Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer’s Disease in Cellular Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Phenolic Compounds
2.2.2. Determination of Total Phenolic Content
2.2.3. Determination of Flavonoid Content
2.2.4. Determination of Profile and Level of Individual Phenolic Compounds
2.2.5. Chemical-Based Antioxidant Assays
2.2.6. Preparation of the Sorghum Extracts for Cell Treatment
2.2.7. Aβ Oligomer Preparation
2.2.8. Cell Culture
2.2.9. Cytotoxicity of Sorghum Extracts on BE (2)-M17 Cells
2.2.10. Cytoprotectively of Sorghum Extracts
- Negative control group (NC) (the cells treated with the treatment media and the equivalent amount of DMSO and phenol red-free f12, without Aβ42, and without extracts);
- Positive control group (PC) (the cells treated with 20 μM Aβ42, without extracts);
- Extract control group (EC) (the cells treated with the optimum dosage or ½ of the optimum dosage of each extract, without Aβ42);
- Extract-treated group (ET) (the cells treated with the optimum dosage or ½ of the optimum dosage of each extract + 20 μM Aβ42);
- Blank group (no cells, just the treatment media).
2.2.11. Thioflavin-T Aggregation Assay
- Positive control (20 μL Aβ-42 solution in DMSO/F12, 10 μL 60 µM Th-T solution, and 70 μL 1 × TBS).
- Negative control (20 μL DMSO/F-12 solvent only, 10 μL 60 µM Th-T solution, and 70 μL 1 × TBS).
- Extract control group (20 μL DMSO/F-12 solvent only, 10 μL 60 µM Th-T solution, and 70 μL of 1 × TBS/PP extracts in 1 × TBS).
- Combination group (20 μL of the prepared peptides, 10 μL of 60 µM Th-T solution, and 70 μL of 1 × TBS/extract dissolved in 1 × TBS).
2.2.12. Cellular ROS Production Assay
- Blank group: no cells, 100 μL of just the extracts or treatment media (no phenol red DMEM/f12 + 1% FBS);
- Treatment groups: the cells treated with 30 μM Aβ + 70 μL of the extracts (at two non-toxic doses) and the cells treated with 50 μM TBHP diluted with the extracts (at two non-toxic doses);
- Negative control group: the cells treated with 70 μL of the treatment media + 30 μL of no phenol red or DMSO.
- Positive control groups: 30 μM Aβ + 70 μL treatment media and 50 μM TBHP diluted with the treatment media [34].
2.2.13. Determination of Mitochondrial Superoxide Production in Live Cells
- Blank group: no cells, 100 μL of the extracts or treatment media (no phenol red DMEM/f12 + 1% FBS);
- Treatment groups: the cells treated with 30 μM Aβ + 70 μL of the extracts (optimum dosage only) and the cells treated with 50 μM TBHP diluted with the extracts (optimum dosage only);
- Negative control group: the cells treated with 70 μL of the treatment media + 30 μL of no phenol red DMEM/f12;
- Positive control group: 30 μM Aβ + 70 μL treatment media and 50 μM TBHP diluted with the treatment media.
