Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota
Abstract
1. Introduction
2. Impacts of Exercise on the Gut
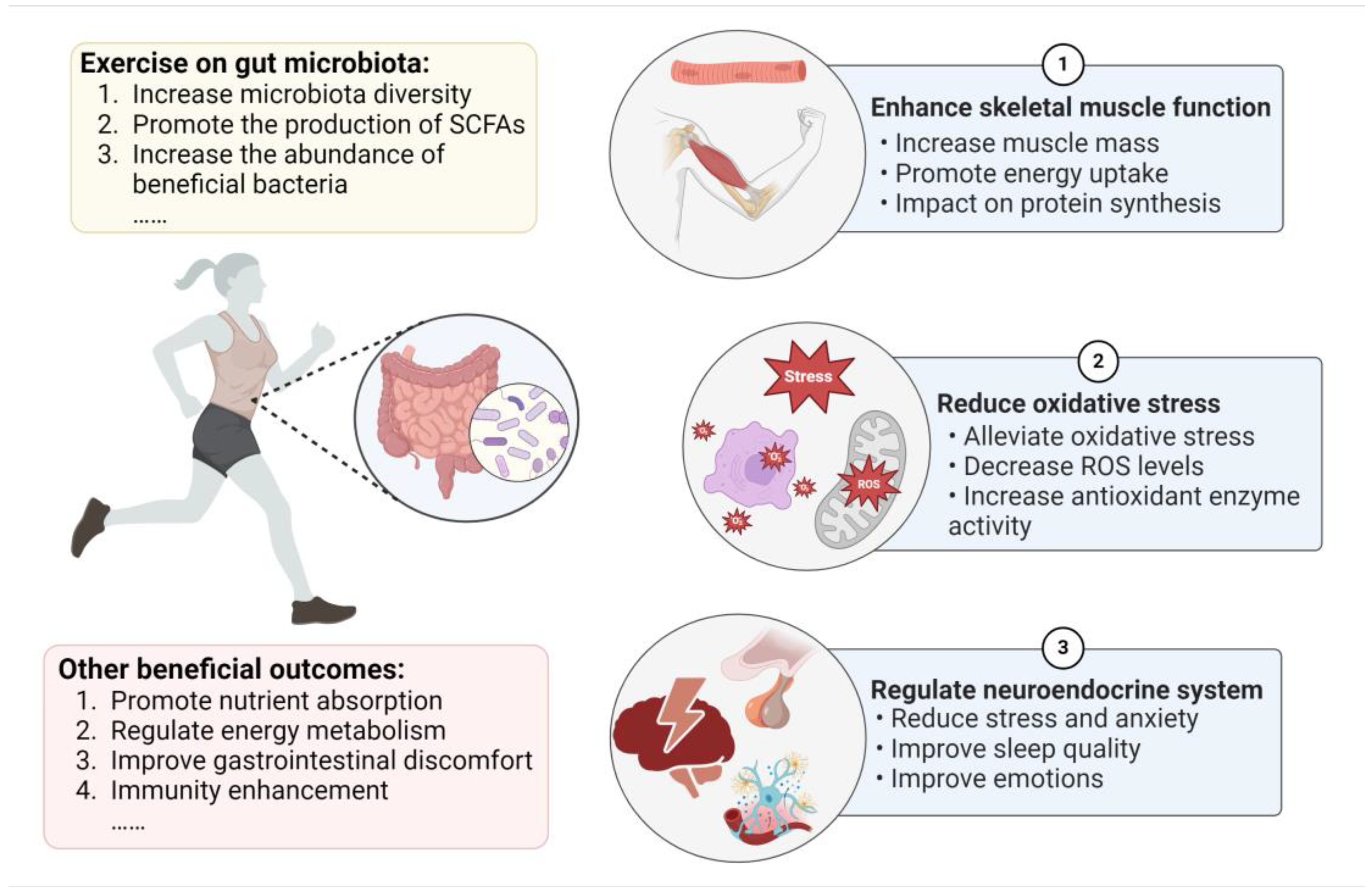
2.1. Exercise Induces Changes in the Gut Microbiota
2.2. Negative Effects of Excessive Exercise on Gut Health
3. The Mechanisms of the Gut Microbiota with Respect to Exercise Performance
3.1. Gut Microbiota Enhances Skeletal Muscle Function
3.2. Gut Microbiota Reduces Oxidative Stress
3.3. Gut Microbiota Impacts the Neuroendocrine System
4. Dietary Recommendations for Targeting of the Gut Microbiota
4.1. Dietary Strategies
4.1.1. Carbohydrates
4.1.2. Protein
4.1.3. Fat
4.2. Probiotics and Synbiotics
| Intervention | Exercise Type | Study Model | Duration | Results | Reference |
|---|---|---|---|---|---|
| Veillonella atypica | Exhaustive treadmill runtime | Mice | 2 days | Blood SCFAs concentration ↑ Lactate utilization ↑ Treadmill performance ↑ The Cori cycle ↑ | Scheiman et al., 2019 [15] |
| Lactobacillus plantarum PS128 | Triathlon championship | Triathletes | 3 weeks | TNF-α, IFN-γ, IL-8, and IL-6 cytokine levels ↓ IL-10 cytokines levels ↑ Oxidative stress ↓ Thioredoxin and component 5a ↑ Free amino acid content ↑ | Huang et al., 2019 [79] |
| Inactivated Bacillus coagulans | A self-defense course | Male soldiers | 2 weeks | IL-10 and IFN-γ concentrations ↑ Vertical jump power ↑ Lower-body power and short-distance speed ↑ | Hoffman et al., 2019 [136] |
| Lactobacillus plantarum PS128 | Usual training | Triathletes | 4 weeks | Acetic acid, propionic acid, and butyric acid ↑ Endurance capacity ↑ No effect on VO2max | Huang et al., 2020 [128] |
| Bifidobacterium longum subsp. longum Olympic No. 1 (OLP-01) | Usual training | Well-trained runners | 5 weeks | 12-min Cooper’s test running distance ↑ No change in the abundance of the gut microbiota. | Lin et al., 2020 [137] |
| Lactobacillus salivarius (SA-03) | Swimming exercise endurance test | Mice | 4 weeks | Average exhaustive swim time ↑ Muscle strength and endurance performance ↑ Hepatic and muscular glycogen storage ↑ Serum lactate, blood urea nitrogen, ammonia, and creatine kinase levels ↓ | Lee et al., 2020 [131] |
| Commercially available probiotic (PRO) | Usual training | Male elite cyclists | 90 days | The incidence and severity of gastrointestinal tract symptoms ↓ No significant changes in VO2max, mean levels of IL-6, or TNF-α. | Pugh et al., 2020 [138] |
| Lactobacillus casei | Usual training | Male badminton players | 6 weeks | Anxiety and stress levels ↓ Aerobic capacity ↑ No significant difference in energy intake and macronutrients. | Salleh et al., 2021 [98] |
| Probiotic blend | Usual training | Male elite cyclists | 6 weeks | Incidence of gastrointestinal Symptoms ↓ No significant changes in IL-6 and TNF-α levels. | Schreiber et al., 2021 [139] |
| Probiotic (Ultrabiotic 60TM) and Saccharomyces boulardii | During competition season | Elite male rugby athletes | 17 weeks | Muscle soreness and leg heaviness scores ↓ Sleep quantity, quality, and motivation scores ↑ | Harnett et al., 2021 [99] |
| Probiotic (SANPROBI BARRIER) | During a marathon | Runners | 3 months | Incidence of constipation ↓ Sodium, potassium, iron concentration, and HDL cholesterol ↑ LDL cholesterol and triglyceride concentration ↓ | Smarkusz-Zarzecka et al., 2022 [140] |
| Yogurt with Bifidobacterium animalis ssp. lactis BB-12 | Usual training | Female taekwondo athletes | 8 weeks | L-arginine biosynthesis I, fatty acid biosynthesis and oxidation, and L-isoleucine biosynthesis III pathways ↑ Tyrosine degradation I ↓ | Zhu et al., 2023 [132] |
