Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Chromatic Determination
2.3. Pretreatments of Kidney Seeds
2.4. Extraction of Free Phenols
2.5. Extraction of Bound Phenols
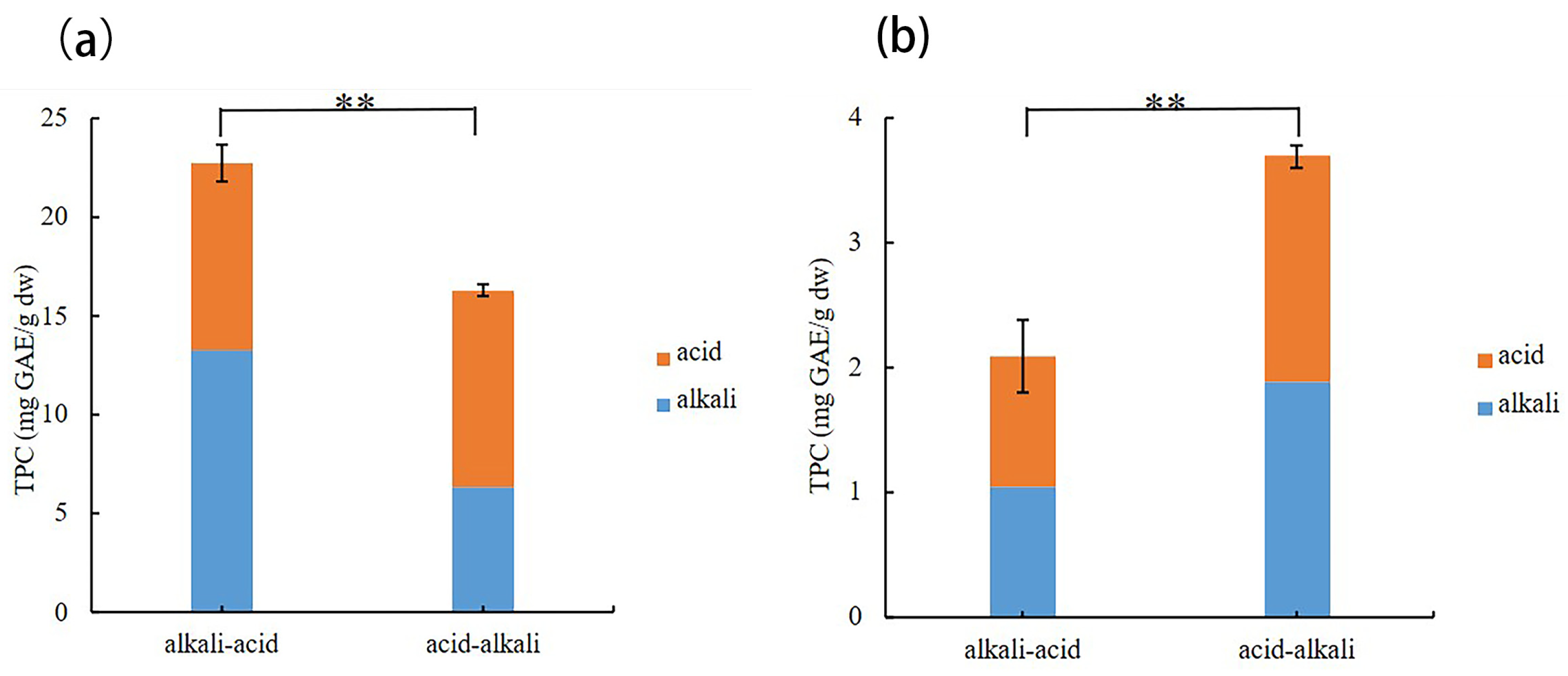
2.5.1. Acid Hydrolysis and Acid-Alkali Sequential Hydrolysis Treatment
2.5.2. Alkaline Hydrolysis and Alkali-Acid Sequential Hydrolysis Treatment
2.6. Determination of the Total Content of Phenol, Flavonoid and Anthocyanin
2.6.1. The Total Phenol Content
2.6.2. The Total Flavonoid Content
2.6.3. The Total Anthocyanin Content
2.7. Determination of Antioxidant Activities
2.7.1. The Ability to Eliminate DPPH Free Radicals
2.7.2. The Ability to Eliminate ABTS Free Radicals
2.7.3. The Ferric-Reducing Antioxidant Power
2.8. Analysis of Phenolic Compounds by UHPLC-QE-MS
2.9. Statistical Analysis
3. Results
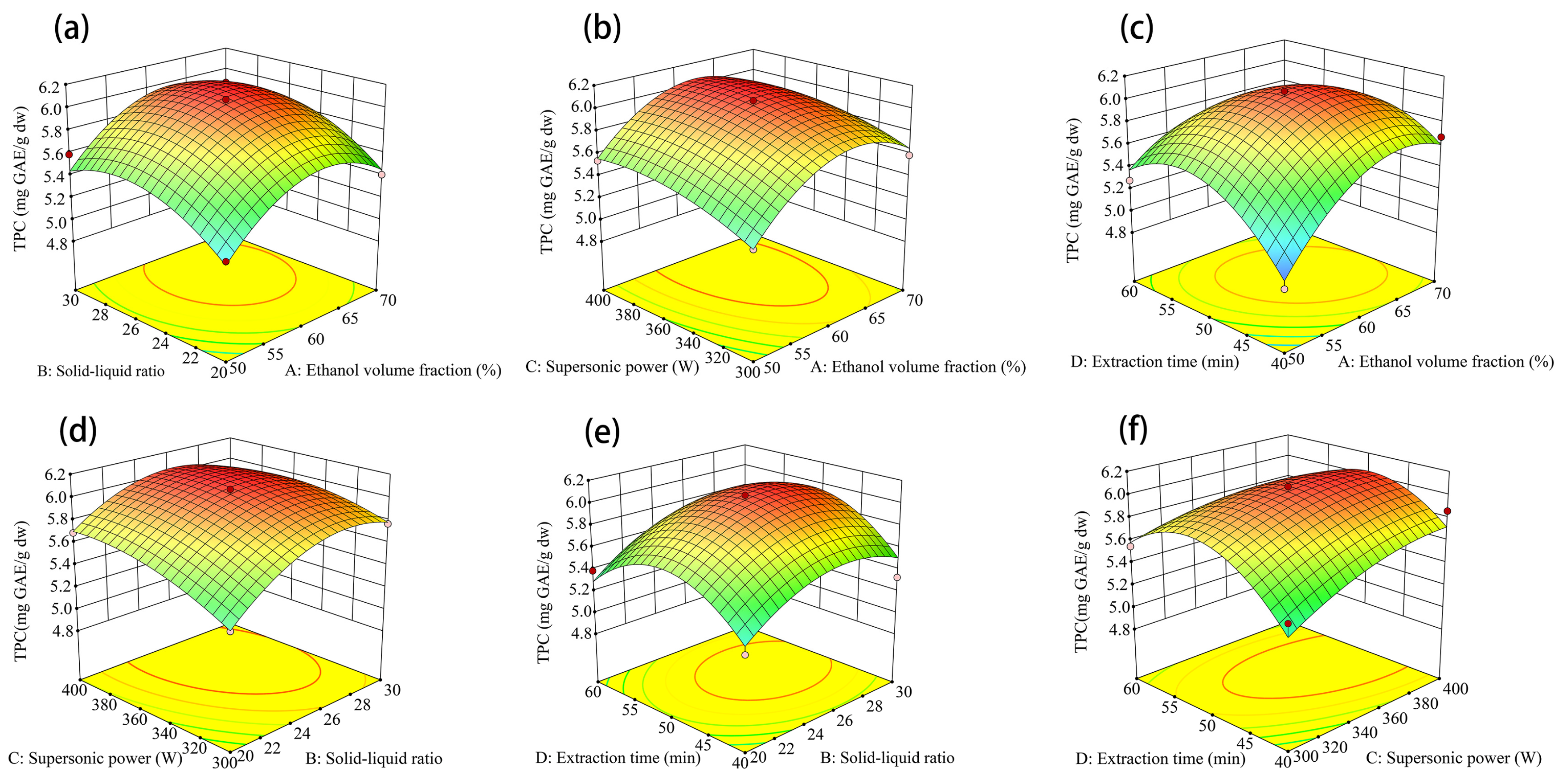
3.1. Optimization of Phenolic Extraction
3.1.1. Optimization of Free Phenols Extraction
3.1.2. Extraction Sequence of Bound Phenols
3.2. Chromatic Feature
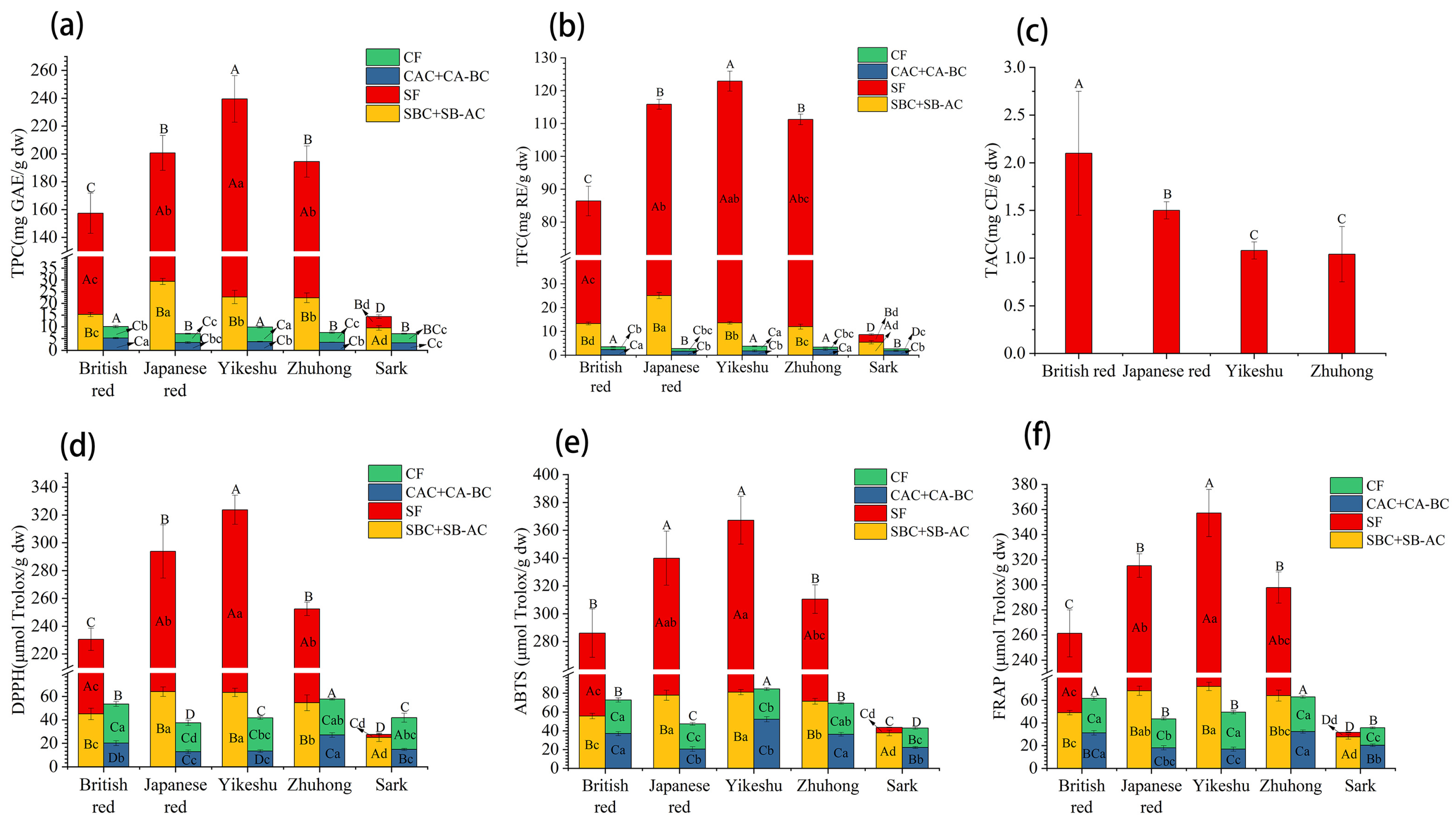
3.3. Total Content and Antioxidant Activities of Phenols in Extractions
3.3.1. Total Phenol Content
3.3.2. Antioxidant Activities of Phenols
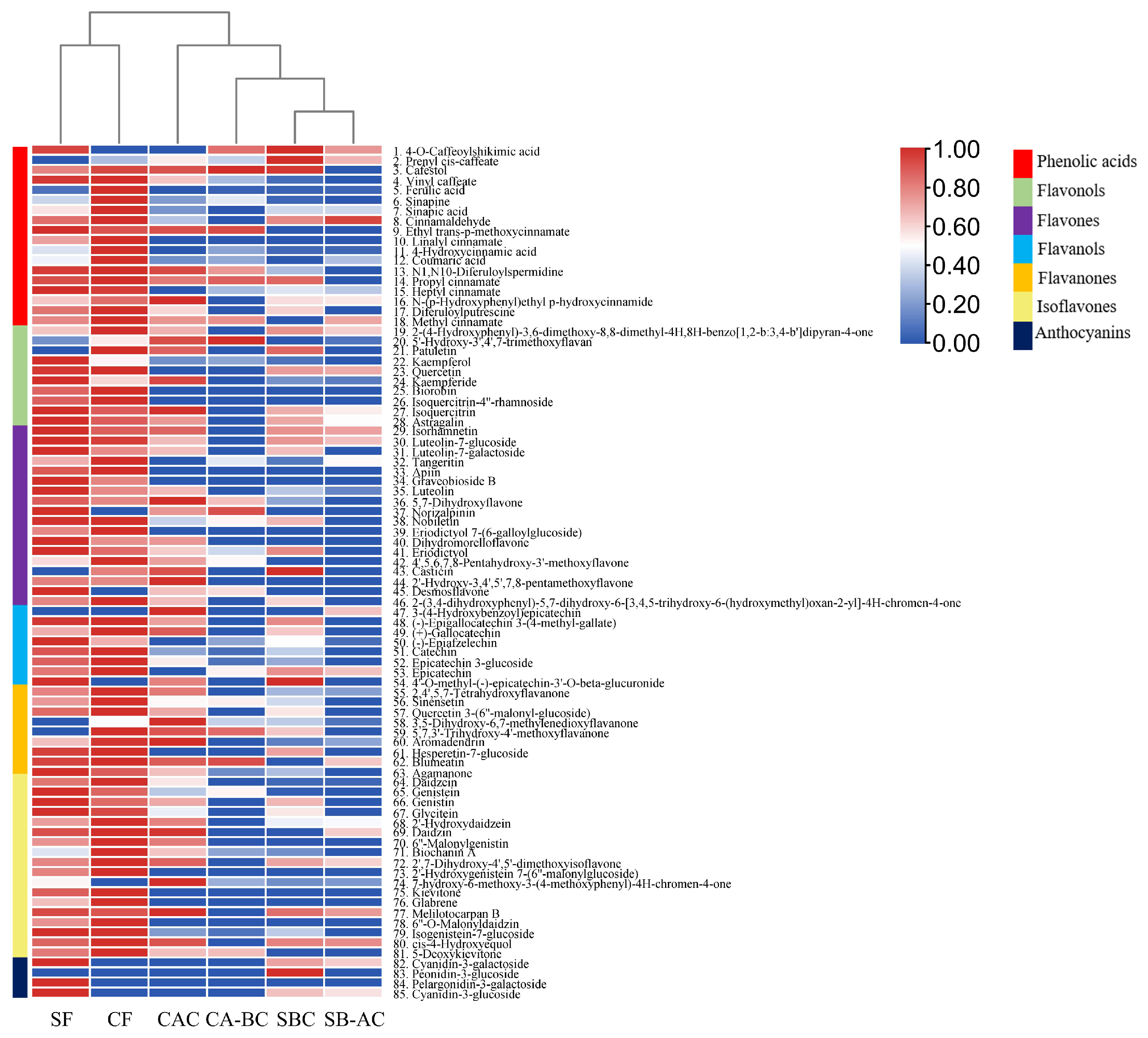
3.4. Analyses of the Composition of Phenols in the ‘Yikeshu’ Seed
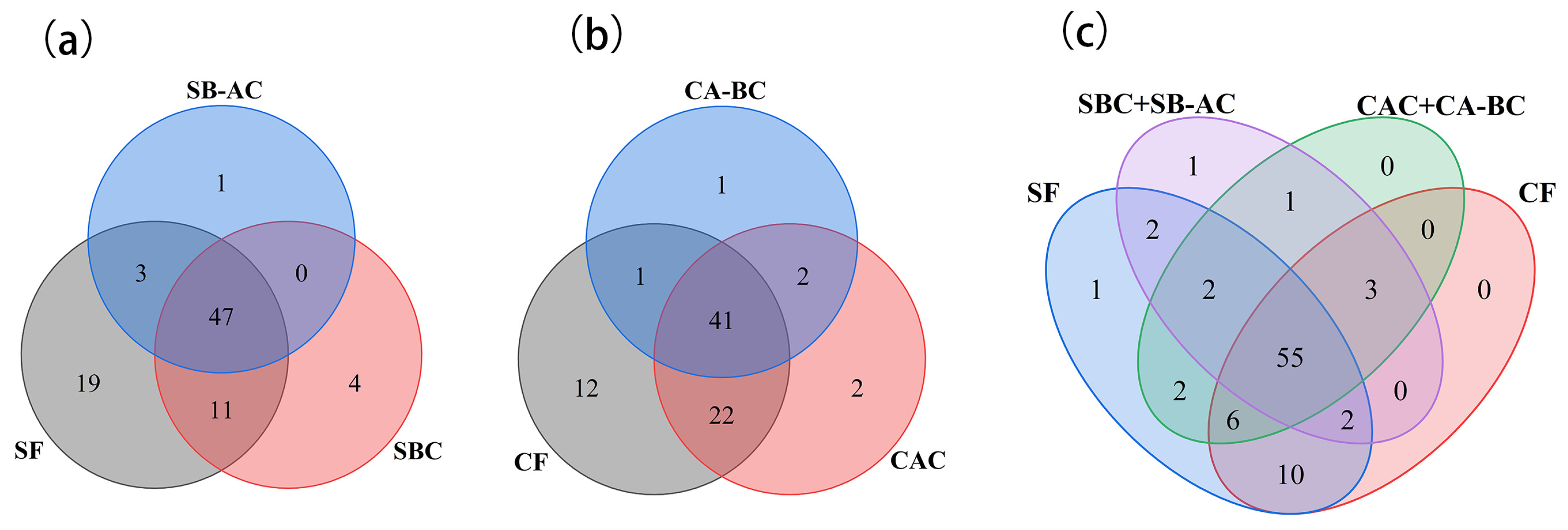
3.4.1. General Analysis of Phenolic Compounds in Different Samples