2.3. Statistical Analysis
3. Results
3.1. Polyphenolic and Flavonoid Contents of Sorghum Grain
3.2. Identification and Quantitation of the Individual Polyphenolic Compounds of Sorghum Extracts
3.3. Free Radical-Scavenging Ability of the Extracts
3.4. Cytotoxicity of Sorghum Extracts on BE(2)-M17 Cells
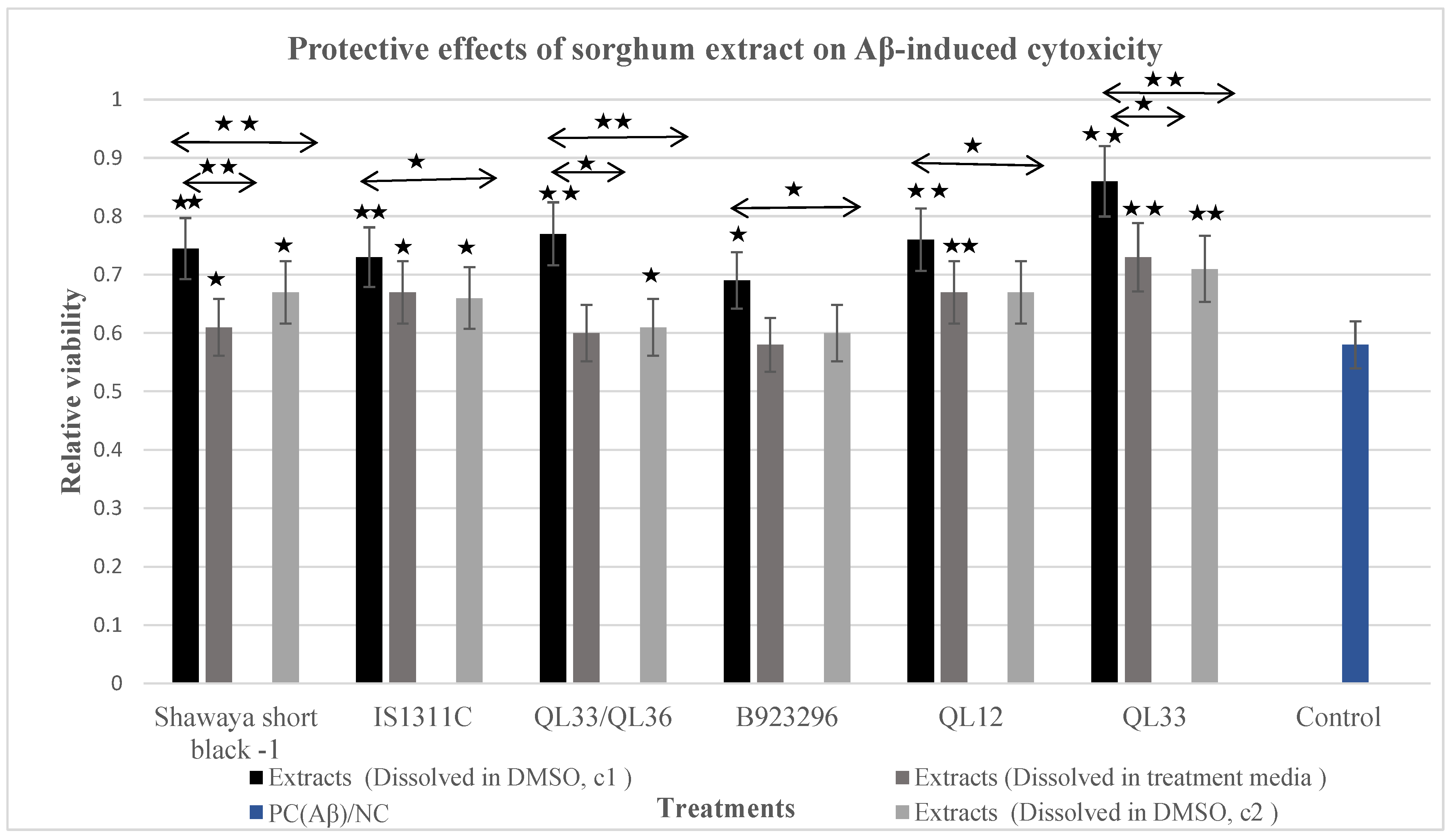
3.5. Protective Effect of DMSO Sorghum Extracts on Aβ-Induced Cytotoxicity
3.6. Protective Effect of Treatment Media Sorghum Extracts on Aβ-Induced Cytotoxicity
3.7. Anti-Aβ Aggregation Effect of Sorghum Extracts
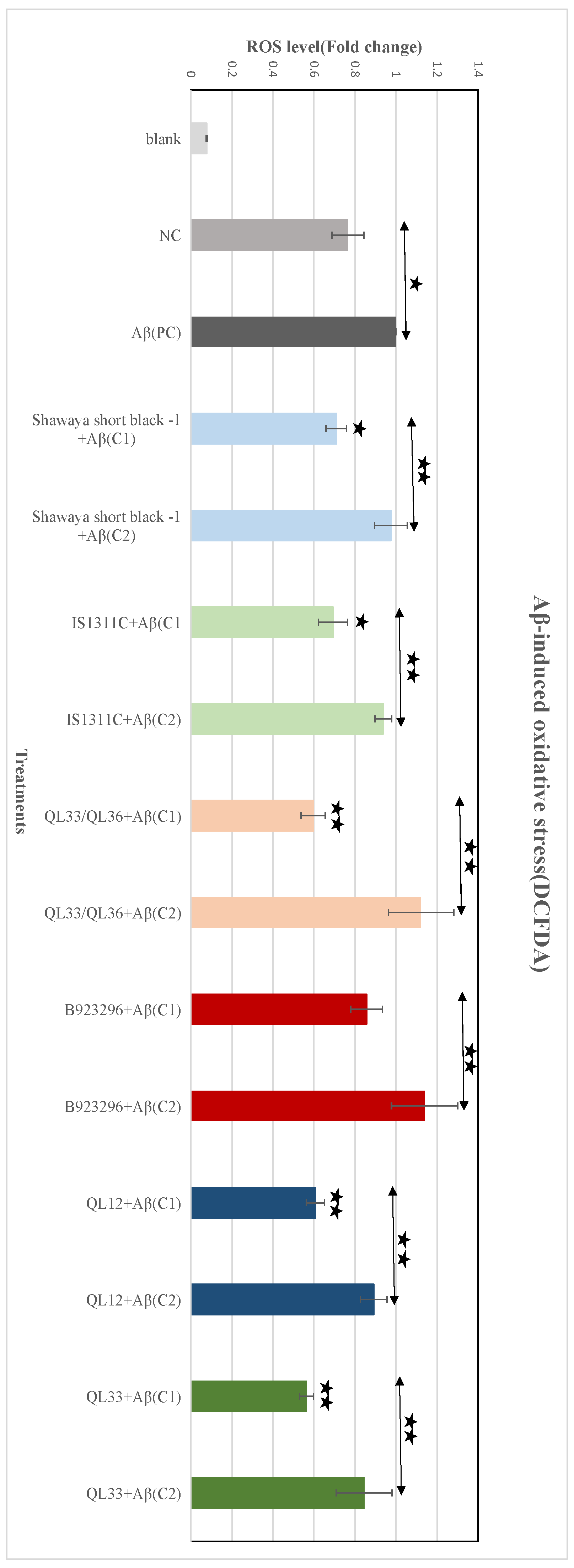
3.8. Effect of PP-Rich Sorghum Extracts on Aβ-Induced ROS in BE (2)-M17 Cells
3.9. Effect of PP-Rich Sorghum Extracts on TBHP-Induced ROS in BE (2)-M17 Cells
3.10. Effect of PP-Rich Sorghum Extracts on Aβ-Induced Mitochondrial Superoxide in BE (2)-M17 Cells
3.11. Effect of PP-Rich Sorghum Extracts on TBHP-Induced Mitochondrial Superoxide in BE (2)-M17 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Tapsell, L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Internat. 2015, 31, 401–437. [Google Scholar] [CrossRef]
- Licata, R.; Chu, J.; Wang, S.; Coorey, R.; James, A.; Zhao, Y.; Johnson, S. Determination of formulation and processing factors affecting slowly digestible starch, protein digestibility and antioxidant capacity of extruded sorghum–maize composite flour. Int. J. Food Sci. Technol. 2014, 49, 1408–1419. [Google Scholar] [CrossRef]
- Kangama, C.O. Importance of Sorghum bicolor in African’s cultures. J. Agric. Enviro. Sci. 2017, 6, 134–137. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, A.A.D.T.; Deshapriya, R.D.U.S.; Udawatte, C. Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Meehan, S.; Carver, J.A.; Wilson, M.R.; Dobson, C.M.; Klenerman, D. Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry 2012, 51, 9270–9276. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.-X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and alpha-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Geldenhuys, W.J.; Van der Schyf, C.J. Oxidative stress and Alzheimer’s disease: Dietary polyphenols as potential therapeutic agents. Expert Rev. Neurother. 2010, 10, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552 Pt 2, 335–344. [Google Scholar] [CrossRef]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 2019, 9, 561. [Google Scholar] [CrossRef]