| Heat-killed Lactiplantibacillus plantarum TWK10 | Fixed-intensity exercise challenge | Healthy males | 6 weeks | Exercise endurance time ↑ Muscle weight and muscle strength ↑ Serum lactate and ammonia levels during exercise ↓ | Cheng et al., 2023 [141] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Grice, E.A.; Segre, J.A. The human microbiome: Our second genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Anton. Leeuw. Int. J. G. 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Lensu, S.; Pekkala, S. Gut microbiota, microbial metabolites and human physical performance. Metabolites 2021, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the microbiota-gut-brain axis: Focus on psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Medina-Rodriguez, E.M.; Cruz, A.A.; De Abreu, J.C.; Beurel, E. Stress, inflammation, microbiome and depression. Pharmacol. Biochem. Behav. 2023, 227–228, 173561. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhang, S.; Peng, X.; Yang, W.; Xu, Y.; Wu, P.; Chen, J.; Cai, Y.; Zhou, J. Characteristics of the gut microbiota in professional martial arts athletes: A comparison between different competition levels. PLoS ONE 2019, 14, e0226240. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omic analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Hughes, R.L.; Holscher, H.D. Fueling gut microbes: A review of the interaction between diet, exercise, and the gut microbiota in athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Plant-derived bioactive components regulate gut microbiota to prevent depression and depressive-related neurodegenerative diseases: Focus on neurotransmitters. Trends Food Sci. Technol. 2022, 129, 581–590. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Cordero, A.; Masiá, M.D.; Galve, E. Physical exercise and health. Rev. Esp. Cardiol. 2014, 67, 748–753. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic exercise training with brisk walking increases intestinal Bacteroides in healthy elderly women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The connection between physical exercise and gut microbiota: Implications for competitive sports athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut microbiota composition is related to cardiorespiratory fitness in healthy young adults. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, M.; Zha, Y.; Yang, K.; Tong, Y.; Wang, S.; Lu, Q.; Ning, K. Gut microbiota and inflammation patterns for specialized athletes: A multi-cohort study across different types of sports. mSystems 2023, 8, e00259-23. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Hansen, E.E.; Manchester, J.K.; Coutinho, P.M.; Henrissat, B.; Fulton, R.; Latreille, P.; Kim, K.; Wilson, R.K.; Gordon, J.I. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. USA 2007, 104, 10643–10648. [Google Scholar] [CrossRef]
- Kern, T.; Blond, M.B.; Hansen, T.H.; Rosenkilde, M.; Quist, J.S.; Gram, A.S.; Ekstrøm, C.T.; Hansen, T.; Stallknecht, B. Structured exercise alters the gut microbiota in humans with overweight and obesity-a randomized controlled trial. Int. J. Obesity 2020, 44, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hampton-Marcell, J.T.; Eshoo, T.W.; Cook, M.D.; Gilbert, J.A.; Horswill, C.A.; Poretsky, R. Comparative analysis of gut microbiota following changes in training volume among swimmers. Int. J. Sports Med. 2020, 41, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, E.V.; Grandy, S.A.; Langille, M.G.I. Moderate exercise has limited but distinguishable effects on the mouse microbiome. mSystems 2017, 2, e00006-17. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Stuempfle, K.J.; Hoffman, M.D. Gastrointestinal distress is common during a 161-km ultramarathon. J. Sports Sci. 2015, 33, 1814–1821. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Vargas, N.; Marino, F. Heat stress, gastrointestinal permeability and interleukin-6 signaling–implications for exercise performance and fatigue. Temperature 2016, 3, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in ultra-marathon running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The influence of gut microbiota on oxidative stress and the immune system. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Imdad, S.; Lim, W.; Kim, J.-H.; Kang, C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef]
- Otsuka, T.; Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin-releasing factor neurons, and anxiety- and depressive-like behaviors in rats. Behav. Brain Res. 2016, 298, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016, 6, 31786. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.; Dejong, C.; Rensen, S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Racine, H.L.; Serrat, M.A. The actions of IGF-1 in the growth plate and its role in postnatal bone elongation. Curr. Osteoporos. Rep. 2020, 18, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Sun, J.; Qiu, X.; Zhang, Y.; Wang, J.; Wang, Q.; Huang, J.; Ge, L.; Liu, Z. The intestinal microbiota contributes to the growth and physiological state of muscle tissue in piglets. Sci. Rep. 2021, 11, 11237. [Google Scholar] [CrossRef] [PubMed]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Kim, Y.J.; Baek, J.H.; Kim, H.S.; Kim, I.Y.; Seong, J.K. Microbiota influences host exercise capacity via modulation of skeletal muscle glucose metabolism in mice. Exp. Mol. Med. 2023, 55, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Dong, Z.; Wang, N.; Wang, W.; Tao, L.; Cao, W.; Liu, Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012, 61, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, M.; Li, T.; Dong, N.; Yi, L.; Zhang, Q.; Mi, M. GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119300. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obesity 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Karaki, S.; Kuwahara, A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion 2014, 89, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guo, Q.; Zhou, X.; Duan, Y.; Yang, Y.; Gong, S.; Han, M.; Liu, Y.; Yang, Z.; Chen, Q.; et al. Role of brain-gut-muscle axis in human health and energy homeostasis. Front. Nutr. 2022, 9, 947033. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-activated protein kinase and stimulation of energy metabolism by acetic acid in L6 myotube cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef]
- Kang, L.; Fang, X.; Song, Y.H.; He, Z.X.; Wang, Z.J.; Wang, S.L.; Li, Z.S.; Bai, Y. Neutrophil-epithelial crosstalk during intestinal inflammation. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 1257–1267. [Google Scholar] [CrossRef]
- Webb, R.; Hughes, M.G.; Thomas, A.W.; Morris, K. The ability of exercise-associated oxidative stress to trigger redox-sensitive signalling responses. Antioxidants 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of running exercise on oxidative stress biomarkers: A systematic review. Front. Physiol. 2021, 11, 610112. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Aoi, W.; Matsumoto, K.; Kato, Y.; Kobayashi, Y.; Kuwahata, M. High-intensity exercise impairs intestinal barrier function by generating oxidative stress. J. Clin. Biochem. Nutr. 2024, 74, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Mazani, M.; Nemati, A.; Baghi, A.N.; Amani, M.; Haedari, K.; Alipanah-Mogadam, R. The effect of probiotic yoghurt consumption on oxidative stress and inflammatory factors in young females after exhaustive exercise. J. Pak. Med. Assoc. 