Preparation and Characterization of Prickly Ash Peel Oleoresin Microcapsules and Flavor Retention Analysis
Abstract
1. Introduction
2. Materials and Methods
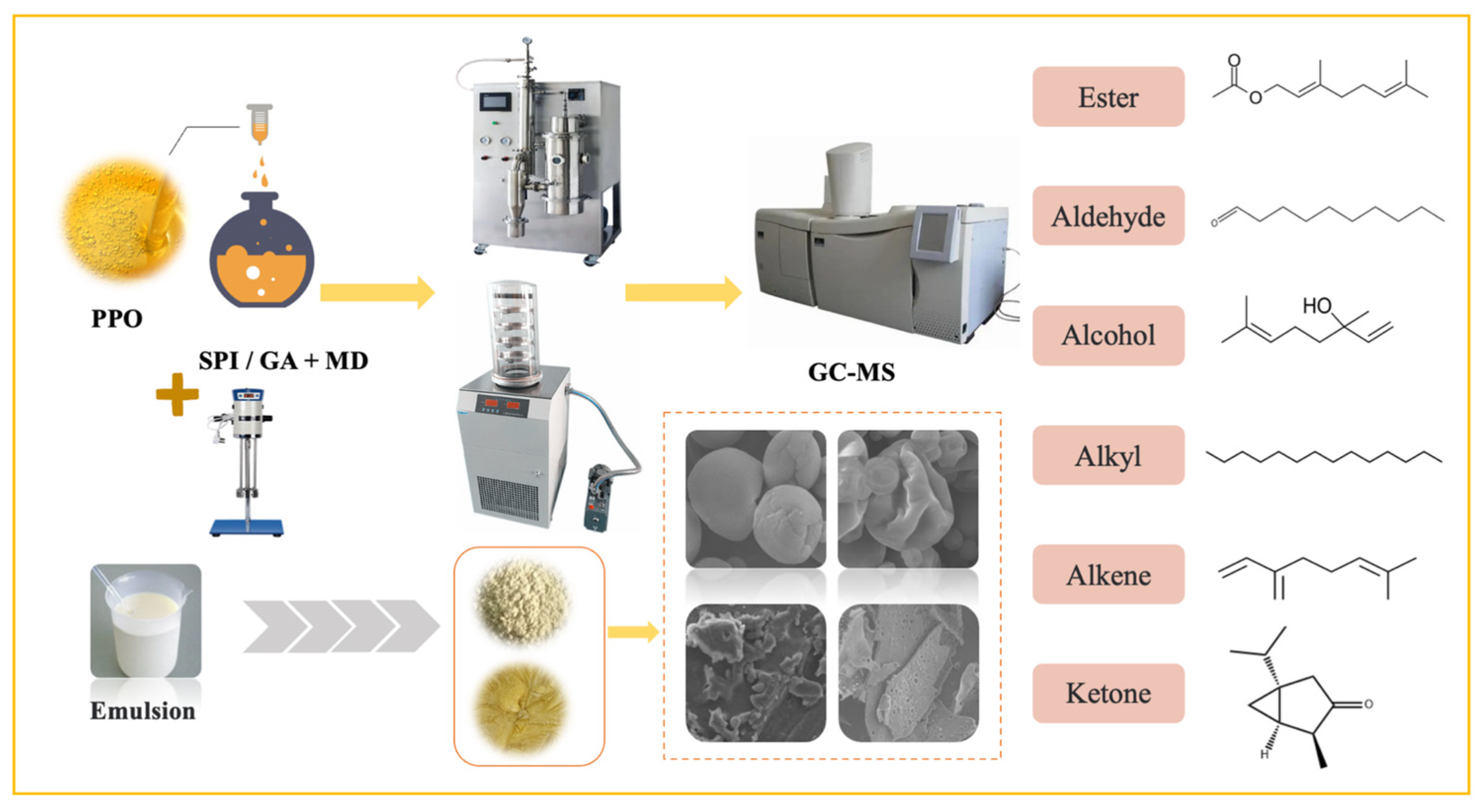
2.1. Emulsion Preparation
2.2. Preparation of Microcapsules
2.3. Microcapsule Characterization
2.3.1. Particle Size Analysis
2.3.2. Encapsulation Efficiency (EE) Determination
2.3.3. Scanning Electron Microscopy (SEM) Analysis
2.3.4. Differential Scanning Calorimetry (DSC) Analysis
2.3.5. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.4. Volatile Compound Analysis
2.4.1. Head Space-Solid Phase Microextraction/Gas Chromatography–Mass Spectroscopy (HS-SPME/GC-MS) Analysis
2.4.2. Principal Component Analysis (PCA)
2.4.3. Calculation of the Odor Activity Value (OAV)
2.5. Determination of Antioxidant Capacity
2.5.1. DPPH Clearance Rate
2.5.2. Hydroxyl Radical Scavenging
2.5.3. Clearance of Lipid Hydroperoxides
2.6. Statistical Analysis
3. Results
3.1. Characteristic Analysis of POMs
3.1.1. Particle Size and EE
3.1.2. SEM Analysis
3.1.3. DSC Analysis
3.1.4. FTIR Analysis
3.2. GC-MS Analysis
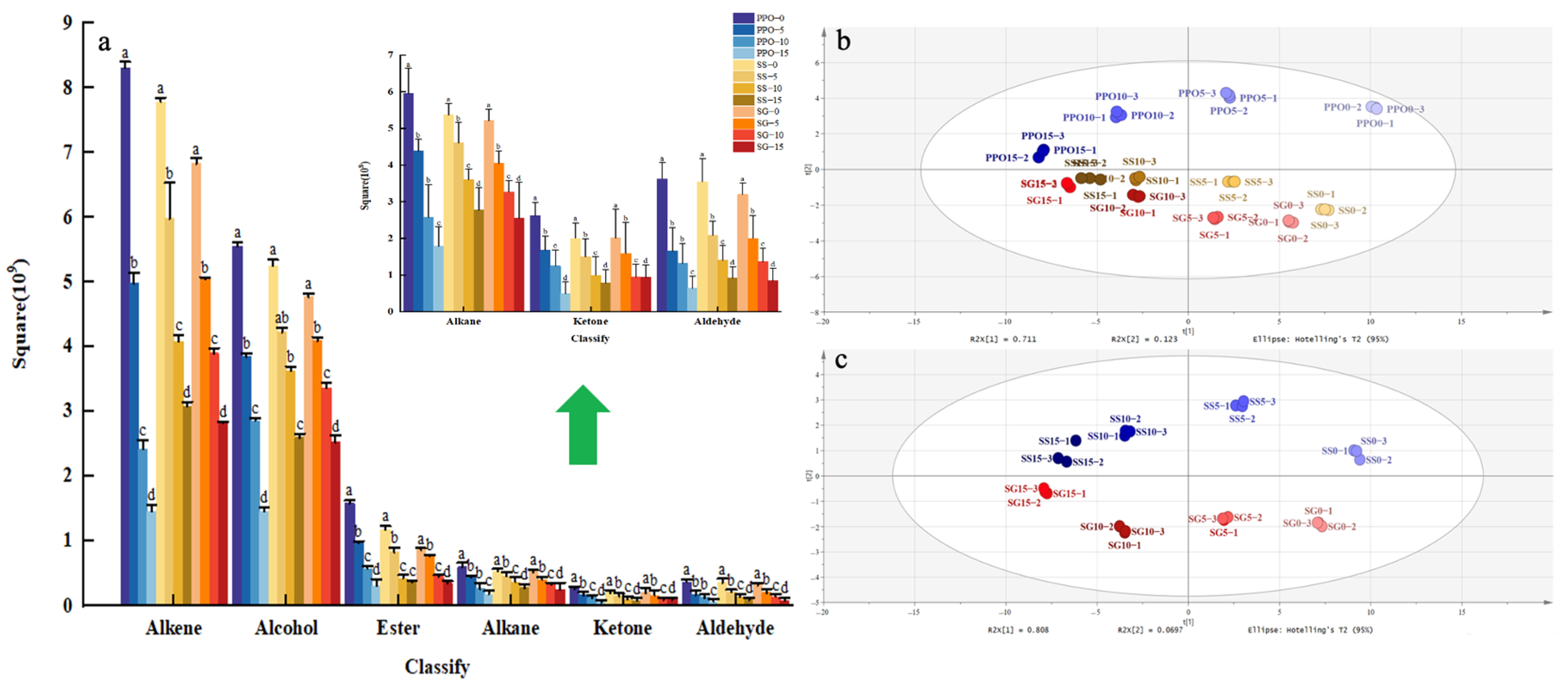
3.2.1. Composition and Content of Volatile Compounds
3.2.2. Principal Component Analysis (PCA)
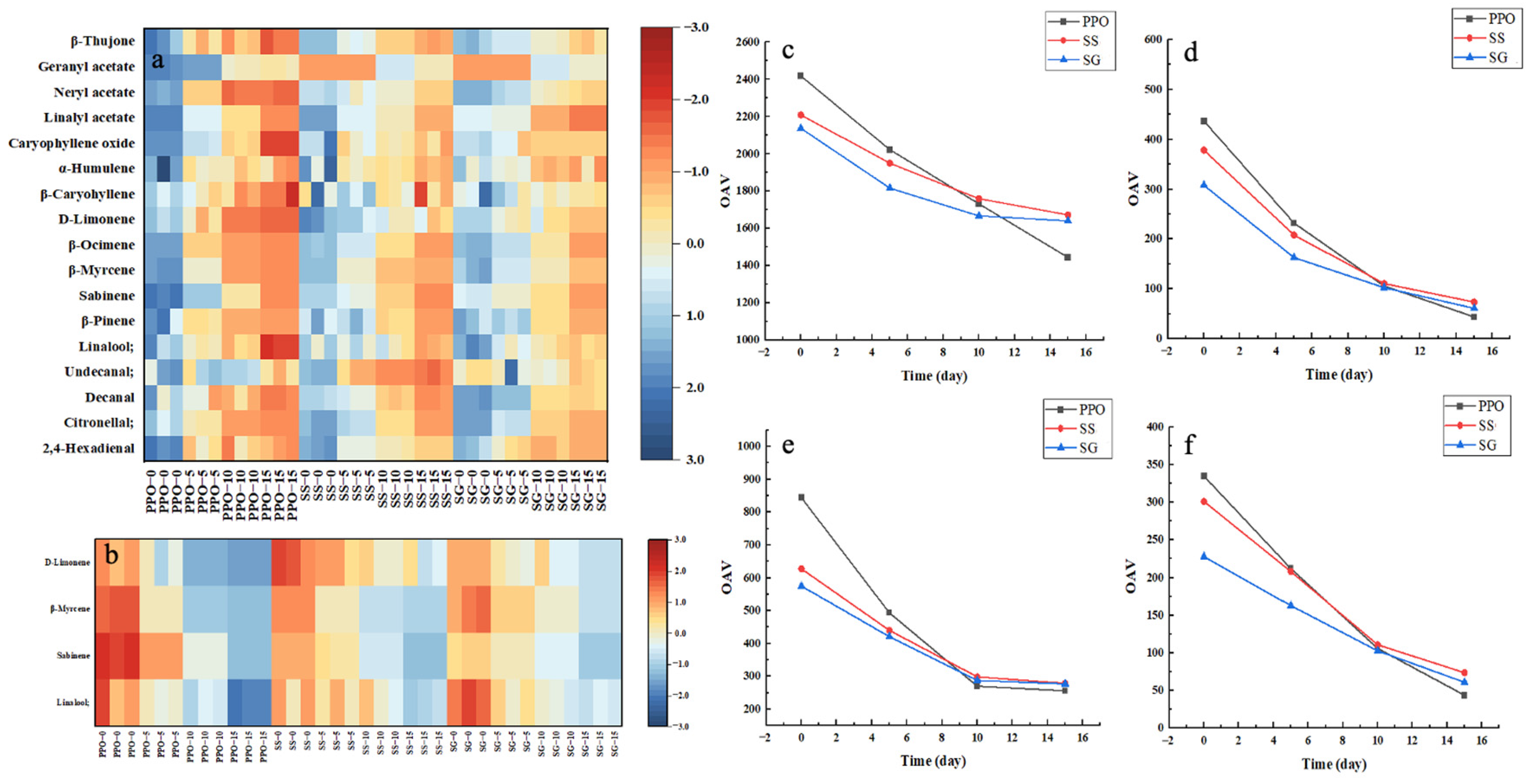
3.2.3. Volatile Flavor Compound Analysis
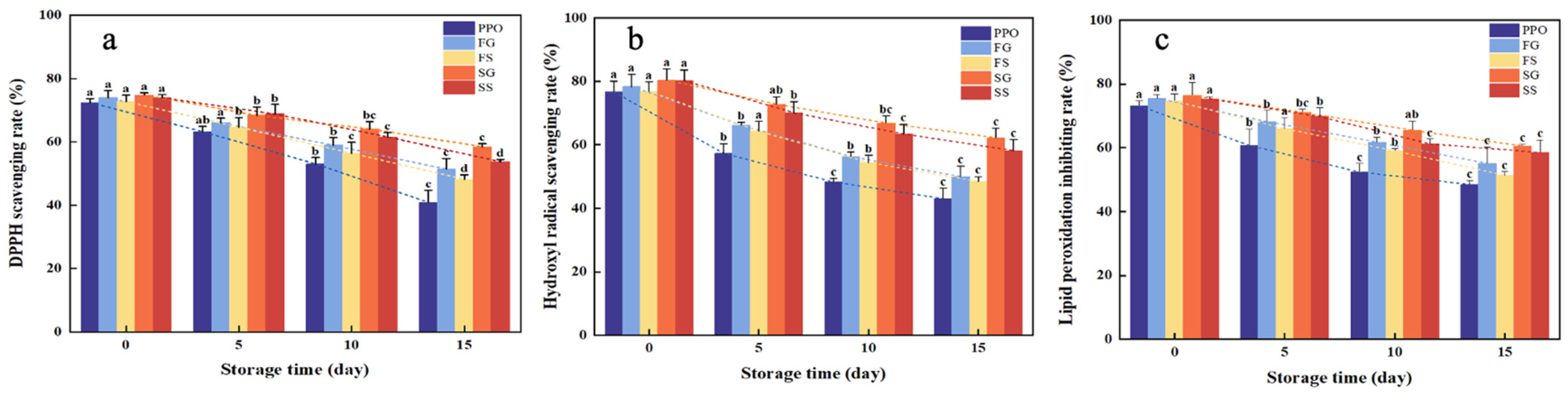
3.3. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Ke, J.; Hou, X.; Li, S.; Luo, Q.; Wu, H.; Shen, G.; Zhang, Z. Composition, Structure and Flavor Mechanism of Numbing Substances in Chinese Prickly Ash in the Genus Zanthoxylum: A Review. Food Chem. 2022, 373, 131454. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, H.; Liu, S.; Wang, G.; Yan, F.; Feng, J. Chemical Constituents from the Leaves of Zanthoxylum Nitidum (Roxb.) DC. Biochem. Syst. Ecol. 2020, 91, 104080. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Chen, K.; Bhandari, B.; Deng, D. Impact of Cooking Methods on the Quality, Sensory and Flavor Compounds of Sichuan Pepper Oleoresin. Food Chem. 2023, 427, 136639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. IJMS 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Chanotiya, C.S.; Yadav, A. Enantioenriched (3S)-(+)-Linalool in the Leaf Oil of Cinnamomum tamala Nees et Eberm. from Kumaon. J. Essent. Oil Res. 2010, 22, 593–596. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent Updates on Bioactive Properties of Linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile Essential Oils, Aromas and Flavours: Degradation Pathways, Effect of Thermal Processing and Alteration of Sensory Quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Cui, T.; Chen, C.; Jia, A.; Li, D.; Shi, Y.; Zhang, M.; Bai, X.; Liu, X.; Liu, C. Characterization and Human Microfold Cell Assay of Fish Oil Microcapsules: Effect of Spray Drying and Freeze-Drying Using Konjac Glucomannan (KGM)-Soybean Protein Isolate (SPI) as Wall Materials. J. Funct. Foods 2021, 83, 104542. [Google Scholar] [CrossRef]
- Li, J.; Hou, X.; Jiang, L.; Xia, D.; Chen, A.; Li, S.; Li, Q.; Gu, X.; Mo, X.; Zhang, Z. Optimization and Characterization of Sichuan Pepper (Zanthoxylum bungeanum Maxim) Resin Microcapsule Encapsulated with β-Cyclodextrin. LWT 2022, 171, 114120. [Google Scholar] [CrossRef]
- Djihad, N.; Naima, F.O.; Petronilho, S.; Hamid, S.; Bedjou, F.N.E.; Coimbra, M.A. Microencapsulation of Citrus limon Essential Oil by Complex Coacervation and Release Behavior of Terpenic and Derived Volatile Compounds. Food Hydrocoll. 2024, 152, 109830. [Google Scholar] [CrossRef]
- Zhang, H.; Song, G.; Ma, W.; Guo, M.; Ling, X.; Yu, D.; Zhou, W.; Li, L. Microencapsulation Protects the Biological Activity of Sea Buckthorn Seed Oil. Front. Nutr. 2023, 9, 1043879. [Google Scholar] [CrossRef] [PubMed]
- Copado, C.N.; Julio, L.M.; Diehl, B.W.K.; Ixtaina, V.Y.; Tomás, M.C. Multilayer Microencapsulation of Chia Seed Oil by Spray-Drying Using Electrostatic Deposition Technology. LWT 2021, 152, 112206. [Google Scholar] [CrossRef]