- Haque, A.M.; Hashimoto, M.; Katakura, M.; Hara, Y.; Shido, O. Green tea catechins prevent cognitive deficits caused by Aβ1–40 in rats. J. Nutr. Biochem. 2008, 19, 619–626. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Recent advances in berry supplementation and age-related cognitive decline. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 91–94. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.I.; Henriques, A.G.; Silva, A.M.; Wiltfang, J.; da Cruz e Silva, O.A. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci. 2014, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedcine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Synergisms in antioxidant and anti-hepatocellular carcinoma activities of artichoke, milk thistle and borututu syrups. Indust. Crops Products 2014, 52, 709–713. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How to improve bayberry (Myrica rubra sieb. et zucc.) juice color quality: Effect of juice processing on bayberry anthocyanins and polyphenolics. J. Agric. Food Chem. 2006, 54, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.; Singh, V.; Fang, Z. Effect of genotype and growth temperature on sorghum grain physical characteristics, polyphenol content, and antioxidant activity. Cereal Chem. 2016, 93, 419–425. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Stine, W.B.; Jungbauer, L.; Yu, C.; LaDu, M.J. Preparing synthetic Abeta in different aggregation states. Methods Mol. Biol. 2011, 670, 13–32. [Google Scholar]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive biophenols reduces Alzheimer’s pathology in SH-SY5Y cells and APPswe mice. Int. J. Mol. Sci. 2019, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.; Barrier, L.; Ingrand, S. The Tyr216 phosphorylated form of GSK3β contributes to tau phosphorylation at PHF-1 epitope in response to Aβ in the nucleus of SH-SY5Y cells. Life Sci. 2016, 158, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M. Sorghum flavonoids: Unusual compounds with promising implications for health. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.A., Piironen, V., Bean, S., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 171–200. [Google Scholar]
- Su, X.; Rhodes, D.; Xu, J.; Chen, X.; Davis, H.; Wang, D.; Herald, T.J.; Wang, W. Phenotypic diversity of anthocyanins in sorghum accessions with various pericarp pigments. J. Nutr. Food Sci. 2017, 7, 610. [Google Scholar] [CrossRef]
- Lietti, A.; Cristoni, A.; Picci, M. Studies on Vaccinium myrtillus anthocyanosides. I. Vasoprotective and anti-inflammatory activity. Arzneim.-Forsch 1976, 26, 829–832. [Google Scholar]
