Supercritical CO2 Extraction of Terpenoids from Indocalamus latifolius Leaves: Optimization, Purification, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. GC-MS Analysis
2.3. Quantification of Diterpenes
2.4. Extraction Methods
2.4.1. Supercritical Carbon Dioxide Extraction (SC-CO2)
2.4.2. Steam Distillation (SD)
2.4.3. Simultaneous Distillation Extraction (SDE)
2.4.4. Ultrasound-Assisted Extraction (UE)
2.4.5. Ultra-High Pressure-Assisted Extraction (UHPE)
2.5. Measurement of Scanning Electron Microscopy (SEM)
2.6. Determination of In Vitro Antioxidant Activity of ILLTs by Different Methods
2.6.1. DPPH Radical Scavenging Activity Assay
2.6.2. Hydroxyl Radical Scavenging Activity Assay
2.7. Optimization of SC-CO2 Extraction
2.7.1. Single-Factor Test
2.7.2. Optimization of Experimental Design
2.8. Calculation of Diterpene Yield and Purity
2.9. Isolation and Purification
2.10. Cell Culture
2.10.1. Cell Viability
2.10.2. Determination of ROS and MDA Levels in Cells
2.10.3. The Production of ROS Was Observed by Microscopy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Impact of Different Extraction Methods on the Composition of I. latifolius Leaf Extracts
3.2. Influence of Different Extraction Methods on the Extraction Rate of I. latifolius Leaf Terpenoids (ILLTs)
3.3. SEM Imaging of I. latifolius Leaves during Extraction Using Different Extraction Methods
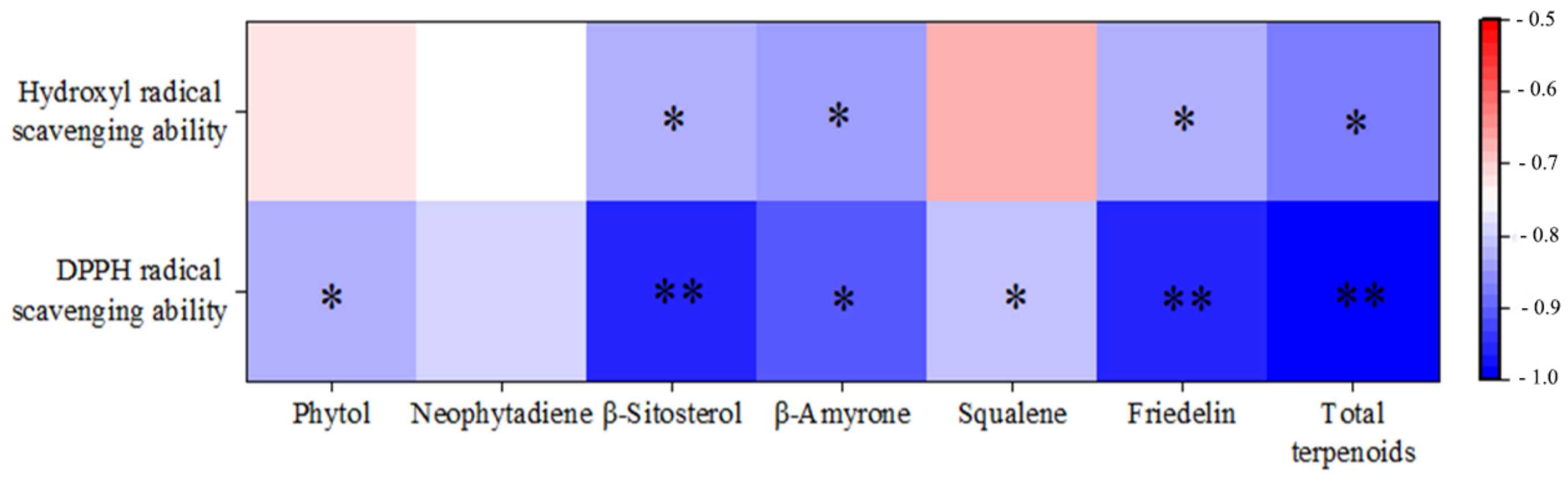
3.4. Study on the Antioxidant Activity of Extracts by Different Methods
3.5. Effects of Operating Conditions of SC-CO2 on the Extraction of ILLTs
3.6. Optimization of Extraction Conditions by CCD
3.7. Purification Results of Terpenoid Compounds from I. latifolius Leaves
3.8. Cytotoxicity Effect of ILLTs on HepG2 Cells
3.9. Effects of ILLTs on ROS and MDA Content of HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Ding, Y.; Elmahadi, E.A.; Zhou, J.; Xu, H. Study on the isolation, purification and physicochemical properties of polysaccharides from Indocalamus tesselatus. Biomed. Chromatogr. 1999, 13, 11–14. [Google Scholar] [CrossRef]
- Sun, J.; Xun, H.; Yu, J.; Tang, F.; Yue, Y.-D.; Guo, X.-F. Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”. Molecules 2015, 20, 15686–15700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Li, Q.; Cheng, J.; Wang, Y.; Zhao, J.; Yuan, S.; Chen, Y.; Shi, R. Optimization of ultrasonic-assisted deep eutectic solvent for the extraction of polysaccharides from Indocalamus tessellatus leaves and their biological studies. Sustain. Chem. Pharm. 2022, 30, 100855. [Google Scholar] [CrossRef]
- Tu, L.; Cai, X.; Zhang, Y.; Tong, Y.; Wang, J.; Su, P.; Lu, Y.; Hu, T.; Luo, Y.; Wu, X.; et al. Mechanistic analysis for the origin of diverse diterpenes in Tripterygium wilfordii. Acta Pharm. Sin. B 2022, 12, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Wardana, A.P.; Aminah, N.S.; Rosyda, M.; Abdjan, M.I.; Kristanti, A.N.; Tun, K.N.W.; Choudhary, M.I.; Takaya, Y. Potential of diterpene compounds as antivirals, a review. Heliyon 2021, 7, e07777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, X.; Qiao, Z.; Yu, X.; Zhu, Y.; Xie, L.; Qiang, L.; Fu, Y. Bioactive daphnane diterpenes from Wikstroemia chuii with their potential anti-inflammatory effects and anti-HIV activities. Bioorg. Chem. 2020, 105, 104388. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, M.; Zhao, C.; Liu, F.; Yang, H.; Li, Z.; Han, T.; Lin, B.; Li, D.; Hua, H. Abietane diterpenes from the twigs and leaves of Cephalotaxus oliveri Mast. with antitumor activity. Phytochemistry 2022, 199, 113187. [Google Scholar] [CrossRef] [PubMed]