- Muhoza, B.; Yuyang, H.; Uriho, A.; Harindintwali, J.D.; Liu, Q.; Li, Y. Spray-and Freeze-Drying of Microcapsules Prepared by Complex Coacervation Method: A Review. Food Hydrocoll. 2023, 140, 108650. [Google Scholar] [CrossRef]
- Anand, V.; Ksh, V.; Kar, A.; Varghese, E.; Vasudev, S.; Kaur, C. Encapsulation Efficiency and Fatty Acid Analysis of Chia Seed Oil Microencapsulated by Freeze-Drying Using Combinations of Wall Material. Food Chem. 2024, 430, 136960. [Google Scholar] [CrossRef] [PubMed]
- Ledri, S.A.; Milani, J.M.; Shahidi, S.-A.; Golkar, A. Comparative Analysis of Freeze Drying and Spray Drying Methods for Encapsulation of Chlorophyll with Maltodextrin and Whey Protein Isolate. Food Chem. X 2024, 21, 101156. [Google Scholar] [CrossRef]
- Wangkulangkool, M.; Ketthaisong, D.; Tangwongchai, R.; Boonmars, T.; Lomthaisong, K. Microencapsulation of Chia Oil Using Whey Protein and Gum Arabic for Oxidation Prevention: A Comparative Study of Spray-Drying and Freeze-Drying Methods. Processes 2023, 11, 1462. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Y.; Ye, J.; Jia, J.; Ma, J.; Ge, F. Preparation of Walnut Oil Microcapsules Employing Soybean Protein Isolate and Maltodextrin with Enhanced Oxidation Stability of Walnut Oil. LWT Food Sci. Technol. 2017, 83, 292–297. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Gao, X. Development of Antioxidant Sodium Alginate Gel Beads Encapsulating Curcumin/Gum Arabic/Gelatin Microcapsules. Food Hydrocoll. 2024, 152, 109901. [Google Scholar] [CrossRef]
- Dong, S.; Hu, S.-M.; Yu, S.-J.; Zhou, S.; Zhou, T. Soybean Protein Isolate/Chitosan Complex-Rutin Microcapsules. Int. J. Biol. Macromol. 2023, 243, 125323. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, H.; Qi, B.; Wang, D.; Wu, Y.; Dai, S.; Xia, J.; Lu, M.; Yao, K.; Ma, A.; et al. Volatile Characterization of Crude and Refined Walnut Oils from Aqueous Enzymatic Extraction by GC-IMS and GC-MS. Arab. J. Chem. 2024, 17, 105404. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Cane, A.; Mulinacci, N.; Zanoni, B. Volatile Profile of Two-Phase Olive Pomace (Alperujo) by HS-SPME-GC–MS as a Key to Defining Volatile Markers of Sensory Defects Caused by Biological Phenomena in Virgin Olive Oil. J. Agric. Food Chem. 2021, 69, 5155–5166. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative Studies on the Physicochemical Properties of Soy Protein Isolate-Maltodextrin and Soy Protein Isolate-Gum Acacia Conjugate Prepared through Maillard Reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Muangrat, R.; Ravichai, K.; Jirarattanarangsri, W. Encapsulation of Polyphenols from Fermented Wastewater of Miang Processing by Freeze Drying Using a Maltodextrin/Gum Arabic Mixture as Coating Material. J. Food Process. Preserv. 2019, 43, e13908. [Google Scholar] [CrossRef]
- Gao, H.; Liu, M.; Zheng, L.; Zhang, T.; Chang, X.; Liu, H.; Zhou, S.; Zhang, Z.; Li, S.; Sun, J. Comparative Analysis of Key Odorants and Aroma Characteristics in Hot-Pressed Yellow Horn (Xanthoceras sorbifolia bunge) Seed Oil Via Gas Chromatography–Ion Mobility Spectrometry and Gas Chromatography–Olfactory-Mass Spectrometry. Foods 2023, 12, 3174. [Google Scholar] [CrossRef] [PubMed]
- Nicolotti, L.; Mall, V.; Schieberle, P. Characterization of Key Aroma Compounds in a Commercial Rum and an Australian Red Wine by Means of a New Sensomics-Based Expert System (SEBES)—An Approach To Use Artificial Intelligence in Determining Food Odor Codes. J. Agric. Food Chem. 2019, 67, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H. Characterization of the Key Aroma Compounds in White Bread by Aroma Extract Dilution Analysis, Quantitation, and Sensory Evaluation Experiments. J. Food Process. Preserv. 2019, 43, e13933. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2003. [Google Scholar]