- Suganyadevi, P.; Saravanakumar, K.M.; Mohandas, S. The antiproliferative activity of 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran through P(53)-dependent and Bcl-2 gene expression in breast cancer cell line. Life Sci. 2013, 92, 379–382. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-deoxyanthocyanins from sorghum. J. Agric. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zang, J.; Guo, L.; Xu, Y.; Sun, L.; Wang, S.; Feng, Y.; Gou, L.; Zhang, L.; Liu, Y. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharmacol. Biochem. Behav. 2014, 126, 122–130. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R.; et al. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, W.Q.; Zha, H.; Yu, H.R. Effect of naringenin on learning and memory ability on model rats with Alzheimer disease. Zhong Yao Cai 2013, 36, 271–276. [Google Scholar] [PubMed]
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic acid activates PPARalpha to stimulate lysosomal biogenesis and lower amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019, 124, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-J.; Jung, J.-S.; Kim, T.-K.; Hasan, M.A.; Hong, C.-W.; Nam, J.-S.; Song, D.-K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Aβ25–35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Jo, S.A. Mechanisms of amyloid-beta peptide clearance: Potential therapeutic targets for Alzheimer’s disease. Biomol. Ther. 2012, 20, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Design 2006, 67, 27–37. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Lin, C.-L.; Huang, W.-N.; Li, H.-H.; Huang, C.-N.; Hsieh, S.; Lai, C.; Lu, F.-J. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. Biol. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J. Cell. Mol. Med. 2018, 22, 1081–1094. [Google Scholar] [CrossRef]
- Gemma, C.; Vila, J.; Bachstetter, A.; Bickford, P.C. Oxidative Stress and the Aging Brain: From Theory to Prevention. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Arbex, P.M.; de Castro Moreira, M.E.; Toledo, R.C.L.; de Morais Cardoso, L.; Pinheiro-Sant, H.M.; Benjamin, L.D.A.; Licursi, L.; Carvalho, C.W.P.; Queiroz, V.A.V.; Martino, H.S.D. Extruded sorghum flour (Sorghum bicolor L.) modulate adiposity and inflammation in high fat diet-induced obese rats. J. Funct. Foods 2018, 42, 346–355. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-inflammatory Properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41. [Google Scholar] [CrossRef]