- Paulous, R.; Zakaria, Z.; Awang, J.; Kamada, T.; Vairappan, C.S. Antifungal diterpene from Rhizome of wild bornean ginger, Hornstedtia havilandii (Zingiberaceae). Biochem. Syst. Ecol. 2022, 105, 104546. [Google Scholar] [CrossRef]
- Herrera, J.G.; Ramos, M.P.; de Lima Albuquerque, B.N.; de Oliveira Farias de Aguiar, J.C.R.; Agra Neto, A.C.; Guedes Paiva, P.M.; do Amaral Ferraz Navarro, D.M.; Pinto, L. Multivariate evaluation of process parameters to obtain essential oil of Piper corcovadensis using supercritical fluid extraction. Microchem. J. 2022, 181, 107747. [Google Scholar] [CrossRef]
- Pizani, R.S.; Viganó, J.; de Souza Mesquita, L.M.; Contieri, L.S.; Sanches, V.L.; Chaves, J.O.; Souza, M.C.; da Silva, L.C.; Rostagno, M.A. Beyond aroma: A review on advanced extraction processes from rosemary (Rosmarinus officinalis) and sage (Salvia officinalis) to produce phenolic acids and diterpenes. Trends Food Sci. Technol. 2022, 127, 245–262. [Google Scholar] [CrossRef]
- Guta, M.; Tan, H.; Zhao, Y. Optimization of supercritical CO2 extraction of incensole-enriched oil from Boswellia papyrifera resin using response surface methodology. J. Supercrit. Fluids 2024, 205, 106154. [Google Scholar] [CrossRef]
- Pereira, G.S.L.; Magalhães, R.d.S.; Fraga, S.; de Souza, P.T.; de Lima, J.P.; Meirelles, A.J.d.A.; Sampaio, K.A. Extraction of bioactive compounds from Butia capitata fruits using supercritical carbon dioxide and pressurized fluids. J. Supercrit. Fluids 2023, 199, 105959. [Google Scholar] [CrossRef]
- Vinitha, U.G.; Sathasivam, R.; Muthuraman, M.S.; Park, S.U. Intensification of supercritical fluid in the extraction of flavonoids: A comprehensive review. Physiol. Mol. Plant Pathol. 2022, 118, 101815. [Google Scholar] [CrossRef]
- Melloul, S.; Zehioua, R.; Meniai, A.-H. Supercritical CO2 extraction of bioactive compounds from local Peganum Harmala plant seeds and optimization of the extraction yield and the antioxidant activities. Sustain. Chem. Pharm. 2022, 28, 101815. [Google Scholar] [CrossRef]
- Alvarez-Henao, M.V.; Cardona, L.; Hincapié, S.; Londoño-Londoño, J.; Jimenez-Cartagena, C. Supercritical fluid extraction of phytosterols from sugarcane bagasse: Evaluation of extraction parameters. J. Supercrit. Fluids 2022, 179, 105427. [Google Scholar] [CrossRef]
- Gong, T.; Liu, S.; Wang, H.; Zhang, M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- Barbosa, H.M.A.; de Melo, M.M.R.; Coimbra, M.A.; Passos, C.P.; Silva, C.M. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluids 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Zulkafli, Z.D.; Wang, H.; Miyashita, F.; Utsumi, N.; Tamura, K. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata). J. Supercrit. Fluids 2014, 94, 123–129. [Google Scholar] [CrossRef]
- Muala, W.C.B.; Desobgo, Z.S.C.; Jong, N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 2021, 7, e06744. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Grao-Cruces, E.; Toscano, R.; Millan-Linares, M.C.; Montserrat-de la Paz, S.; Martin, M.E. Acyclic Diterpene Phytol from Hemp Seed Oil (Cannabis sativa L.) Exerts Anti-Inflammatory Activity on Primary Human Monocytes-Macrophages. Foods 2022, 11, 2366. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Ono, A.; Kawaguchi, K.; Teraoka, S.; Harada, M.; Sumi, K.; Ando, M.; Tsukamasa, Y.; Ninomiya, M.; Koketsu, M.; et al. Phytol isolated from watermelon (Citrullus lanatus) sprouts induces cell death in human T-lymphoid cell line Jurkat cells via S-phase cell cycle arrest. Food Chem. Toxicol. 2018, 115, 425–435. [Google Scholar] [CrossRef]
