Electrical Stimulation Induces Activation of Mitochondrial Apoptotic Pathway and Down-Regulates Heat Shock Proteins in Pork: An Innovative Strategy for Enhancing the Ripening Process and Quality of Dry-Cured Loin Ham
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation of Fresh Meat and Cured Meat
2.2. Measurement of Quality Indicators
2.3. Measurement of HSP27 and HSP70 Expression
2.4. Mitochondrial Extraction
2.5. Measurement of Mitochondrial Structure Indicators
2.6. Measurement of Mitochondrial Oxidative Stress
2.7. Measurement of Bcl-2 Family Protein Content
2.8. Measurement of Caspase-3 and Caspase-9 Activity
2.9. Measurement of Microstructure (SEM)
2.10. HE Stain
2.11. Measurement of Apoptosis Rate
2.12. Statistical Analysis
3. Results and Discussion
3.1. Quality Indicators
3.2. Microstructure
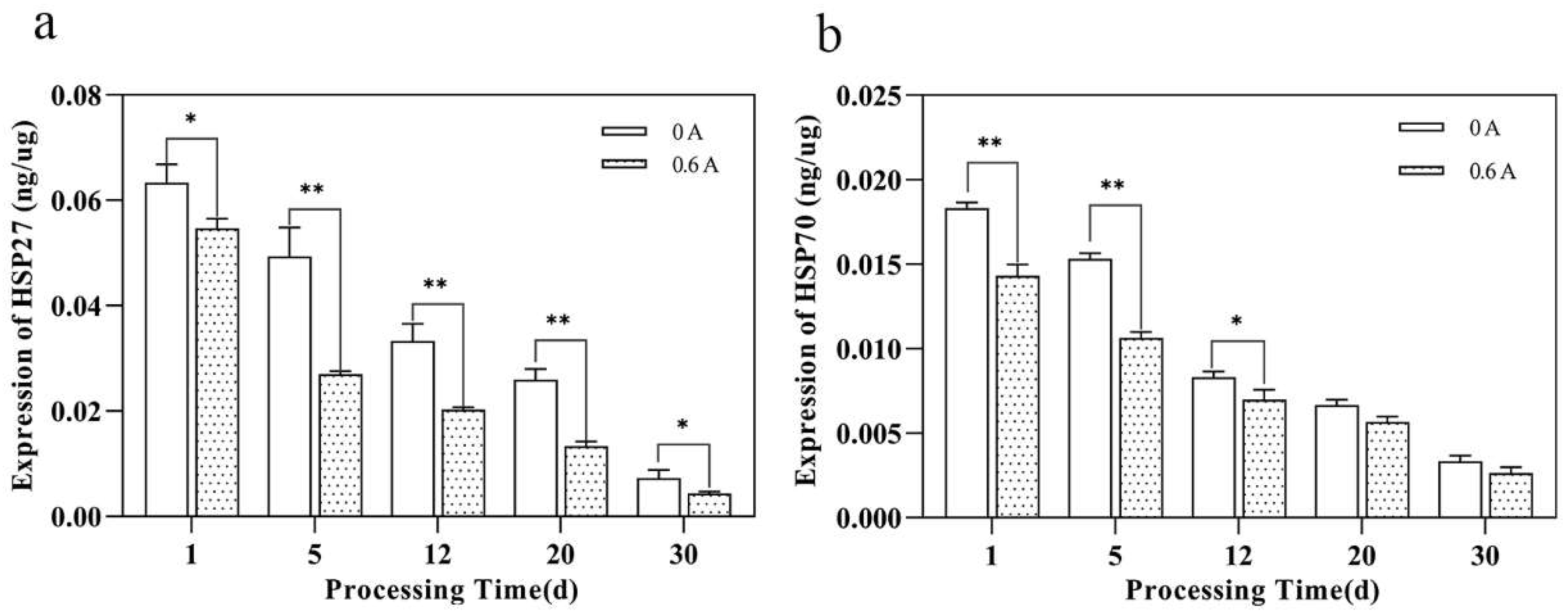
3.3. Expression Level of HSP27, HSP70
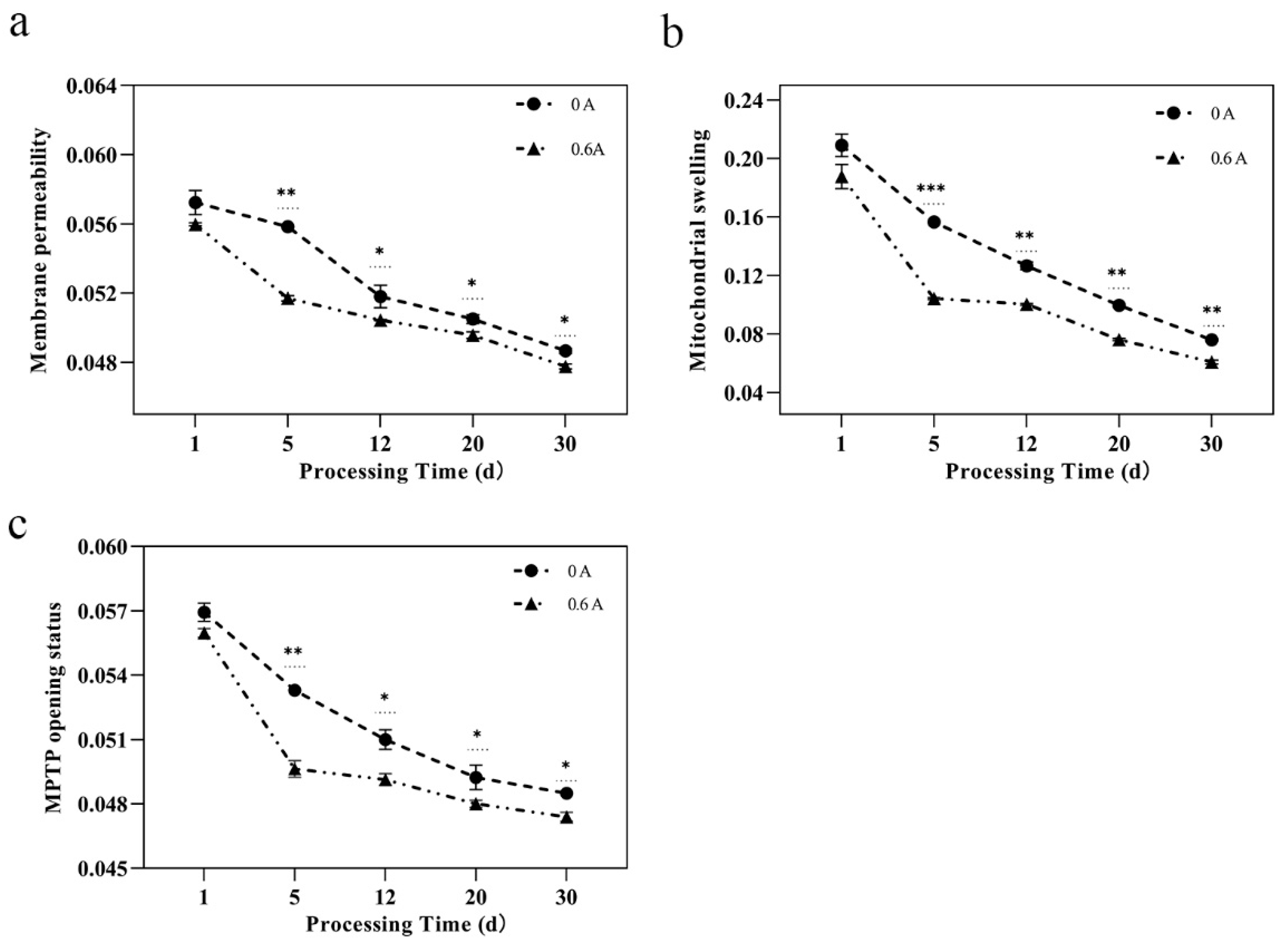
3.4. Mitochondrial Structure
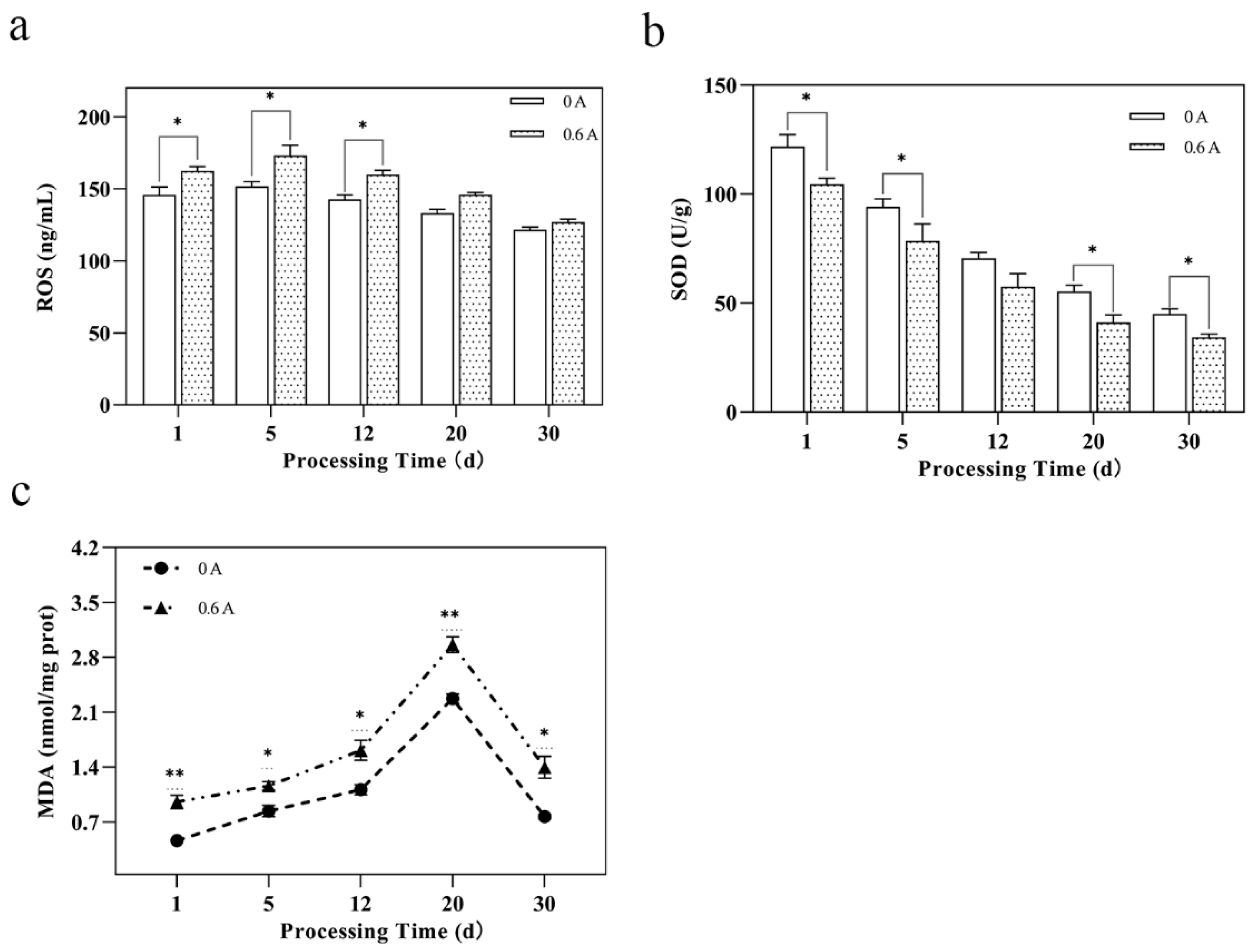
3.5. Mitochondrial Oxidative Stress and Damage
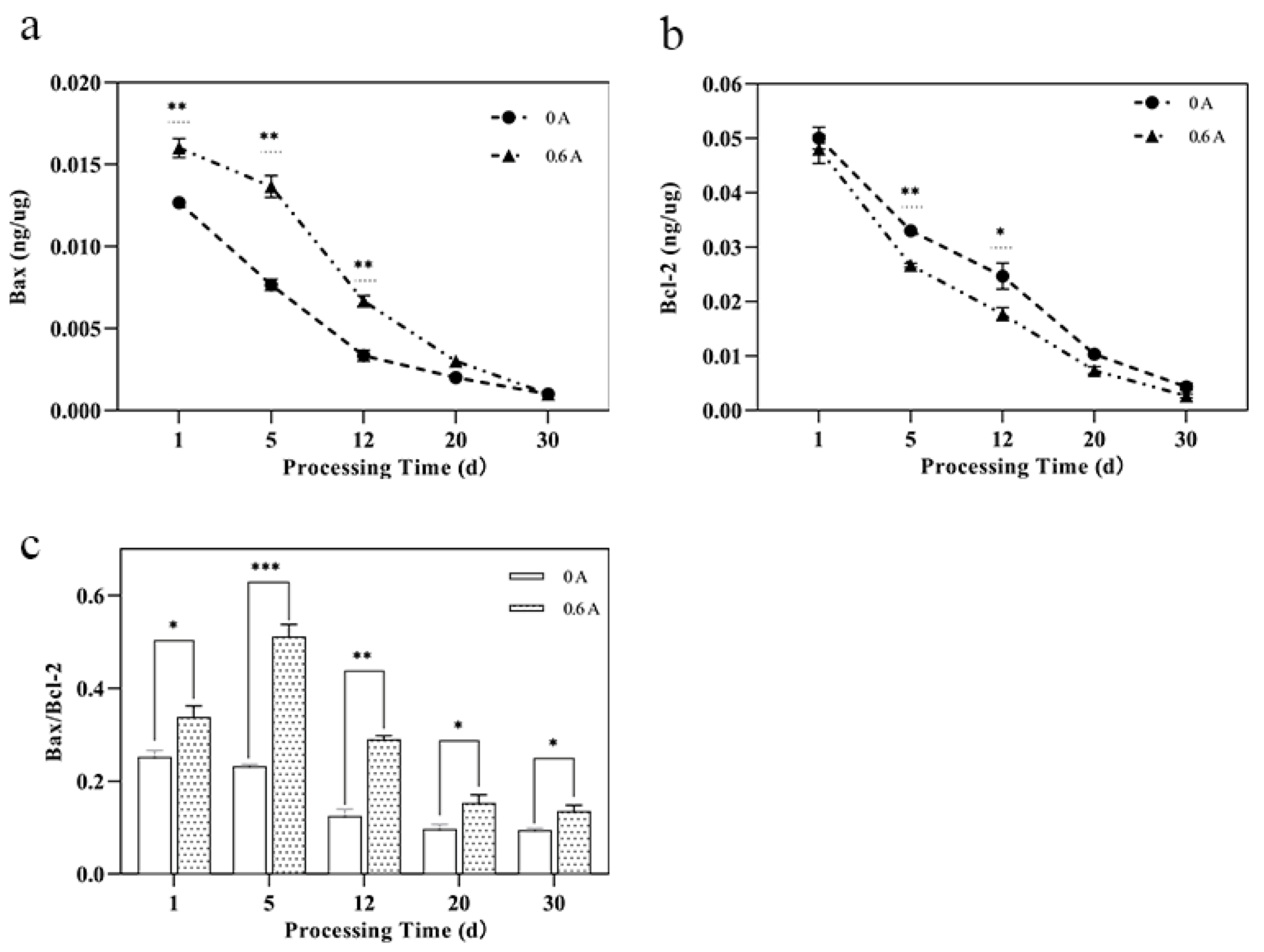
3.6. Bcl-2 Family Protein Content
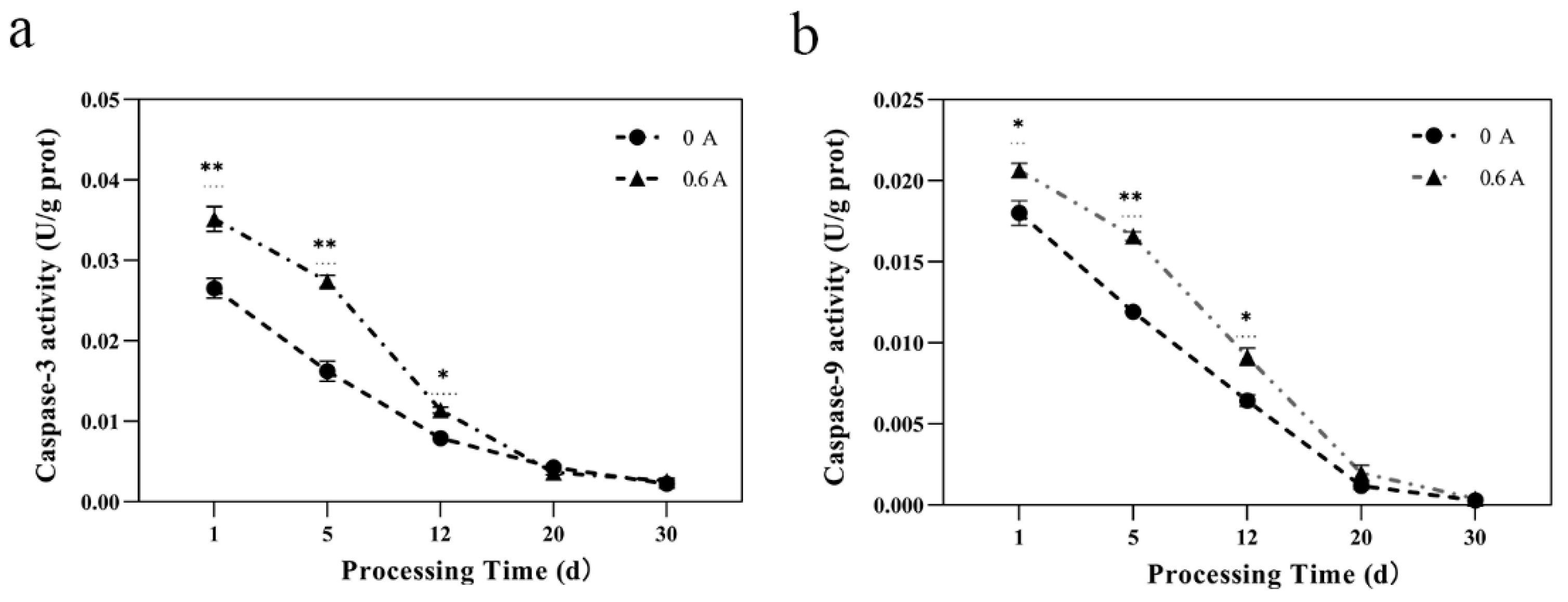
3.7. Caspase-3 and Caspase-9 Activity
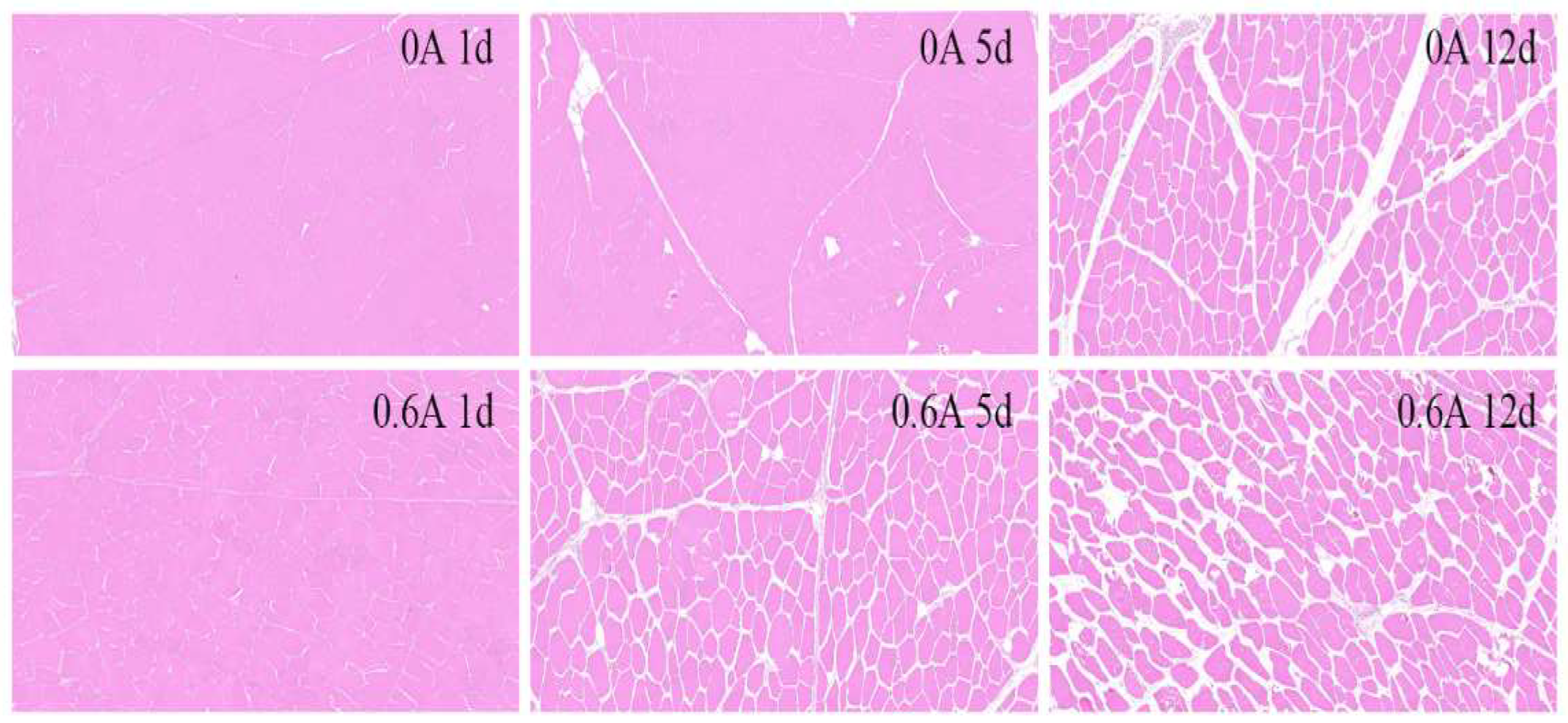
3.8. HE Stain
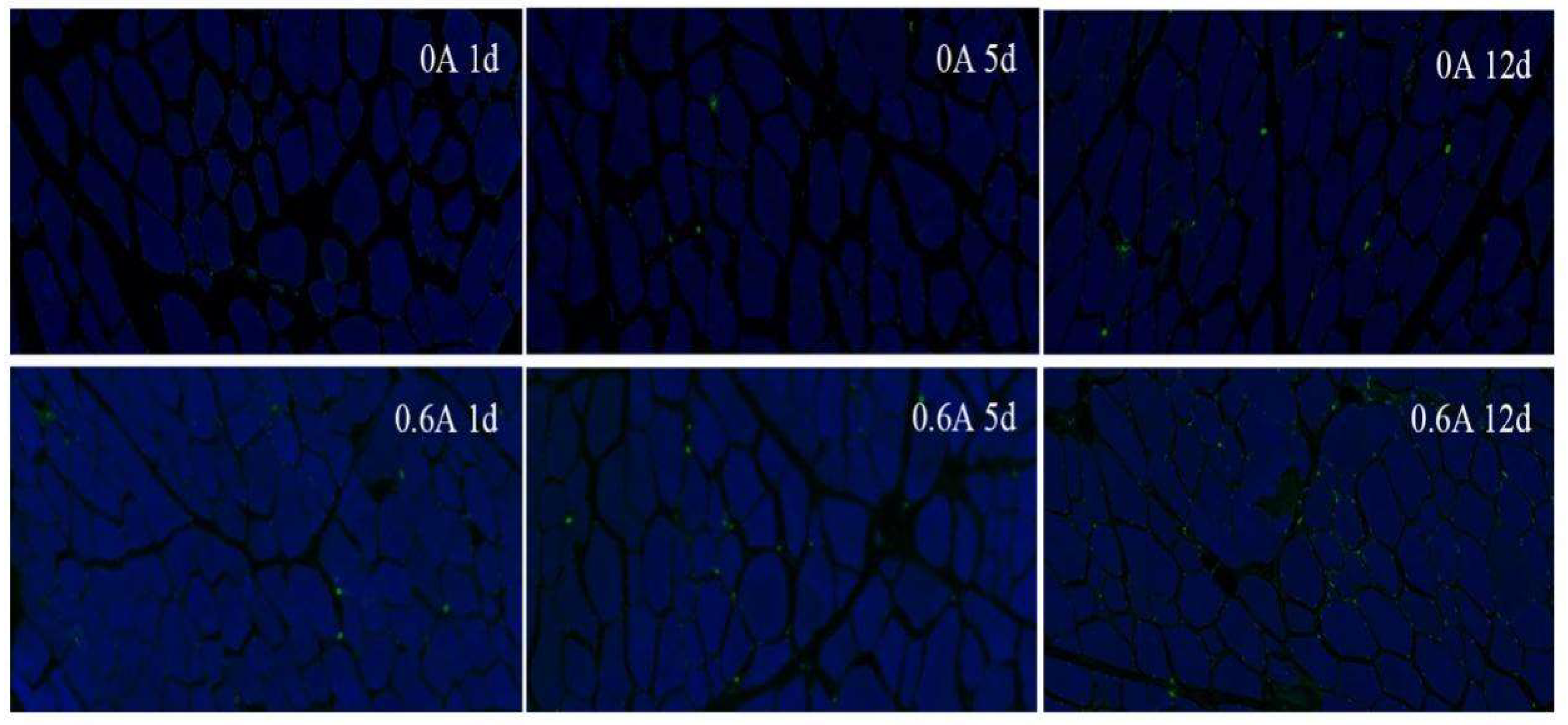
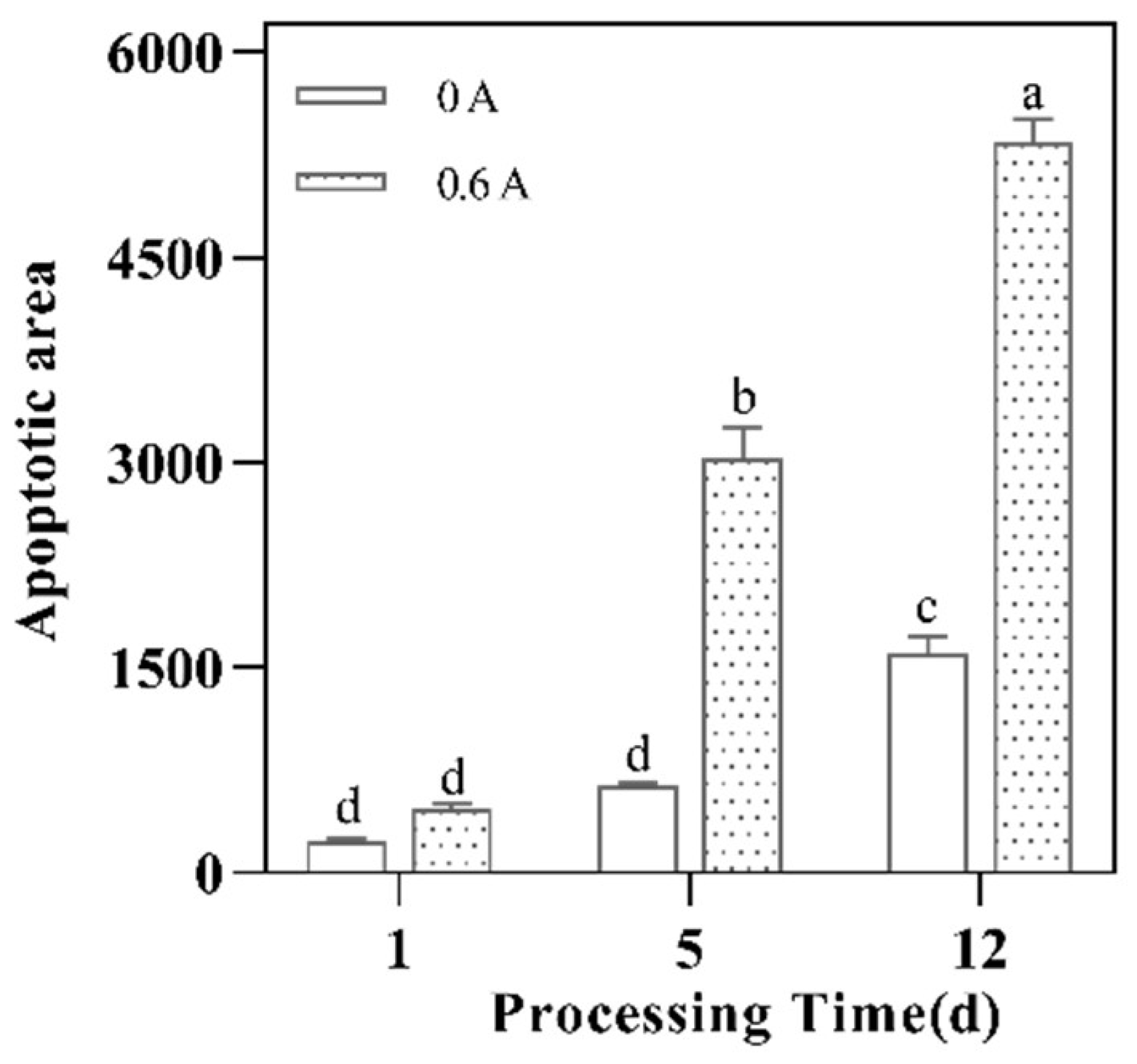
3.9. Apoptosis Rate
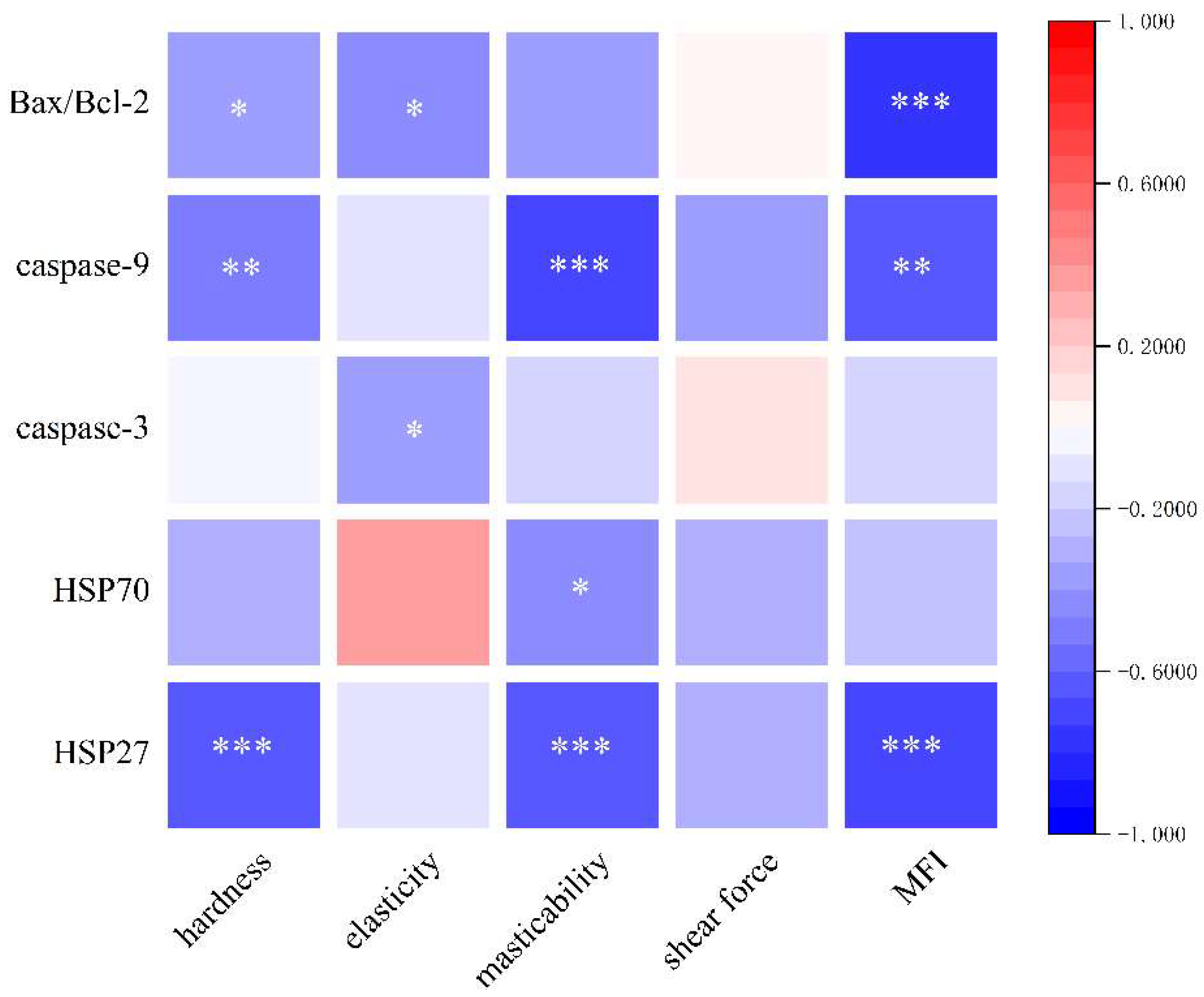
3.10. Analysis of Potential Relationships between Heat Shock Proteins, Apoptosis, and Quality of Dry-Cured Pork Loin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kemp, C.M.; Sensky, P.L.; Bardsley, R.G.; Buttery, P.J.; Parr, T. Tenderness—An enzymatic view. Meat Sci. 2010, 84, 248–256. [Google Scholar] [CrossRef]
- Banach, J.K.; Zywica, R.; Matusevicius, P. Influence of various chilling methods on sustainable beef production based on high voltage electrical stimulation. PLoS ONE 2021, 16, e0240639. [Google Scholar] [CrossRef]
- Carvalho, M.E.; Gasparin, G.; Poleti, M.D.; Rosa, A.F.; Carvalho Balieiro, J.C.; Labate, C.A.; Nassu, R.T.; Tullio, R.R.; de Almeida Regitano, L.C.; Mourao, G.B.; et al. Heat shock and structural proteins associated with meat tenderness in Nellore beef cattle, a Bos indicus breed. Meat Sci. 2014, 96, 1318–1324. [Google Scholar] [CrossRef]