2018, 68, 1748–1754. [Google Scholar] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Macarro, M.; Ávila-Gandía, V.; Pérez-Piñero, S.; Cánovas, F.; García-Muñoz, A.M.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; Luque-Rubia, A.J.; Climent, E.; Genovés, S.; et al. Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants 2021, 10, 323. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- MacKinnon, L.T. Special feature for the Olympics: Effects of exercise on the immune system: Overtraining effects on immunity and performance in athletes. Immunol. Cell Biol. 2000, 78, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Buguet, A.; Cespuglio, R.; Radomski, M.W. Sleep and stress in man: An approach through exercise and exposure to extreme environments. Can. J. Physiol. Pharmacol. 1998, 76, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L.; Juliff, L.E. Sleep, sport, and the brain. Prog. Brain Res. 2017, 234, 13–31. [Google Scholar] [PubMed]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, I.C.P.D.M.; Mara, L.S.D.; Lima, W.C.D.; Carvalho, T.D. Relationship of the overtraining syndrome with stress, fatigue, and serotonin. Rev. Bras. Med. Esporte 2005, 11, 367–372. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Brain-gut-microbiota axis and mental health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zhu, H.; Zou, R.; Kong, Q.; Xu, M.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. An in vitro screening method for probiotics with antidepressant-like effect using the enterochromaffin cell model. Food Funct. 2021, 12, 646–655. [Google Scholar] [CrossRef]
- Dey, S.; Singh, R.H.; Dey, P.K. Exercise training: Significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol. Behav. 1992, 52, 1095–1099. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Exercise, serum free tryptophan, and central fatigue. J. Nutr. 2006, 136, 553S–559S. [Google Scholar] [CrossRef] [PubMed]
- Vargas, N.T.; Marino, F. A Neuroinflammatory model for acute fatigue during exercise. Sports Med. 2014, 44, 1479–1487. [Google Scholar] [CrossRef]
- Proschinger, S.; Freese, J. Neuroimmunological and neuroenergetic aspects in exercise-induced fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zou, R.; Wang, L.; Chen, Y.; Qian, X.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Wang, G.; et al. Multi-probiotics ameliorate major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J. Adv. Res. 2023, 45, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Liao, S.; Lai, M.; Peng, L.; Song, G. Reduction in the choking phenomenon in elite diving athletes through changes in gut microbiota induced by yogurt containing Bifidobacterium animalis subsp. lactis BB-12: A quasi experimental study. Microorganisms 2020, 8, 597. [Google Scholar] [PubMed]
- Zhu, J.; Zhu, Y.; Song, G. Effect of probiotic yogurt supplementation (Bifidobacterium animalis ssp. lactis BB-12) on gut microbiota of female taekwondo athletes and its relationship with exercise-related psychological fatigue. Microorganisms 2023, 11, 1403. [Google Scholar]