- He, B.; Chen, L.; Chen, C.; Zeng, J.; Xu, J.; He, X.; Wang, Y. Anti-inflammatory terpenoids from the seeds of Eucommia ulmoides. Phytochem. Lett. 2024, 61, 44–51. [Google Scholar] [CrossRef]
- Zhang ZDai, X. Preparation of alginate oligosaccharide nanoliposomes and an analysis of their inhibitory effects on Caco-2 cells. IET Nanobiotechnol. 2018, 12, 946–950. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Hao, X.; Yan, Y.; Hong, M.; Wei, S.; Zhou, Y.; Wang, Q.; Cheng, Y.; Liu, Y. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, N.; Gao, Y.; Han, R.; Wang, X.; Tian, J.; Gao, J. Phytosterol profiles and iridoids of the edible Eucommia ulmoides Oliver seeds and their anti-inflammatory potential. Food Biosci. 2021, 43, 101295. [Google Scholar] [CrossRef]
- He, D.; Wang, S.; Fang, G.; Zhu, Q.; Wu, J.; Li, J.; Shi, D.; Lian, X. LXRs/ABCA1 activation contribute to the anti-inflammatory role of phytosterols on LPS-induced acute lung injury. J. Funct. Foods 2022, 89, 104966. [Google Scholar] [CrossRef]
- Glisic, S.B.; Ristic, M.; Skala, D.U. The combined extraction of sage (Salvia officinalis L.): Ultrasound followed by supercritical CO2 extraction. Ultrason. Sonochem. 2011, 18, 318–326. [Google Scholar] [CrossRef]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.-S.; Romdhane, M. Optimization of accelerated solvent extraction of bioactive compounds from Eucalyptus intertexta using response surface methodology and evaluation of its phenolic composition and biological activities. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100464. [Google Scholar] [CrossRef]
- Luca, S.V.; Kittl, T.; Minceva, M. Supercritical CO2 extraction of hemp flowers: A systematic study to produce terpene-rich and terpene-depleted cannabidiol fractions. Ind. Crops Prod. 2022, 187, 115395. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, Z.; Mo, M.; Duan, W.; Bi, Y.; Kong, F. Essential oils from sugarcane molasses: Chemical composition, optimization of microwave-assisted hydrodistillation by response surface methodology and evaluation of its antioxidant and antibacterial activities. Ind. Crops Prod. 2020, 156, 112875. [Google Scholar] [CrossRef]
- Bajer, T.; Surmová, S.; Eisner, A.; Ventura, K.; Bajerová, P. Use of simultaneous distillation-extraction, supercritical fluid extraction and solid-phase microextraction for characterisation of the volatile profile of Dipteryx odorata (Aubl.) Willd. Ind. Crops Prod. 2018, 119, 313–321. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, F.; Ke, B.; Zhang, L.; Yan, X.; Deng, G. Sequential two-stage extraction of Xanthoceras sorbifolia seed oil using supercritical CO2 and CO2-expanded ethanol. J. Supercrit. Fluids 2023, 200, 105977. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, X.; Liu, H.; Qian, J. In vitro and in vivo anti-inflammatory models demonstrate oligopeptides play a significant role in anti-inflammatory properties of white tea. J. Funct. Foods 2024, 112, 105983. [Google Scholar] [CrossRef]