3.4.2. Screening of Characteristic Phenolic Compounds in Free or Bound States Extracted from the Seed Coat and the Cotyledon
4. Discussion
4.1. Extraction and Total Contents of Phenols in Kidney Bean Seeds
4.2. Correlation among Total Phenol Contents, Antioxidant Activities and Color Attributes
4.3. Composition of Free and Bound Phenolic Compounds in the Seed Coat and Cotyledon Represented by ‘Yikeshu’
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choe, U.; Chang, L.; Ohm, J.B.; Chen, B.; Rao, J. Structure modification, functionality and interfacial properties of kidney bean (Phaseolus vulgaris L.) protein concentrate as affected by post-extraction treatments. Food Hydrocoll. 2022, 133, 108000. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Han, J.; Zheng, W.B.; Wang, Y.; Zhang, H.W.; Zhang, L.P.; Zuo, Y.H.; Cui, S.P. Evaluation of protein nutritional value of kidney bean in Heilongjiang province. Food Sci. Technol. 2016, 41, 99–104. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green pea (Pisum sativum L.) hull polyphenol extracts ameliorate dss-induced colitis through keap1/nrf2 pathway and gut microbiota modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef]
- Kawahara, S.I.; Ishihara, C.; Matsumoto, K.; Senga, S.; Kawaguchi, K.; Yamamoto, A.; Suwannachot, J.; Hamauzu, Y.; Makabe, H.; Fujii, H. Identification and characterization of oligomeric proanthocyanidins with significant anti-cancer activity in adzuki beans (Vigna angularis). Heliyon 2019, 5, e02610. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; De Mejía, E.G.; Martín-Cabrejas, M.A. Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef]