- Salleh, R.M.; Kuan, G.; Aziz, M.N.A.; Rahim, M.R.A.; Rahayu, T.; Sulaiman, S.; Kusuma, D.W.Y.; Adikari, A.M.G.C.P.; Razam, M.S.M.; Radhakrishnan, A.K.; et al. Effects of probiotics on anxiety, stress, mood and fitness of badminton players. Nutrients 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.E.; Pyne, D.B.; McKune, A.J.; Penm, J.; Pumpa, K.L. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J. Sci. Med. Sport 2021, 24, 195–199. [Google Scholar] [CrossRef]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential health effects on inflammatory, immunological and stress parameters in professional soccer players and sedentary individuals after consuming a synbiotic. A triple-blinded, randomized, placebo-controlled pilot study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Cermak, N.M.; Van Loon, L.J.C. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkienė, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Yin, M.O.L.; Heaney, L.M. The athlete and gut microbiome: Short-chain fatty acids as potential ergogenic aids for exercise and training. Int. J. Sports Med. 2021, 42, 1143–1158. [Google Scholar] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nut. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Donatto, F.F.; Prestes, J.; Frollini, A.B.; Palanch, A.C.; Verlengia, R.; Cavaglieri, C.R. Effect of oat bran on time to exhaustion, glycogen content and serum cytokine profile following exhaustive exercise. J. Int. Soc. Sports Nutr. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.P.; Koido, M.; Kawaguchi, M.; Timm, D.; Ozeki, M.; Yamada, M.; Mitsuya, T.; Okubo, T. Lifestyle related changes with partially hydrolyzed guar gum dietary fiber in healthy athlete individuals—A randomized, double-blind, crossover, placebo-controlled gut microbiome clinical study. J. Funct. Foods 2020, 72, 104067. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose-glucose composite carbohydrates and endurance performance: Critical review and future perspectives. Sports Med. 2015, 45, 1561–1576. [Google Scholar] [CrossRef]
- Odell, O.J.; Wallis, G.A. The application of lactose in sports nutrition. Int. Dairy J. 2021, 116, 104970. [Google Scholar] [CrossRef]
- Mancin, L.; Rollo, I.; Mota, J.F.; Piccini, F.; Carletti, M.; Susto, G.A.; Valle, G.; Paoli, A. Optimizing microbiota profiles for athletes. Exerc. Sport Sci. Rev. 2021, 49, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Egan, B. Protein intake for athletes and active adults: Current concepts and controversies. Nutr. Bulletin 2016, 41, 202–213. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Z.; Wu, W.; Lin, Q.; Liang, Y. High animal protein diet and gut microbiota in human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 6225–6237. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Sun, Y.; Zhang, X. Combined physical exercise and diet: Regulation of gut microbiota to prevent and treat of metabolic disease: A review. Nutrients 2022, 14, 4774. [Google Scholar] [CrossRef]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a protein supplement on the gut microbiota of endurance athletes: A randomized, controlled, double-blind pilot study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Zhu, W. Temporal microbiota changes of high-protein diet intake in a tat model. Anaerobe 2017, 47, 218–225. [Google Scholar] [CrossRef]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef]
- Murtaza, N.; Burke, L.; Vlahovich, N.; Charlesson, B.; O’ Neill, H.; Ross, M.; Campbell, K.; Krause, L.; Morrison, M. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients 2019, 11, 261. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Mancin, L.; Wu, G.D.; Paoli, A. Gut microbiota–bile acid–skeletal muscle axis. Trends. Microbiol. 2023, 31, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Santibañez-Gutierrez, A.; Fernández-Landa, J.; Calleja-González, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of probiotic supplementation on exercise with predominance of aerobic metabolism in trained population: A systematic review, meta-analysis and meta-regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.S.F.; Resende Master Student, A.S.; West, N.P.; Lancha, A.H. Probiotics and sports: A new magic bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef]