- Díaz-Viciedo, R.; Hortelano, S.; Girón, N.; Massó, J.M.; Rodriguez, B.; Villar, A.; de las Heras, B. Modulation of inflammatory responses by diterpene acids from Helianthus annuus L. Biochem. Biophys. Res. Commun. 2008, 369, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Antioxidant, free radical scavenging and liver protective effects of friedelin isolated from Azima tetracantha Lam. leaves. Food Chem. 2013, 139, 860–865. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Wu, Q.; Jin, Y.; Ning, C.; Wang, R.; Mao, J.; Chen, M. The hepatoprotective effects of Sedum sarmentosum extract and its isolated major constituent through Nrf2 activation and NF-κB inhibition. Phytomedicine 2019, 53, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem.-Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Rath, D.; Kar, D.M.; Pattanaik, S. Hepatoprotective potency of Litsea glutinosa (L.) C.B. Rob. leaf methanol extract on H2O2-induced toxicity in HepG2 cells. J. Ethnopharmacol. 2023, 304, 116076. [Google Scholar] [CrossRef] [PubMed]
- Dumandan, N.G.; Kagaoan, A.C.T.; Acda, R.D.P.; Tumambing, C.R.; Pham, L.J.; Mohamed, S.A. Extraction, Profiling, and Characterization of Phytosterols and Triterpenoids from Pili (Canarium ovatum Engl.) Pulp Oil Exhibiting Antioxidant and Antibacterial Properties. Biochem. Res. Int. 2022, 2022, 6604984. [Google Scholar] [CrossRef]
- Marzlan, A.A.; Muhialdin, B.J.; Zainal Abedin, N.H.; Mohammed, N.K.; Abadl, M.M.T.; Mohd Roby, B.H.; Meor Hussin, A.S. Optimized supercritical CO2 extraction conditions on yield and quality of torch ginger (Etlingera elatior (Jack) R.M. Smith) inflorescence essential oil. Ind. Crops Prod. 2020, 154, 112581. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wei, S.; Yan, Z. Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn. Food Chem. 2012, 132, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Rout, P.K.; Naik, S.N.; Rao, Y.R.; Jadeja, G.; Maheshwari, R.C. Extraction and composition of volatiles from Zanthoxylum rhesta: Comparison of subcritical CO2 and traditional processes. J. Supercrit. Fluids 2007, 42, 334–341. [Google Scholar] [CrossRef]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors that influence the extraction methods of terpenes from natural sources. Chem. Pap. 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Gasparini, A.; Ferrentino, G.; Angeli, L.; Morozova, K.; Zatelli, D.; Scampicchio, M. Ultrasound assisted extraction of oils from apple seeds: A comparative study with supercritical fluid and conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2023, 86, 103370. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, L.; Liang, Z.; Chen, J.; Zhao, M.; Tang, Q. Comparative analysis of flavonoids extracted from Dendrobium chrysotoxum flowers by supercritical fluid extraction and ultrasonic cold extraction. Sustain. Chem. Pharm. 2023, 36, 101267. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, T.H.; Nguyen, T.H.; Do, T.H. Cytotoxic sesquiterpenes and diterpenes from the rhizomes of Curcuma zedoaroides Chaveer. & Tanee. Biochem. Syst. Ecol. 2024, 112, 104781. [Google Scholar]
- Tao, L.; Gu, F.; Liu, Y.; Yang, M.; Wu, X.-Z.; Sheng, J.; Tian, Y. Preparation of antioxidant peptides from Moringa oleifera leaves and their protection against oxidative damage in HepG2 cells. Front. Nutr. 2022, 9, 1062671. [Google Scholar] [CrossRef]








| Group | Samples, Vc | Ethyl Alcohol | DPPH Ethanol Solution (0.1 mmol/L) |
|---|---|---|---|
| Asample | 1 mL | - | 1 mL |
| Ablank | 1 mL | 1 mL | - |
| Acontrol | - | 1 mL | 1 mL |
| Factor | Units | Level of Factor | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: Extraction stress | MPa | 10 | 20 | 30 |