- Swatland, H.J. Cellular heterogeneity in the response of beef to electrical stimulation. Meat Sci. 1981, 5, 451–455. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Yang, J.; Dashdorj, D.; Hwang, I. Volatile Flavor Components as a Function of Electrical Stimulation and Chiller Aging for M. longissimus and biceps femoris of Hanwoo Beef. Food Sci. Anim. Resour. 2019, 39, 474–493. [Google Scholar] [CrossRef]
- Hopkins, L.D.; Holman, W.B.; Ven, D. Modeling lamb carcass pH and temperature decline parameters: Relationship to shear force and abattoir variation. Meat Sci. 2015, 100, 85–90. [Google Scholar] [CrossRef]
- Ouali, A.; Herrera-Mendez, C.H.; Coulis, G.; Becila, S.; Boudjellal, A.; Aubry, L.; Sentandreu, M.A. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 2006, 74, 44–58. [Google Scholar] [CrossRef]
- Xu, A.; Chang, R.; Zhu, Q. Effect of electrical stimulation on red meat Neu5Gc content reduction: A combined experimental and DFT study. Food Sci. Hum. Wellness 2022, 11, 982–991. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, S.; Li, M.; Yue, X.; Wan, J.; Zhou, Y.; Liu, Y.; Zhu, Q. Study on activation strategy and effect of protease activity during the post-processing stage of dry-cured meat based on electrical stimulation. Food Control 2024, 161, 110363. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhou, X.; Li, C.; Liu, Y. Changes in the extent and products of In vitro protein digestion during the ripening periods of Chinese dry-cured hams. Meat Sci. 2021, 171, 108290. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadottir, S.G.; Hollung, K.; Hoy, M.; Bendixen, E.; Codrea, M.C.; Veiseth-Kent, E. Changes in protein abundance between tender and tough meat from bovine Longissimus thoracis muscle assessed by isobaric Tag for Relative and Absolute Quantitation (iTRAQ) and 2-dimensional gel electrophoresis analysis. J. Anim. Sci. 2012, 90, 2035–2043. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Wiklund, E.; Young, O.A. Small heat shock proteins and their role in meat tenderness: A review. Meat Sci. 2014, 96, 26–40. [Google Scholar] [CrossRef]