- Inman, C.; Lourith, N.; Kanlayavattanakul, M. Alternative application approach on black bean: Hair coloring product. Chem. Biol. Technol. Agric. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Rojasawasthien, T.; Shirakawa, T.; Washio, A.; Tsujisawa, T.; Matsubara, T.; Inoue, A.; Takahama, U.; Nakashima, K.; Kokabu, S. Vignacyanidin polyphenols isolated from Vigna Angularis bean promote osteoblast differentiation. In Vivo 2021, 35, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Plaza, A.; Méndez, D.; Carrasco, B.; Tellería, F.; Palomo, I.; Fuentes, E. Antioxidant capacity and antiplatelet activity of aqueous extracts of common bean (Phaseolus vulgaris L.) obtained with microwave and ultrasound assisted extraction. Plants 2022, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.S.; Barros, A.I. Nutrients, antinutrients, phenolic composition, and antioxidant activity of common bean cultivars and their potential for food applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Cui, S.W.; Li, Y.; Xu, S.; Wu, Y.; Wang, J.; Bai, Z.; et al. Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food Chem. Toxicol. 2017, 108, 467–477. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Ge, Y.Y.; Gunaratne, A.; Kong, K.W.; Li, H.B.; Gul, K.; Kumara, K.; Arachchi, L.V.; Zhu, F.; Corke, H.; et al. Phenolic profiles, antioxidant activities, and antiproliferative activities of different mung bean (Vigna radiata) varieties from Sri Lanka. Food Biosci. 