| B: Extraction temperature | °C | 32 | 37 | 42 |
| C: Cosolvent | % (v/w) | 0 | 10 | 20 |
| D: Time | h | 3 | 4 | 5 |
| No. | Compound | Molecular Formula | Molecular Weight | CAS | Absolute Content * (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | SDE | UHPE-EtOH | UHPE-Hex | UE-EtOH | UE-Hex | SC-CO2 | |||||||
| 1 | Isophytol | C20H40O | 297 | 505-32-8 | 0.069 ± 0.001 | 0.036 ± 0.001 | |||||||
| 2 | Phytol | C20H40O | 297 | 150-86-7 | 0.677 ± 0.043 | 0.438 ± 0.061 | 0.219 ± 0.017 | 1.553 ± 0.083 | 2.932 ± 0.096 | 5.38 ± 0.089 | 5.745 ± 0.073 | ||
| 3 | Heneicosanol | C21H44O | 313 | 3381-26-8 | 1.948 ± 0.101 | 0.086 ± 0.060 | |||||||
| 4 | Octacosanol | C28H58O | 411 | 557-61-9 | 0.148 ± 0.001 | 0.167 ± 0.001 | 0.071 ± 0.029 | ||||||
| 5 | Lignocerol | C24H50O | 355 | 506-51-4 | 0.094 ± 0.031 | ||||||||
| 6 | Campesterol | C28H48O | 401 | 474-62-4 | 0.196 ± 0.037 | 0.346 ± 0.052 | 0.422 ± 0.047 | ||||||
| 7 | Stigmasterol | C29H48O | 413 | 83-48-7 | 0.104 ± 0.018 | 0.171 ± 0.046 | 0.269 ± 0.051 | ||||||
| 8 | β-Sitosterol | C29H50O | 415 | 5779-62-4 | 0.019 ± 0.006 | 3.094 ± 0.122 | 8.686 ± 0.141 | 9.909 ± 0.901 | |||||
| 9 | Lupeol | C30H50O | 427 | 545-47-1 | 0.074 ± 0.061 | ||||||||
| 10 | 2-(Octadecyloxy)-ethanol | C20H42O2 | 533 | 2136-72-3 | 0.013 ± 0.041 | ||||||||
| 11 | 2-Ethyl-1-decanol | C12H26O | 186 | 21078-65-9 | 0.011 ± 0.001 | ||||||||
| 12 | Tetrahydrofurfuryl alcohol | C5H10O2 | 102 | 97-99-4 | 0.061 ± 0.008 | ||||||||
| 13 | 1-Docosanol | C22H46O | 326 | 661-19-8 | 0.285 ± 0.067 | ||||||||
| 14 | Sclareol | C20H36O2 | 308 | 515-03-7 | 0.227 ± 0.001 | 0.084 ± 0.033 | |||||||
| 15 | (E, E)-10,12-Hexadecadien-1-ol acetat | C16H30O | 238 | 765-19-5 | 0.033 ± 0.012 | ||||||||
| 16 | Heptacosanol | C27H56O | 397 | 2004-39-9 | 0.124 ± 0.092 | ||||||||
| 17 | Nonacosanol | C29H60O | 425 | 25154-56-7 | 0.279 ± 0.082 | ||||||||
| 18 | Glutina-5-ene-3β-ol | C30H50O | 427 | 545-24-4 | 0.072 ± 0.041 | ||||||||
| 19 | Glycerin | C3H8O3 | 92 | 30918-77-5 | 12.36 ± 1.073 | ||||||||
| 20 | Hexadecanal | C16H32O | 240 | 629-80-1 | 0.064 ± 0.005 | 0.124 ± 0.076 | |||||||
| 21 | Octadecanal | C18H36O | 268 | 638-66-4 | 4.050 ± 0.475 | 0.036 ± 0.018 | 0.041 ± 0.021 | ||||||
| 22 | Hexanal | C6H12O | 100 | 66-25-1 | 1.18 ± 0.089 | ||||||||
| 23 | 2-Hexenal | C6H10O | 98 | 505-57-7 | 0.220 ± 0.074 | ||||||||
| 24 | (E, E)-2,4-Heptadienal | C7H10O | 110 | 4313-3-5 | 0.061 ± 0.023 | ||||||||
| 25 | Benzeneacetaldehyde | C8H8O | 120 | 122-78-1 | 1.648 ± 0.001 | ||||||||
| 26 | Nonanal | C9H18O | 142 | 124-19-6 | 0.916 ± 0.149 | ||||||||
| 27 | β-Cyclocitral | C10H16O | 152 | 432-25-7 | 0.102 ± 0.013 | ||||||||
| 28 | (Z)-7-Tetradecenal | C14H26O | 210 | 65128-96-3 | 0.127 ± 0.016 | ||||||||
| 29 | α-Ionone | C13H20O | 192 | 127-41-3 | 0.003 ± 0.001 | 0.491 ± 0.165 | 0.024 ± 0.007 | ||||||
| 30 | β-Apo-13-carotenone | C18H26O | 258 | 17974-57-1 | 0.097 ± 0.011 | 0.072 ± 0.031 | 0.059 ± 0.023 | ||||||
| 31 | 2-Nonadecanone | C19H38O | 282 | 629-66-3 | 0.057 ± 0.009 | ||||||||
| 32 | Dotriacontanal | C32H64O | 465 | 57878-00-9 | 2.016 ± 0.822 | ||||||||
| 33 | 2-Nonacosanone | C29H58O | 423 | 17600-99-6 | 0.099 ± 0.021 | ||||||||
| 34 | Phytone | C18H36O | 268 | 502-69-2 | 0.709 ± 0.173 | 0.211 ± 0.054 | 0.032 ± 0.007 | ||||||
| 35 | Trans-β-Ionone | C13H20O | 192 | 14901-07-6 | 0.032 ± 0.008 | 0.070 ± 0.011 | |||||||
| 36 | Hydroxyacetone | C3H6O2 | 74 | 116-09-6 | 9.461 ± 0.363 | ||||||||