- Contreras-Castillo, C.J.; Lomiwes, D.; Wu, G.; Frost, D.; Farouk, M.M. The effect of electrical stimulation on post-mortem myofibrillar protein degradation and small heat shock protein kinetics in bull beef. Meat Sci. 2016, 113, 65–72. [Google Scholar] [CrossRef]
- Lung, C.; Chang, C.; Cheng, F.; Hou, C.; Chen, M.; Santoso, S.P.; Yudhistira, B.; Hsieh, C. Effects of pulsed electric field-assisted thawing on the characteristics and quality of Pekin duck meat. Food Chem. 2022, 390, 133137. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ye, C.; Zhou, Y.; Liu, Y.; Wan, J.; Liu, L.; Lu, K.; Bi, S.; Jie, G.; Zhu, Q. Systematic studies on improving structural properties of myofibrillar proteins and pork quality based on electrical stimulation. Int. J. Food Sci. Technol. 2023, 58, 2258–2269. [Google Scholar] [CrossRef]
- Guillemin, N.; Bonnet, M.; Jurie, C.; Picard, B. Functional analysis of beef tenderness. J. Proteom. 2012, 75, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Yu, Q.; Han, L.; Ma, Z. Effects of Lysosomal-Mitochondrial Apoptotic Pathway on Tenderness in Post-Mortem Bovine Longissimus Muscle. J. Agric. Food. Chem. 2019, 67, 4578–4587. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Floros, K.V.; Chipuk, J.E. BAK/BAX activation and cytochrome c release assays using isolated mitochondria. Methods 2013, 61, 146–155. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Wang, Z.; Xie, Y.; Tang, C.; Li, C.; Xu, F.; Zhou, H.; Xu, B. New perspective for Calpain-Mediated regulation of meat Quality: Unveiling the impact on mitochondrial pathway apoptosis in post-mortem. Food Chem. 2024, 441, 138287. [Google Scholar] [CrossRef]
- Wang, L.; Han, L.; Ma, X.; Yu, Q.; Zhao, S. Effect of mitochondrial apoptotic activation through the mitochondrial membrane permeability transition pore on yak meat tenderness during postmortem aging. Food Chem. 2017, 234, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, W.; Xian, Z.; Liu, Q.; Hui, A.; Zhang, W. The impact of quick-freezing methods on the quality, moisture distribution, and microstructure of prepared ground pork during storage duration. Ultrason. Sonochem. 2021, 78, 105707. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, P.; Brueggemann, D.A.; Henckel, P.; Vyberg, M.; Groves, K.; Oksbjerg, N.; Therkildsen, M. Performance of conventional histochemical methods relative to a novel immunolabeling technique in assessing the degree of degradation in comminuted chicken meat. Food Control 2017, 73, 133–139. [Google Scholar] [CrossRef]
