. 11: 1670. https://doi.org/10.3390/foods13111670