| 37 | D-Friedoolean-14-en-3-one | C30H48O | 425 | 514-07-8 | 0.062 ± 0.013 | 0.296 ± 0.047 | |||||||
| 38 | 4-(1,5-Dihydroxy-2,6,6-trimethylcyclohex-2-enyl) but-3-en-2-one | C13H20O3 | 208 | 38963-41-6 | 0.035 ± 0.014 | ||||||||
| 39 | 4-(3-Hydroxybutyl)-3,5,5-trimethyl-2-cyclohexen-1-one | C13H22O2 | 210 | 36151-02-7 | 0.041 ± 0.016 | ||||||||
| 40 | β-Amyrone | C30H48O | 425 | 638-97-1 | 0.517 ± 0.001 | 1.583 ± 0.891 | 1.591 ± 0.131 | ||||||
| 41 | Lup-20(29)-en-3-one | C30H48O | 425 | 1617-70-5 | 0.061 ± 0.012 | 0.071 ± 0.019 | |||||||
| 42 | Friedelin | C30H50O | 427 | 559-74-0 | 0.062 ± 0.018 | 1.405 ± 0.521 | 6.699 ± 1.091 | 4.100 ± 0.941 | |||||
| 43 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one | C6H8O4 | 144 | 28564-83-2 | 6.588 ± 0.873 | ||||||||
| 44 | 17-Pentatriacontene | C15H30O | 226 | 2345-28-0 | 0.050 ± 0.016 | ||||||||
| 45 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 7786-61-0 | 0.256 ± 0.068 | ||||||||
| 46 | 3,5-Di-tert-butylphenol | C14H22O | 206 | 1138-52-9 | 0.114 ± 0.070 | ||||||||
| 47 | 2,4-Di-t-butylphenol | C14H22O | 206 | 96-76-4 | 0.059 ± 0.017 | 0.024 ± 0.013 | |||||||
| 48 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) | C23H32O2 | 340 | 119-47-1 | 0.405 ± 0.026 | 0.452 ± 0.095 | |||||||
| 49 | 2,6-Di-tert-butylphenol | C14H22O | 206 | 128-39-2 | 0.561 ± 0.064 | ||||||||
| 50 | γ-Tocopherol | C28H48O2 | 417 | 54-28-4 | 0.042 ± 0.015 | 0.080 ± 0.021 | |||||||
| 51 | (Z, Z)-9,12-Octadecadienoic acid | C20H36O2 | 424 | 544-35-4 | 0.546 ± 0.088 | ||||||||
| 52 | 5,6,7,7a-Tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | C11H16O2 | 180 | 15356-74-8 | 0.024 ± 0.006 | ||||||||
| 53 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 112-39-0 | 0.132 ± 0.025 | 1.329 ± 0.471 | 0.357 ± 0.081 | ||||||
| 54 | Ethyl palmitate | C18H36O2 | 284 | 628-97-7 | 0.096 ± 0.041 | 0.717 ± 0.092 | |||||||
| 55 | Octacosyl acetate | C30H60O2 | 452 | 18206-97-8 | 0.012 ± 0.001 | ||||||||
| 56 | Methyl salicylate | C8H8O3 | 152 | 119-36-8 | 0.104 ± 0.019 | ||||||||
| 57 | Benzyl salicylate | C14H12O3 | 228 | 118-58-1 | 0.036 ± 0.017 | ||||||||
| 58 | 1-Heneicosyl formate | C22H44O2 | 341 | 77899-03-7 | 0.013 ± 0.003 | ||||||||
| 59 | (Z)-7-Hexadecenoic acid, methyl ester | C17H32O2 | 268 | 56875-67-3 | 0.074 ± 0.015 | ||||||||
| 60 | 9-Hexadecenoic acid, ethyl ester | C18H34O2 | 282 | 54546-22-4 | 0.118 ± 0.027 | ||||||||
| 61 | Heptadecanoic acid, ethyl ester | C19H38O2 | 299 | 14010-23-2 | 0.044 ± 0.009 | 0.046 ± 0.011 | |||||||
| 62 | Elaidic acid ethyl ester | C20H38O2 | 311 | 6114-18-7 | 0.763 ± 0.039 | ||||||||
| 63 | Ethyl icosanoate | C22H44O2 | 341 | 18281-05-5 | 0.060 ± 0.017 | 0.103 ± 0.005 | |||||||
| 64 | 1-Hexadecanol, acetate | C18H36O2 | 284 | 629-70-9 | 0.072 ± 0.033 | ||||||||
| 65 | Butyrolactone | C4H6O2 | 86 | 3068-88-0 | 1.402 ± 0.107 | ||||||||
| 66 | Ethyl stearate | C20H40O2 | 313 | 111-61-5 | 0.035 ± 0.007 | ||||||||
| 67 | Methyl octadeca-9,12-dienoate | C19H34O2 | 294 | 2566-97-4 | 0.162 ± 0.017 | ||||||||
| 68 | 1,2,3-Trielaidoyl glycerol | C57H104O6 | 885 | 537-39-3 | 0.496 ± 0.065 | ||||||||
| 69 | Phytyl acetate | C22H42O2 | 339 | 10236-16-5 | 0.044 ± 0.013 | ||||||||
| 70 | β-Sitosterol acetate | C31H52O2 | 457 | 915-05-9 | 0.086 ± 0.014 | ||||||||
| 71 | α-Tocopheryl acetate | C31H52O3 | 473 | 7695-91-2 | 0.059 ± 0.005 | ||||||||
| 72 | Ethyl linolenate | C20H34O2 | 306 | 1191-41-9 | 1.007 ± 0.085 | ||||||||