2020, 37, 100705. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Gao, F.; Liu, Y.; Lang, S.; Wang, C.; Zhang, D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023, 94, 106311. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Lin, H.F.; Wang, X.; Yuan, Y.; Yin, J.Y.; Song, X.X. Comprehensive analysis in the nutritional composition, phenolic species and in vitro antioxidant activities of different pea cultivars. Food Chem. X 2023, 17, 100599. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Paranavitana, L.; Oh, W.Y.; Yeo, J.; Shahidi, F. Determination of soluble and insoluble-bound phenolic compounds in dehulled, whole, and hulls of green and black lentils using electrospray ionization (ESI)-MS/MS and their inhibition in DNA strand scission. Food Chem. 2021, 361, 130083. [Google Scholar] [CrossRef]
- Yuan, L.; Niu, H.; Yun, Y.; Tian, J.; Lao, F.; Liao, X.; Gao, Z.; Ren, D.; Zhou, L. Analysis of coloration characteristics of Tunisian soft-seed pomegranate arils based on transcriptome and metabolome. Food Chem. 2022, 370, 131270. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, W.; Li, H.; Deng, Z.; Zhang, B. Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). J. Funct. Foods 2017, 32, 296–312. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, L.; Zhan, C.; Wu, X.; Li, X. Extraction optimization and antioxidant activity assessment of polyphenols from seed coat of kidney bean. China Food Addit. 