- Cao, J.; Sun, W.; Zhou, G.; Xu, X.; Peng, Z.; Hu, Z. Morphological and biochemical assessment of apoptosis in different skeletal muscles of bulls during conditioning. J. Anim. Sci. 2010, 88, 3439–3444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Pan, D.; Bai, Y.; Li, C.; Xu, X.; Zhou, G.; Cao, J. Evaluating endogenous protease of salting exudates during the salting process of Jinhua ham. LWT Food Sci. Technol. 2019, 101, 76–82. [Google Scholar] [CrossRef]
- Leighton, P.L.A.; Lopez-Campos, O.; Chabot, B.; Scott, H.R.; Zawadski, S.; Barragan-Herandez, W.; Aarhus, J.L.; Prieto, N. Impact of a constant current electrical stimulation (CCES) system and hormonal growth-promoting (HGP) implants on meat quality and palatability of finished steers. Meat Sci. 2023, 205, 109297. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Ordonez, J.A.; de Avila, R.; Herranz, B.; de la Hoz, L.; Cambero, M.I. Breaking strength of dry fermented sausages and their correlation with texture profile analysis (TPA) and physicochemical characteristics. Meat Sci. 2007, 77, 331–338. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, G.L.; Hoffman, L.C.; Strydom, P.E.; Frylinck, L. Effect of sex (ram or wether) and short duration, high volt electrical stimulation on tenderisation of Longissimus thoracis et lumborum and Semimembranosus muscles derived from Boer Goat and large frame Indigenous Veld Goat. Meat Sci. 2023, 204, 109271. [Google Scholar] [CrossRef] [PubMed]
- Ngapo, T.M.; Vachon, L. The impact of homogenizer speed, dispersing aggregate size, and centrifugation on particle size analyses of pork as a measure of myofibrillar fragmentation. Meat Sci. 2017, 133, 166–172. [Google Scholar] [CrossRef]
- Lang, Y.; Sha, K.; Zhang, R.; Xie, P.; Luo, X.; Sun, B.; Li, H.; Zhang, L.; Zhang, S.; Liu, X. Effect of electrical stimulation and hot boning on the eating quality of Gannan yak longissimus lumborum. Meat Sci. 2016, 112, 3–8. [Google Scholar] [CrossRef]
- Koohmaraie, M. Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci. 1996, 43 (Suppl. S1), 193–201. [Google Scholar] [CrossRef]