| 73 | Linoleic acid ethyl ester | C20H36O2 | 309 | 544-35-4 | 0.579 ± 0.053 | 0.702 ± 0.074 | |||||||
| 74 | 2,2-Dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)-oxirane | C30H50O | 427 | 7200-26-2 | 0.053 ± 0.006 | 0.056 ± 0.011 | |||||||
| 75 | 1-Pentadecene | C15H30 | 210 | 13360-61-7 | 0.130 ± 0.025 | ||||||||
| 76 | (E)-3-Eicosene | C20H40 | 281 | 74685-33-9 | 0.077 ± 0.012 | ||||||||
| 77 | (E)-5-Eicosene | C20H40 | 281 | 74685-30-6 | 0.159 ± 0.034 | ||||||||
| 78 | 1-Docosene | C22H44 | 309 | 1599-67-3 | 0.084 ± 0.013 | 0.433 ± 0.058 | |||||||
| 79 | Squalene | C30H50 | 411 | 111-02-4 | 0.006 ± 0.001 | 0.075 ± 0.009 | 0.054 ± 0.005 | 0.144 ± 0.012 | |||||
| 80 | 1,19-Eicosadiene | C20H38 | 279 | 14811-95-1 | 0.020 ± 0.001 | 0.380 ± 0.016 | |||||||
| 81 | 8-Heptadecene | C17H34 | 238 | 2579-4-6 | 0.438 ± 0.081 | ||||||||
| 82 | 7-Methyl-6-Tridecene | C14H28 | 196 | 24949-42-6 | 0.149 ± 0.036 | ||||||||
| 83 | (Z)-9-Tricosene | C23H46 | 323 | 27519-02-4 | 0.246 ± 0.056 | ||||||||
| 84 | Neophytadiene | C20H38 | 279 | 504-96-1 | 0.002 ± 0.001 | 0.016 ± 0.002 | 0.383 ± 0.027 | 0.633 ± 0.036 | 0.073 ± 0.008 | 0.122 ± 0.015 | |||
| 85 | Nonadecane | C19H40 | 269 | 629-92-5 | 0.247 ± 0.062 | 0.027 ± 0.004 | |||||||
| 86 | Heptacosane | C27H56 | 381 | 593-49-7 | 0.080 ± 0.017 | 0.197 ± 0.016 | |||||||
| 87 | Triacontane | C30H62 | 423 | 638-68-6 | 0.224 ± 0.078 | ||||||||
| 88 | Heneicosane | C21H44 | 297 | 629-94-7 | 1.960 ± 0.106 | 0.099 ± 0.012 | 0.187 ± 0.009 | 0.289 ± 0.017 | |||||
| 89 | Pentacosane | C25H52 | 353 | 629-99-2 | 0.036 ± 0.011 | 0.050 ± 0.007 | 0.342 ± 0.029 | 0.209 ± 0.071 | |||||
| 90 | Tricosane | C23H48 | 325 | 638-67-5 | 0.062 ± 0.008 | 0.443 ± 0.058 | |||||||
| 91 | Undecane | C11H24 | 156 | 1120-21-4 | |||||||||
| 92 | Hexadecane | C16H34 | 226 | 544-76-3 | 0.034 ± 0.016 | ||||||||
| 93 | Docosane | C22H46 | 311 | 629-97-0 | 0.027 ± 0.002 | ||||||||
| 94 | Tetratetracontane | C44H90 | 619 | 7098-22-8 | 0.049 ± 0.007 | ||||||||
| 95 | Hentriacontane | C31H64 | 437 | 630-04-6 | 1.074 ± 0.006 | ||||||||
| 96 | 2-Methylhexacosane | C27H56 | 381 | 1561-02-0 | 0.292 ± 0.021 | ||||||||
| 97 | 3,5,24-Trimethyl-tetracontane | C43H88 | 605 | 55162-61-3 | 0.017 ± 0.008 | ||||||||
| 98 | 2,6,10,15-Tetramethyl-heptadecane | C21H44 | 297 | 54833-48-6 | 0.013 ± 0.002 | ||||||||
| 99 | 5,14-Dibutyloctadecane | C26H54 | 367 | 55282-13-8 | 0.022 ± 0.002 | ||||||||
| 100 | 7-Hexyltridecane | C19H40 | 269 | 7225-66-3 | 1.458 ± 0.075 | 0.022 ± 0.003 | |||||||
| 101 | 8-Hexyl-pentadecane | C21H44 | 297 | 13475-75-7 | 0.499 ± 0.035 | 0.210 ± 0.048 | |||||||
| 102 | Dotriacontane | C32H66 | 451 | 544-85-4 | 0.049 ± 0.006 | ||||||||
| 103 | Tetracosane | C24H50 | 339 | 646-31-1 | 0.062 ± 0.014 | 0.084 ± 0.022 | 0.044 ± 0.006 | 0.215 ± 0.061 | |||||
| 104 | Octadecane | C18H38 | 254 | 593-45-3 | 0.016 ± 0.003 | 0.192 ± 0.047 | |||||||
| 105 | 2,6,10,15-Tetramethyl heptadecane | C21H44 | 297 | 54833-48-6 | 0.054 ± 0.007 | ||||||||
| 106 | Phytonadione | C31H46O2 | 451 | 84-80-0 | 0.092 ± 0.011 | ||||||||
| 107 | Cholesterol | C27H46O | 387 | 57-88-5 | 0.136 ± 0.038 | ||||||||
| 108 | 2-Palmitoylglycerol | C19H38O4 | 331 | 23470-00-0 | 0.304 ± 0.068 | ||||||||
| 109 | Hexadecanamide | C16H33NO | 255 | 629-54-9 | 0.968 ± 0.056 | ||||||||
| 110 | (Z)-9-Octadecenamide | C18H35NO | 281 | 301-02-0 | 0.706 ± 0.090 | 0.084 ± 0.029 | 0.115 ± 0.024 | ||||||