- Zhang, Z.-H.; Yu, B.; Xu, Q.; Bai, Z.; Ji, K.; Gao, X.; Wang, B.; Aadil, R.M.; Ma, H.; Xiao, R. The Physicochemical Properties and Antioxidant Activity of Spirulina (Artrhospira platensis) Chlorophylls Microencapsulated in Different Ratios of Gum Arabic and Whey Protein Isolate. Foods 2022, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bao, Y.; Chai, Y. Preparation of Microcapsule Antioxidative Wall Materials of Pine Nut Oil by the Maillard Reaction. J. Sci. Food Agric. 2019, 99, 2793–2801. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Wang, Y.; Bhandari, B.; Wang, M. Oral Soluble Shell Prepared from OSA Starch Incorporated with Tea Polyphenols for the Microencapsulation of Sichuan Pepper Oleoresin: Characterization, Flavor Stability, Release Mechanisms and Its Application in Mooncake. Food Chem. 2024, 451, 139478. [Google Scholar] [CrossRef]
- Tao, Y.; Tang, Z.; Huang, Q.; Xu, X.; Cheng, X.; Zhang, G.; Jing, X.; Li, X.; Liang, J.; Granato, D.; et al. Effects of Spray Drying Temperature on Physicochemical Properties of Grapeseed Oil Microcapsules and the Encapsulation Efficiency of Pterostilbene. LWT 2024, 193, 115779. [Google Scholar] [CrossRef]
- Aminikhah, N.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S.; Khoobbakht, F.; Hosseini, S.M. Investigation of Structural and Physicochemical Properties of Microcapsules Obtained from Protein-Polysaccharide Conjugate via the Maillard Reaction Containing Satureja Khuzestanica Essential Oil. Int. J. Biol. Macromol. 2023, 252, 126468. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.-H.; Li, X.-D.; Wei, A.-C.; Wang, X.-D.; Wang, D.-Y. Characterization and Oxidative Stability of Cold-Pressed Sesame Oil Microcapsules Prepared by Complex Coacervation. J. Oleo Sci. 2020, 69, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, M.; Buera, M.D.P.; Rodríguez, S.D. Evaluation of SIMCA and PLS Algorithms to Detect Adulterants in Canola Oil by FT-IR. Int. J. Food Sci. Tech. 2021, 56, 2596–2603. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical Composition, Enantiomeric Analysis and Anticholinesterase Activity of Lepechinia betonicifolia Essential Oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, S.; Xi, W.; Yang, J.; Liao, Q.; Lan, J.; Lv, Y.; Tang, J. Comparison of Volatile Components in Fresh and Dried Zanthoxylum Bungeanum Maxim. Food Sci. Biotechnol. 