- George, A.R.; Bendall, J.R.; Jones, R.C. The tenderizing effect of electrical stimulation of beef carcasses. Meat Sci. 1980, 4, 51–68. [Google Scholar] [CrossRef]
- Bar, H.; Strelkov, S.V.; Sjoberg, G.; Aebi, U.; Herrmann, H. The biology of desmin filaments: How do mutations affect their structure, assembly, and organization? J. Struct. Biol. 2004, 148, 137–152. [Google Scholar] [CrossRef]
- Clark, J.I.; Muchowski, P.J. Small heat-shock proteins and their potential role in human disease. Curr. Opin. Struct. Biol. 2000, 10, 52–59. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meriin, A.B.; Mosser, D.D.; Caron, A.W.; Rits, S.; Shifrin, V.I.; Sherman, M.Y. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J. Biol. Chem. 1997, 272, 18033–18037. [Google Scholar] [CrossRef]
- Rizvi, S.F.; Hasan, A.; Parveen, S.; Mir, S.S. Untangling the complexity of heat shock protein 27 in cancer and metastasis. Arch. Biochem. Biophys. 2023, 736, 109537. [Google Scholar] [CrossRef]
- Ke, Y.; Mitacek, R.M.; Abraham, A.; Mafi, G.G.; VanOverbeke, D.L.; DeSilva, U.; Ramanathan, R. Effects of Muscle-Specific Oxidative Stress on Cytochrome c Release and Oxidation Reduction Potential Properties. J. Agric. Food. Chem. 2017, 65, 7749–7755. [Google Scholar] [CrossRef]
- Bekhit, A.E.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative Processes in Muscle Systems and Fresh Meat: Sources, Markers, and Remedies. Compr. Rev. Food. Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, G.; Guo, Z.; Yu, Q.; Han, L.; Han, M.; Zhu, Y. Study on the apoptosis mediated by apoptosis-inducing-factor and influencing factors of bovine muscle during postmortem aging. Food Chem. 2018, 266, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Cléach, J.; Pasdois, P.; Marchetti, P.; Watier, D.; Duflos, G.; Goffier, E.; Lacoste, A.; Slomianny, C.; Grard, T.; Lencel, P. Mitochondrial activity as an indicator of fish freshness. Food Chem. 2019, 287, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, Y.; Yu, Q.; Han, L.; Zhao, S.; Song, R. Effect of a low-voltage electrical stimulation on yak meat tenderness during postmortem aging. Anim. Sci. J. 2020, 91, e13410. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Moon, G.; Shim, D.; Kim, J.C.; Lee, K.J.; Chung, K.; An, J.H. Neuroprotective effects of fermented tea in MPTP-induced Parkinson’s disease mouse model via MAPK signaling-mediated regulation of inflammation and antioxidant activity. Food Res. Int. 2023, 164, 112133. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A. Current and future prospects for the use of pulsed electric fields in the meat industry. Crit. Rev. Food. Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Cui, J.; Liu, H.; Liu, Y.; Qiao, W.; Sun, H.; Yan, C. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J. Gastroenterol. 2013, 19, 9439–9446. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease, and aging. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Garcia-Ruiz, C.; Mari, M.; Fernandez-Checa, J.C. Mitochondrial permeability transition induced by reactive oxygen species is independent of cholesterol-regulated membrane fluidity. FEBS Lett. 2004, 560, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Biffin, T.E.; Smith, M.A.; Bush, R.D.; Collins, D.; Hopkins, D.L. The effect of combining tender stretching and electrical stimulation on alpaca (Vicugna pacos) meat tenderness and eating quality. Meat Sci. 2018, 145, 127–136. [Google Scholar] [CrossRef]
- Huang, X.; Choi, J.; Park, S.R.; Ha, Y.; Park, H.; Yoon, S.H.; Park, H.C.; Park, J.O.; Choi, B.H. GM-CSF inhibits apoptosis of neural cells by regulating the expression of apoptosis-related proteins. Neurosci. Res. 2007, 58, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.X.; Ou, C.R.; Zou, Y.F. Activation of caspase-3 and its correlation with shear force in bovine skeletal muscles during postmortem conditioning. J. Anim. Sci. 2013, 9, 4547–4552. [Google Scholar] [CrossRef]
- You, Z.; Savitz, S.I.; Yang, J.; Degterev, A.; Yuan, J.; Cuny, G.D.; Moskowitz, M.A.; Whalen, M.J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008, 28, 1564–1573. [Google Scholar] [CrossRef]
- Chen, L.; Chai, Y.; Luo, J.; Wang, J.; Liu, X.; Wang, T.; Xu, X.; Zhou, G.; Feng, X. Apoptotic changes and myofibrils degradation in post-mortem chicken muscles by ultrasonic processing. LWT Food Sci. Technol. 2021, 142, 110985. [Google Scholar] [CrossRef]