2022, 33, 1–7. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bakar, M.F.A.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-throughput screening and characterization of phenolic compounds in stone fruits waste by lc-esi-qtof-ms/ms and their potential antioxidant activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Xie, X.; Cai, Y.; Li, J.; Feng, Y.; Zhang, Y. Integrated metabolomics and transcriptomics analyses reveal the molecular mechanisms underlying the accumulation of anthocyanins and other flavonoids in cowpea pod (Vigna unguiculata L.). J. Agric. Food Chem. 2020, 68, 9260–9275. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.N.; Willett, J.L.; Lesch, W.C.; Tangsrud, R.R.; Biswas, A. Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res. Int. 2010, 43, 516–519. [Google Scholar] [CrossRef]
- Capistrán-Carabarin, A.; Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular-and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef]
- Wang, Y.K.; Zhang, X.; Chen, G.L.; Yu, J.; Yang, L.Q.; Gao, Y.Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Hung, P.V.; Yen, N.T.H.; Phi, N.T.L.; Tien, N.P.H.; Trung, N.T.T. Nutritional composition, enzyme activities and bioactive compounds of mung bean (Vigna radiata L.) germinated under dark and light conditions. LWT 2020, 133, 110100. [Google Scholar] [CrossRef]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic profiles of ten Australian faba bean varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Characterization of bioactive phenolics and antioxidant capacity of edible bean extracts of 50 Fabaceae populations grown in Thailand. Foods 2021, 10, 3118. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ci, Z.; Feng, S.; Wu, S.; Kojima, M. Characteristics of functional components and antioxidant activity of 28 common beans. J. Food Nutr. Res. 2018, 6, 439–444. [Google Scholar] [CrossRef]
- Bouhafsoun, A.; Yilmaz, M.A.; Boukeloua, A.; Temel, H.; Harche, M.K. Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci. Technol. 2018, 38, 242–247. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, S.M.; Lee, J.H.; Lim, S.T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xie, L.; Deng, Z.; Zhang, B.; Li, H. Stability and antioxidant activity of 10 isoflavones and anthocyanidins during in vitro digestion. Food Biosci. 2023, 56, 103189. [Google Scholar] [CrossRef]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, M.P.; Carretero, M.E. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar] [CrossRef]
- Pitura, K.; Arntfield, S.D. Characteristics of flavonol glycosides in bean (Phaseolus vulgaris L.) seed coats. Food Chem. 2019, 272, 26–32. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT 2020, 122, 109024. [Google Scholar] [CrossRef]


| Samples | British Red | Japanese Red | Yikeshu | Zhuhong | Sark | |