2019, 28, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Heckert Bastos, L.P.; Vicente, J.; Corrêa Dos Santos, C.H.; Geraldo De Carvalho, M.; Garcia-Rojas, E.E. Encapsulation of Black Pepper (Piper nigrum L.) Essential Oil with Gelatin and Sodium Alginate by Complex Coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Zhang, R.; Liu, S.; Yang, S.; Li, Y.; Li, J. Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods 2023, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Özek, T.; Konakchiev, A. Enantiomeric Distribution of Linalool, Linalyl Acetate and Camphor in Bulgarian Lavender Oil. J. Essent. Oil Res. 2005, 17, 135–136. [Google Scholar] [CrossRef]
- Niu, W.; Tian, H.; Zhan, P. The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method. Molecules 2022, 27, 7760. [Google Scholar] [CrossRef]
- Durço, A.O.; De Souza, D.S.; Heimfarth, L.; Miguel-dos-Santos, R.; Rabelo, T.K.; Oliveira Barreto, T.D.; Rhana, P.; Santos Santana, M.N.; Braga, W.F.; Santos Cruz, J.D.; et al. d-Limonene Ameliorates Myocardial Infarction Injury by Reducing Reactive Oxygen Species and Cell Apoptosis in a Murine Model. J. Nat. Prod. 2019, 82, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, T.; Yin, D.; Song, P.; Hou, X.; Qi, Q.; Qi, Z. Major Fatty Acid Profiles and Bioactivity of Seed Oils from Ten Tree Peony Cultivars as a Potential Raw Material Source for the Cosmetics and Healthy Products. Chem. Biodivers. 2020, 17, e2000469. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative Effects of Curcumin, β-Myrcene and 1,8-Cineole against 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Induced Oxidative Stress in Rats Liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bahrampour, Z.; Peighambardoust, S.H.; Amini, A.M.; Soltanzadeh, M. Application of Low-, and Medium-Molecular Weight Chitosan for Preparation of Spray-Dried Microparticles Loaded with Ferulago Angulata Essential Oil: Physicochemical, Antioxidant, Antibacterial and in-Vitro Release Properties. Int. J. Biol. Macromol. 2023, 253, 126554. [Google Scholar] [CrossRef] [PubMed]