- Morzel, M.; Terlouw, C.; Chambon, C.; Micol, D.; Picard, B. Muscle proteome and meat eating qualities of Longissimus thoracis of “Blonde d’Aquitaine” young bulls: A central role of HSP27 isoforms. Meat Sci. 2008, 78, 297–304. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, Q.; Zhang, C.; Zhang, H.; Huang, F. Influence of oxidation on heat shock protein 27 translocations, caspase-3 and calpain activities and myofibrils degradation in postmortem beef muscles. Food Chem. 2021, 340, 127914. [Google Scholar] [CrossRef]
- Zapata, I.; Zerby, H.N.; Wick, M. Functional Proteomic Analysis Predicts Beef Tenderness and the Tenderness Differential. J. Agric. Food. Chem. 2009, 57, 4956–4963. [Google Scholar] [CrossRef]
- Blunt, B.C.; Creek, A.T.; Henderson, D.C.; Hofmann, P.A. H2O2 activation of HSP25/27 protects desmin from calpain proteolysis in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1518–H1525. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.; Terlouw, C.E.M. Inverse Relationships between Biomarkers and Beef Tenderness According to Contractile and Metabolic Properties of the Muscle. J. Agric. Food. Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef]
- Stankiewicz, A.R.; Lachapelle, G.; Foo, C.P.Z.; Radicioni, S.M.; Mosser, D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005, 280, 38729–38739. [Google Scholar] [CrossRef] [PubMed]
- Tai-Nagara, I.; Matsuoka, S.; Ariga, H.; Suda, T. Mortalin and DJ-1 coordinately regulate hematopoietic stem cell function through the control of oxidative stress. Blood 2014, 123, 41–50. [Google Scholar] [CrossRef] [PubMed]

| Group | Processing Time (d) | |||||
|---|---|---|---|---|---|---|
| 1 | 5 | 12 | 20 | 30 | ||
| pH | 0 A | 5.47 ± 0.01 g | 5.53 ± 0.03 f | 5.63 ± 0.01 d | 5.92 ± 0.01 bc | 6.10 ± 0.01 a |
| 0.6 A | 5.45 ± 0.01 g | 5.46 ± 0.00 g | 5.56 ± 0.00 e | 5.90 ± 0.01 c | 5.93 ± 0.01 b | |
| Hardness (gf) | 0 A | 79.5 ± 5.04 d | 36.8 ± 0.69 f | 82.9 ± 4.49 d | 111.5 ± 1.29 c | 3984.1 ± 269.53 a |
| 0.6 A | 76.1 ± 6.33 d | 29.8 ± 2.69 f | 56.1 ± 3.96 e | 77.2 ± 2.26 d | 1533.6 ± 211.57 b | |
| Elasticity (mm) | 0 A | 0.3 ± 0.02 g | 0.7 ± 0.02 ef | 0.7 ± 0.02 de | 0.8 ± 0.02 bc | 0.6 ± 0.03 f |
| 0.6 A | 0.3 ± 0.03 g | 0.8 ± 0.03 cd | 0.9 ± 0.02 ab | 0.9 ± 0.03 a | 0.7 ± 0.03 cd | |