- Paterniti, I.; Cordaro, M.; Campolo, M.; Siracusa, R.; Cornelius, C.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental Alzheimer’s disease models: The control of neuroinflammation. CNS Neurol. Disord. Drug Targets 2014, 13, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Kim, D.-O.; Shin, S.C.; Kim, M.J.; Kim, B.G.; Shin, D.-H. Effect of Antioxidant Flavanone, Naringenin, from Citrus junos on Neuroprotection. J. Agric. Food Chem. 2004, 52, 1520–1525. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Milton Prabu, J.R.S.; Shagirtha, K. In Vivo and In Vitro Antioxidative Efficacy of Naringenin on Cadmium -Induced Toxicity in Rats. RRJoT 2013, 3, 3. [Google Scholar]
- Hamaguchi, T.; Ono, K.; Yamada, M. Anti-amyloidogenic therapies: Strategies for prevention and treatment of Alzheimer’s disease. Cell. Mol. Life Sci. 2006, 63, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Analy. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holding, A.J.; Parviainen, A.; Kilpeläinen, I.; Soto, A.; King, A.W.T.; Rodríguez, H. Efficiency of hydrophobic phosphonium ionic liquids and DMSO as recyclable cellulose dissolution and regeneration media. RSC Advan. 2017, 7, 17451–17461. [Google Scholar] [CrossRef]
- Ouattara, N.; Meda, R.N.-T.; Hilou, A.; Guenné, S.; Konaté, K.; Coulibaly, A.Y.; Kiendrébeogo, M.; Millogo, J.F.; Nacoulma, O.G. Anti-acetylcholinesterase and antioxidant activities and HPLC-MS analysis of polyphenol from extracts of Nelsonia canescens (Lam.) Spreng. Asian Pac. J. Trop. Dis. 2013, 3, 382–388. [Google Scholar] [CrossRef]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentao, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A comparative study on phenolic content, antioxidant activity and anti-Inflammatory capacity of aqueous and ethanolice extracts of sorghum in lipopolysaccharide-Induced RAW 264.7 macrophages. Antioxidant 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Komolafe, Y.O.; Oloyede, O.B.; Ogunbode, S.M.; Adeoye, M.D.; Abdulsalami, I.O.; Nurudeen, Q.O. Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats. Food Sci. Human Well. 2013, 2, 39–45. [Google Scholar] [CrossRef]
- Rezaee, N.; Fernando, W.M.A.D.B.; Hone, E.; Sohrabi, H.R.; Johnson, S.K.; Gunzburg, S.; Martins, R.N. Potential of sorghum polyphenols to prevent and treat alzheimer’s disease: A review article. Front. Aging Neurosci. 2021, 6, 729949. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-based flow cytometry for detecting mitochondrial ROS. Reactive Oxy. Spec. 2016, 2, 361–370. [Google Scholar]
- Mukhopadhyay, P.; Rajesh, M.; Yoshihiro, K.; Haskó, G.; Pacher, P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem. Biophys. Res. Comm. 2007, 358, 203–208. [Google Scholar] [CrossRef]