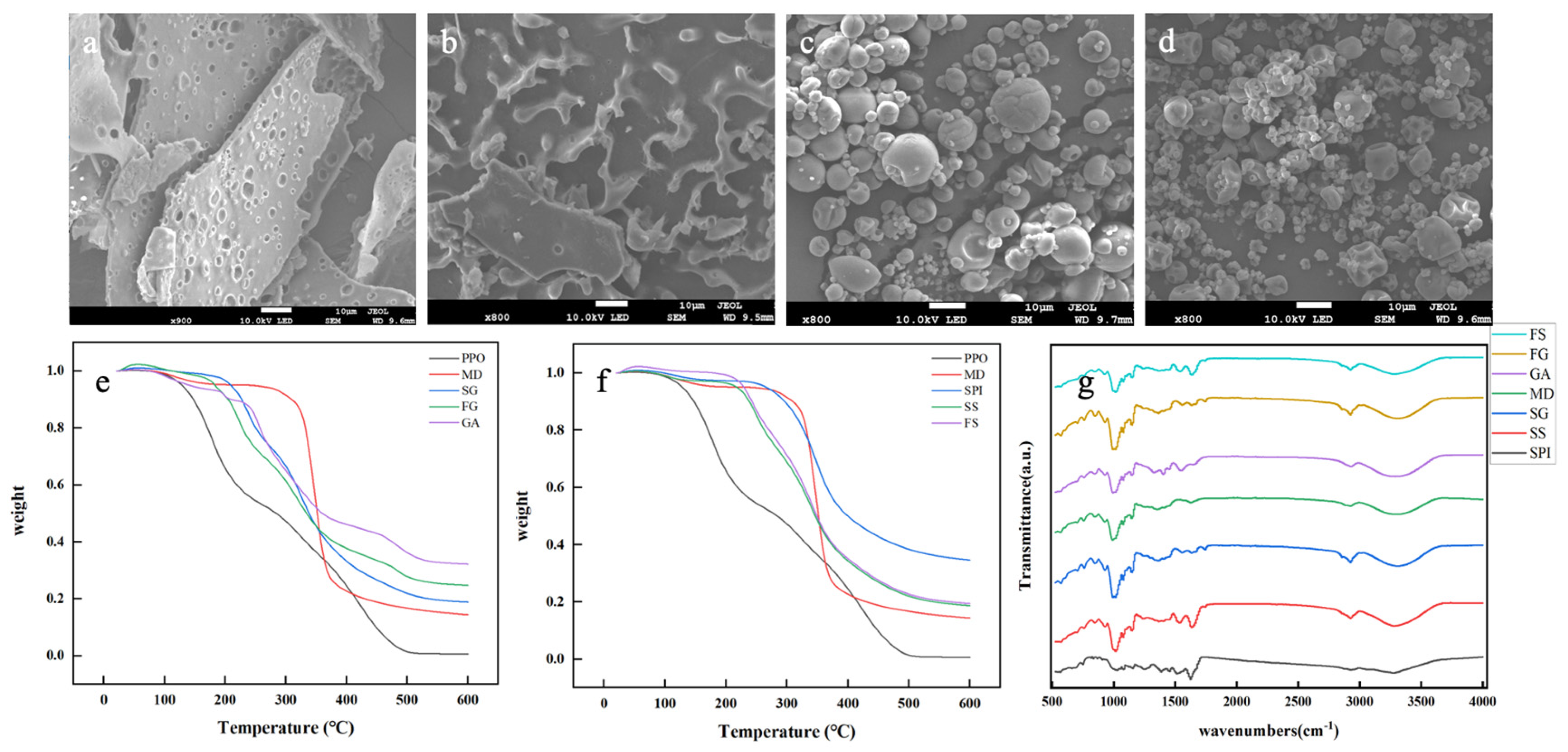
| Name | Method | Wall Material | Surface Oil Content | EE | Size (μm) |
|---|---|---|---|---|---|
| FS | Freeze-drying | SPI, MD | 2.50 ± 0.42% a | 86.60 ± 0.38% a | 3.26 ± 0.09 c |
| SS | Spray-drying | SPI, MD | 1.89 ± 0.20% b | 88.07 ± 0.26% a | 2.42 ± 0.08 a |
| FG | Freeze-drying | GA, MD | 2.48 ± 0.33% a | 87.71 ± 0.10% a | 2.94 ± 0.12 b |
| SG | Spray-drying | GA, MD | 0.60 ± 0.11% c | 92.31 ± 0.31% b | 2.39 ± 0.15 a |
| Number | Name | Chemical Formula | CAS Number | Concentration (μg/g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 10 | Day 15 | ||||||||||||
| PPO | SS | SG | PPO | SS | SG | PPO | SS | SG | PPO | SS | SG | ||||
| 1 | Linalool | C10H18O | 78-70-6 | 14.51 ± 3.40 a | 13.26 ± 0.83 a | 12.82 ± 2.38 a | 12.13 ± 1.96 a | 10.09 ± 0.70 a | 9.70 ± 1.00 b | 9.91 ± 1.21 ab | 10.09 ± 0.70 ab | 7.29 ± 0.20 bc | 7.75 ± 0.95 b | 8.47 ± 0.23 b | 8.64 ± 0.86 c |
| 2 | D-Limonene | C10H16 | 5989-27-5 | 11.38 ± 1.68 a | 11.26 ± 0.52 a | 8.73 ± 1.81 a | 7.21 ± 1.75 a | 7.08 ± 1.06 b | 5.54 ± 0.73 ab | 3.60 ± 0.24 ab | 3.77 ± 1.25 c | 3.50 ± 0.82 ab | 1.49 ± 0.05 b | 2.51 ± 1.88 d | 2.08 ± 0.29 b |
| 3 | Sabinene | C10H16 | 3387-41-5 | 6.99 ± 1.45 a | 4.29 ± 0.34 a | 3.25 ± 0.24 a | 3.71 ± 0.82 b | 3.33 ± 0.03 b | 2.61 ± 0.15 b | 1.69 ± 0.47 c | 1.77 ± 0.10 c | 1.64 ± 0.06 bc | 0.70 ± 0.07 c | 1.18 ± 0.06 c | 0.98 ± 0.23 c |
| 4 | Linalyl acetate | C12H20O2 | 115-95-7 | 4.96 ± 0.92 a | 3.80 ± 0.30 a | 3.24 ± 0.23 a | 3.00 ± 0.45 b | 2.56 ± 0.06 b | 2.41 ± 0.33 b | 2.13 ± 0.28 c | 2.09 ± 0.09 c | 1.74 ± 0.43 bc | 1.24 ± 0.10 c | 1.34 ± 0.23 d | 1.01 ± 0.29 c |
| 5 | β-Myrcene | C10H16 | 123-35-3 | 4.13 ± 0.78 a | 3.07 ± 0.23 a | 2.82 ± 0.15 a | 2.42 ± 0.36 b | 2.16 ± 0.03 b | 2.06 ± 0.11 b | 1.32 ± 0.17 c | 1.46 ± 0.07 c | 1.66 ± 0.05 c | 1.25 ± 0.09 c | 1.37 ± 0.23 d | 1.36 ± 0.21 d |