| Masticability (mJ) | 0 A | 1.8 ± 0.07 g | 28.4 ± 3.44 e | 52.5 ± 3.63 d | 71.2 ± 2.21 c | 1460.0 ± 83.81 a |
| 0.6 A | 0.9 ± 0.08 g | 13.0 ± 1.48 f | 28.7 ± 2.60 e | 50.8 ± 5.93 d | 749.3 ± 42.88 b | |
| Shear force (N) | 0 A | 58.4 ± 2.44 d | 35.9 ± 1.53 ef | 47.4 ± 2.46 de | 107.1 ± 3.25 c | 210.3 ± 5.96 a |
| 0.6 A | 53.1 ± 4.00 d | 28.7 ± 2.69 f | 36.1 ± 0.96 ef | 63.3 ± 8.88 d | 140.7 ± 5.92 b | |
| MFI | 0 A | 27.9 ± 0.48 e | 50.8 ± 1.04 b | 44.2 ± 0.45 c | 22.3 ± 0.44 f | 18.6 ± 0.09 g |
| 0.6 A | 33.0 ± 0.52 d | 68.6 ± 1.94 a | 52.3 ± 0.40 b | 27.6 ± 0.12 e | 22.4 ± 0.21 f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Bi, S.; Li, X.; Zhang, X.; Lan, L.; Chen, L.; Zhang, Z.; Liu, Y.; Zhou, Y.; Ye, C.; et al. Electrical Stimulation Induces Activation of Mitochondrial Apoptotic Pathway and Down-Regulates Heat Shock Proteins in Pork: An Innovative Strategy for Enhancing the Ripening Process and Quality of Dry-Cured Loin Ham. Foods 2024, 13, 1717. https://doi.org/10.3390/foods13111717
Yue X, Bi S, Li X, Zhang X, Lan L, Chen L, Zhang Z, Liu Y, Zhou Y, Ye C, et al. Electrical Stimulation Induces Activation of Mitochondrial Apoptotic Pathway and Down-Regulates Heat Shock Proteins in Pork: An Innovative Strategy for Enhancing the Ripening Process and Quality of Dry-Cured Loin Ham. Foods. 2024; 13(11):1717. https://doi.org/10.3390/foods13111717
Chicago/Turabian StyleYue, Xi, Shenghui Bi, Xiangrui Li, Xinxin Zhang, Lisha Lan, Li Chen, Zhili Zhang, Yuanyuan Liu, Ying Zhou, Chun Ye, and et al. 2024. "Electrical Stimulation Induces Activation of Mitochondrial Apoptotic Pathway and Down-Regulates Heat Shock Proteins in Pork: An Innovative Strategy for Enhancing the Ripening Process and Quality of Dry-Cured Loin Ham" Foods 13, no. 11: 1717. https://doi.org/10.3390/foods13111717
APA StyleYue, X., Bi, S., Li, X., Zhang, X., Lan, L., Chen, L., Zhang, Z., Liu, Y., Zhou, Y., Ye, C., & Zhu, Q. (2024). Electrical Stimulation Induces Activation of Mitochondrial Apoptotic Pathway and Down-Regulates Heat Shock Proteins in Pork: An Innovative Strategy for Enhancing the Ripening Process and Quality of Dry-Cured Loin Ham. Foods, 13(11), 1717. https://doi.org/10.3390/foods13111717