|---|---|---|---|---|---|---|
| Appearance |  |  |  |  |  | |
| Fitting graph |  |  |  |  |  | |
| Color attributes | L* | 38.45 ± 2.06 b | 37.25 ± 1.23 bc | 38.76 ± 1.73 b | 34.57 ± 2.12 c | 80.50 ± 2.15 a |
| a* | 28.49 ± 1.13 a | 21.51 ± 1.31 b | 18.77 ± 2.64 b | 20.77 ± 1.82 b | 7.74 ± 0.98 c | |
| b* | 6.88 ± 0.61 b | 3.61 ± 0.20 c | 6.09 ± 0.23 b | 7.32 ± 0.90 b | 21.68 ± 0.96 a | |
| C* | 29.00 ± 0.69 a | 21.80 ± 1.28 b | 19.95 ± 2.12 b | 22.01 ± 2.00 b | 22.71 ± 1.55 b | |
| H* | 4.06 ± 0.54 b | 5.90 ± 0.41 a | 2.97 ± 0.31 c | 2.72 ± 0.47 c | −2.82 ± 0.49 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhan, C.; Yu, X.; Hu, X.; Gao, S.; Zang, Y.; Yao, D.; Wang, C.; Xu, J. Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region. Foods 2024, 13, 1704. https://doi.org/10.3390/foods13111704
Zhu L, Zhan C, Yu X, Hu X, Gao S, Zang Y, Yao D, Wang C, Xu J. Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region. Foods. 2024; 13(11):1704. https://doi.org/10.3390/foods13111704
Chicago/Turabian StyleZhu, Lei, Chuan Zhan, Xinchu Yu, Xixi Hu, Sibo Gao, Yanqing Zang, Di Yao, Changyuan Wang, and Jingyu Xu. 2024. "Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region" Foods 13, no. 11: 1704. https://doi.org/10.3390/foods13111704
APA StyleZhu, L., Zhan, C., Yu, X., Hu, X., Gao, S., Zang, Y., Yao, D., Wang, C., & Xu, J. (2024). Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region. Foods, 13(11), 1704. https://doi.org/10.3390/foods13111704