- Tavares-Silva, E.; Caris, A.V.; Santos, S.A.; Ravacci, G.R.; Thomatieli-Santos, R.V. Effect of multi-strain probiotic supplementation on URTI symptoms and cytokine production by monocytes after a marathon race: A randomized, double-blind, placebo study. Nutrients 2021, 13, 1478. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. Lactobacillus plantarum PS128 improves physiological adaptation and performance in triathletes through gut microbiota modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hutchinson, N.; Yin, J.; Guan, M.; Wang, Z.; Chen, P.; Yang, S.; Crane, J.D.; Zhang, K.; Li, J. Engineered Bacillus subtilis as oral probiotics to target circulating lactic acid. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Zhao, L.; Liu, Z.; Chen, L.; Liu, C. Comparison of the effects of prebiotics and synbiotics supplementation on the immune function of male university football players. Nutrients 2023, 15, 1158. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Hoffman, M.W.; Zelicha, H.; Gepner, Y.; Willoughby, D.S.; Feinstein, U.; Ostfeld, I. The effect of 2 weeks of inactivated probiotic Bacillus coagulans on endocrine, inflammatory, and performance responses during self-defense training in soldiers. J. Strength Cond. Res. 2019, 33, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Hsu, Y.J.; Ho, H.H.; Chang, Y.C.; Kuo, Y.W.; Yeh, Y.T.; Tsai, S.Y.; Chen, C.W.; Chen, J.F.; Huang, C.C.; et al. Bifidobacterium longum subsp. longum OLP-01 supplementation during endurance running training improves exercise performance in middle- and long-distance runners: A double-blind controlled trial. Nutrients 2020, 12, 1972. [Google Scholar] [PubMed]
- Pugh, J.N.; Wagenmakers, A.J.M.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: A randomized, double-blind, placebo-controlled crossover trial. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Tamir, S.; Golan, R.; Weinstein, A.; Weinstein, Y. The effect of probiotic supplementation on performance, inflammatory markers and gastro-intestinal symptoms in elite road cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 36. [Google Scholar] [CrossRef]
- Smarkusz-Zarzecka, J.; Ostrowska, L.; Leszczyńska, J.; Cwalina, U. Effect of a multi-strain probiotic supplement on gastrointestinal symptoms and serum biochemical parameters of long-distance runners: A randomized controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 9363. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lee, C.C.; Lee, M.C.; Hsu, H.Y.; Lin, J.S.; Huang, C.C.; Watanabe, K. Effects of heat-killed Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, and muscle growth in healthy male adults. Physiol. Rep. 2023, 11, e15835. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, H.; Song, Z.; Liu, Y.; Zhang, X. Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota. Foods 2024, 13, 1680. https://doi.org/10.3390/foods13111680
Zhang L, Li H, Song Z, Liu Y, Zhang X. Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota. Foods. 2024; 13(11):1680. https://doi.org/10.3390/foods13111680
Chicago/Turabian StyleZhang, Li, Haoyu Li, Zheyi Song, Yanan Liu, and Xin Zhang. 2024. "Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota" Foods 13, no. 11: 1680. https://doi.org/10.3390/foods13111680
APA StyleZhang, L., Li, H., Song, Z., Liu, Y., & Zhang, X. (2024). Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota. Foods, 13(11), 1680. https://doi.org/10.3390/foods13111680