| Varieties | ||||||
|---|---|---|---|---|---|---|
| Shawaya Short Black-1 | IS1311C | QL33/QL36 | B923296 | QL12 | QL33 | |
| Phenolics | 7.14 ± 0.67 c | 4.77 ± 0.41 b | 1.59 ± 0.23 a | 1.97 ± 0.57 a | 1.62 ± 0.25 a | 2.13 ± 0.36 a |
| Flavonoids | 5.24 ± 0.01 d | 3.3 ± 0.4 c | 1.28 ± 0.08 b | 0.81 ± 0.05 a | 0.83 ± 0.01 a | 1.19 ± 0.14 a |
| Variety | ||||||
|---|---|---|---|---|---|---|
| Shawaya Short Black-1 | IS1311C | QL33/QL36 | B923296 | QL12 | QL33 | |
| Luteolinidin | 38.0 ± 7.4 d | 3.2 ± 0.7 a | 6.3 ± 0.3 b | Nd | 8.7 ± 0.4 c | 6.6 ± 0.2 b |
| Apigeninidin | 120.5 ± 17.7 e | 3.8 ± 0.4 b | 28.9 ± 3.0 d | 8.0 ± 2.30 c | 1.4 ± 0.4 a | 7.2 ± 0.9 c |
| Luteolin | 12.6 ± 1.4 | nd | nd | nd | nd | nd |
| Apigenin | 9.0 ± 2.2 a | 14.2 ± 0.1 b | Nd | 17.8 ± 0.59 c | nd | 38.6 ± 0.3 d |
| Taxifolin | 15.2 ± 0.5 a | 16.2 ± 0.2 a | nd | nd | nd | nd |
| Naringenin | 11.9 ± 2.3 c | 10.7 ± 0.1 c | 6.2 ± 0.10 b | 5.3 ± 0.07 a | nd | 6.6 ± 0.3 b |
| Cinnamic acid * | 6.0 ± 0.1 a | nd | 5.7 ± 0.08 a | nd | 6.1 ± 0.3 a | 6.1 ± 0.3 a |
| Ferulic acid | 15.8 ± 1.7 c | 28.3 ± 0.2 e | 4.8 ± 0.15 a | 19.9 ± 0.40 d | 6.4 ± 0.3 b | 55.8 ± 0.5 f |
| Caffeic acid | 38.9 ± 2.9 b | 32.1 ± 0.0 b | 35.2 ± 0.48 b | 30.7 ± 0.30 a | 33.16 ± 1.4 b | 34.7 ± 0.3 b |
| Variety | ||||||
|---|---|---|---|---|---|---|
| Shawaya Short Black -1 | IS1311C | QL33/QL36 | B923296 | QL12 | QL33 | |
| DPPH | 20.36 ± 0.152 d | 22.09 ± 0.04 e | 1.12 ± 0.02 b | 0.65 ± 0.04 a | 0.65 ± 0.00 a | 1.52 ± 0.02 c |
| ABTS+ | 45.01 ± 0.216 e | 45.35 ± 0.14 f | 3.04 ± 0.14 b | 3.65 ± 0.00 c | 2.64 ± 0.00 a | 4.24 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaee, N.; Hone, E.; Sohrabi, H.R.; Johnson, S.; Zhong, L.; Chatur, P.; Gunzburg, S.; Martins, R.N.; Fernando, W.M.A.D.B. Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer’s Disease in Cellular Assays. Foods 2024, 13, 1716. https://doi.org/10.3390/foods13111716
Rezaee N, Hone E, Sohrabi HR, Johnson S, Zhong L, Chatur P, Gunzburg S, Martins RN, Fernando WMADB. Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer’s Disease in Cellular Assays. Foods. 2024; 13(11):1716. https://doi.org/10.3390/foods13111716
Chicago/Turabian StyleRezaee, Nasim, Eugene Hone, Hamid R. Sohrabi, Stuart Johnson, Leizhou Zhong, Prakhar Chatur, Stuart Gunzburg, Ralph N. Martins, and W. M. A. D. Binosha Fernando. 2024. "Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer’s Disease in Cellular Assays" Foods 13, no. 11: 1716. https://doi.org/10.3390/foods13111716