| 111 | Vitamin E | C29H50O2 | 431 | 2074-53-5 | 0.166 ± 0.012 | 0.448 ± 0.034 | 0.531 ± 0.078 | ||||||
| Run | A 1 | B 1 | C 1 | D 1 | Yield of Diterpenes (mg/g) | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| 1 | 0 | 0 | 0 | 0 | 0.75 | 0.76 |
| 2 | −1 | −1 | 0 | 0 | 0.28 | 0.31 |
| 3 | 0 | 0 | 1 | −1 | 0.58 | 0.57 |
| 4 | 0 | 0 | −1 | −1 | 0.49 | 0.48 |
| 5 | 0 | 0 | 0 | 0 | 0.77 | 0.76 |
| 6 | 1 | −1 | 0 | 0 | 0.42 | 0.40 |
| 7 | 1 | 1 | 0 | 0 | 0.74 | 0.72 |
| 8 | −1 | 1 | 0 | 0 | 0.19 | 0.23 |
| 9 | 0 | 0 | −1 | 1 | 0.47 | 0.47 |
| 10 | 0 | 0 | 1 | 1 | 0.81 | 0.80 |
| 11 | 1 | 0 | 0 | 1 | 0.60 | 0.61 |
| 12 | 0 | 0 | 0 | 0 | 0.78 | 0.76 |
| 13 | −1 | 0 | 0 | −1 | 0.22 | 0.21 |
| 14 | 0 | 1 | −1 | 0 | 0.63 | 0.61 |
| 15 | 0 | 1 | 1 | 0 | 0.78 | 0.78 |
| 16 | 0 | −1 | −1 | 0 | 0.47 | 0.44 |
| 17 | 1 | 0 | 0 | −1 | 0.40 | 0.40 |
| 18 | −1 | 0 | 0 | 1 | 0.24 | 0.23 |
| 19 | 0 | 0 | 0 | 0 | 0.72 | 0.76 |
| 20 | 0 | −1 | 1 | 0 | 0.72 | 0.70 |
| 21 | 1 | 0 | −1 | 0 | 0.52 | 0.52 |
| 22 | −1 | 0 | 1 | 0 | 0.49 | 0.45 |
| 23 | 0 | 0 | 0 | 0 | 0.75 | 0.76 |
| 24 | 0 | 0 | 0 | 0 | 0.77 | 0.76 |
| 25 | −1 | 0 | −1 | 0 | 0.35 | 0.31 |
| 26 | 0 | 1 | 0 | −1 | 0.47 | 0.45 |
| 27 | 0 | −1 | 0 | 1 | 0.43 | 0.44 |
| 28 | 0 | −1 | 0 | −1 | 0.37 | 0.38 |
| 29 | 1 | 0 | 1 | 0 | 0.83 | 0.81 |
| 30 | 0 | 1 | 0 | 1 | 0.63 | 0.61 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.09 | 14 | 0.0777 | 117.88 | <0.0001 ** |
| A | 0.2458 | 1 | 0.2458 | 372.87 | <0.0001 ** |
| B | 0.0464 | 1 | 0.0464 | 70.32 | <0.0001 ** |
| C | 0.1374 | 1 | 0.1374 | 208.49 | <0.0001 ** |
| D | 0.0361 | 1 | 0.0361 | 54.84 | <0.0001 ** |
| AB | 0.0428 | 1 | 0.0428 | 64.97 | <0.0001 ** |
| AC | 0.0062 | 1 | 0.0062 | 9.48 | 0.0081 |
| AD | 0.0084 | 1 | 0.0084 | 12.7 | 0.0031 * |
| BC | 0.0017 | 1 | 0.0017 | 2.58 | 0.1271 |
| BD | 0.0024 | 1 | 0.0024 | 3.66 | 0.0744 |
| CD | 0.0153 | 1 | 0.0153 | 23.21 | 0.0003 |
| A2 | 0.3478 | 1 | 0.3478 | 527.59 | <0.0001 ** |
| B2 | 0.0943 | 1 | 0.0943 | 143.02 | <0.0001 ** |
| C2 | 0.0004 | 1 | 0.0004 | 0.6609 | 0.4245 |
| D2 | 0.1999 | 1 | 0.1999 | 303.31 | <0.0001 ** |
| Residual | 0.0086 | 13 | 0.0007 | ||
| Lack of Fit | 0.0079 | 10 | 0.0008 | 3.8 | 0.2791 |
| Pure Error | 0.0006 | 3 | 0.0002 | ||
| Cor Total | 1.1 | 29 | 117.88 | ||
| R2 = 0.9738 R2adj = 0.9455 | 2.560 × 10−4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, Y.; He, L.; Wang, L.; Zhao, J.; Yang, Z.; Li, Q.; Shi, R. Supercritical CO2 Extraction of Terpenoids from Indocalamus latifolius Leaves: Optimization, Purification, and Antioxidant Activity. Foods 2024, 13, 1719. https://doi.org/10.3390/foods13111719
Chen Y, Wang Y, He L, Wang L, Zhao J, Yang Z, Li Q, Shi R. Supercritical CO2 Extraction of Terpenoids from Indocalamus latifolius Leaves: Optimization, Purification, and Antioxidant Activity. Foods. 2024; 13(11):1719. https://doi.org/10.3390/foods13111719
Chicago/Turabian StyleChen, Yadan, Yanbin Wang, Liang He, Liling Wang, Jianchen Zhao, Zhenya Yang, Qin Li, and Rui Shi. 2024. "Supercritical CO2 Extraction of Terpenoids from Indocalamus latifolius Leaves: Optimization, Purification, and Antioxidant Activity" Foods 13, no. 11: 1719. https://doi.org/10.3390/foods13111719
APA StyleChen, Y., Wang, Y., He, L., Wang, L., Zhao, J., Yang, Z., Li, Q., & Shi, R. (2024). Supercritical CO2 Extraction of Terpenoids from Indocalamus latifolius Leaves: Optimization, Purification, and Antioxidant Activity. Foods, 13(11), 1719. https://doi.org/10.3390/foods13111719