| 6 | β-Copaene | C15H24 | 18252-44-3 | 1.96 ± 0.25 a | 1.32 ± 0.18 a | 1.10 ± 0.06 a | 1.28 ± 0.16 ab | 1.03 ± 0.15 a | 0.87 ± 0.13 b | 1.17 ± 0.14 ab | 0.91 ± 0.21 ab | 0.77 ± 0.12 b | 0.88 ± 0.24 b | 0.75 ± 0.06 b | 0.70 ± 0.10 b |
| 7 | α-Terpineol | C10H18O | 98-55-5 | 1.73 ± 0.17 a | 1.36 ± 0.26 a | 1.02 ± 0.09 a | 1.37 ± 0.33 a | 1.18 ± 0.12 a | 0.87 ± 0.08 a | 0.62 ± 0.19 b | 0.76 ± 0.07 b | 0.70 ± 0.05 b | 0.27 ± 0.03 b | 0.36 ± 0.09 c | 0.31 ± 0.05 c |
| 8 | β-Caryophyllene | C15H24 | 87-44-5 | 1.53 ± 0.24 a | 1.23 ± 0.13 a | 1.08 ± 0.07 a | 1.25 ± 0.15 b | 1.29 ± 0.06 a | 1.07 ± 0.13 b | 1.17 ± 0.14 b | 1.11 ± 0.07 b | 1.01 ± 0.06 c | 1.09 ± 0.16 b | 0.95 ± 0.08 b | 1.00 ± 0.10 c |
| 9 | Spathulenol | C15H24O | 6750-60-3 | 1.28 ± 0.23 a | 0.93 ± 0.10 a | 0.86 ± 0.05 a | 1.11 ± 0.16 a | 0.72 ± 0.01 b | 0.64 ± 0.15 b | 0.10 ± 0.01 b | 0.42 ± 0.02 b | 0.42 ± 0.01 c | 0.09 ± 0.01 b | 0.12 ± 0.01 c | 0.09 ± 0.01 d |
| 10 | Caryophyleneoxide | C15H24O | 1139-30-6 | 1.28 ± 0.37 a | 0.95 ± 0.24 a | 0.65 ± 0.07 a | 0.69 ± 0.10 b | 0.62 ± 0.04 b | 0.59 ± 0.05 b | 0.65 ± 0.08 bc | 0.57 ± 0.02 b | 0.46 ± 0.02 c | 0.61 ± 0.14 c | 0.47 ± 0.03 b | 0.44 ± 0.13 d |
| 11 | α-Pinene | C10H16 | 80-56-8 | 1.09 ± 0.24 a | 0.78 ± 0.04 a | 0.68 ± 0.06 a | 0.64 ± 0.08 b | 0.61 ± 0.13 a | 0.57 ± 0.07 b | 0.30 ± 0.07 c | 0.32 ± 0.04 b | 0.30 ± 0.04 c | 0.13 ± 0.08 c | 0.16 ± 0.03 c | 0.17 ± 0.05 d |
| 12 | β-Ocimene | C10H16 | 13877-91-3 | 0.90 ± 0.25 a | 0.70 ± 0.07 a | 0.63 ± 0.06 a | 0.40 ± 0.07 b | 0.52 ± 0.01 b | 0.51 ± 0.05 b | 0.22 ± 0.04 b | 0.29 ± 0.01 c | 0.30 ± 0.01 c | 0.13 ± 0.01 b | 0.17 ± 0.04 d | 0.16 ± 0.04 c |
| 13 | 2,4-Hexadienal | C6H8O | 142-83-6 | 0.86 ± 0.14 a | 0.65 ± 0.07 a | 0.47 ± 0.07 a | 0.40 ± 0.10 b | 0.37 ± 0.01 b | 0.23 ± 0.06 b | 0.21 ± 0.12 bc | 0.30 ± 0.02 b | 0.16 ± 0.03 b | 0.12 ± 0.06 c | 0.16 ± 0.01 c | 0.13 ± 0.03 b |
| 14 | α-Ocimene | C10H16 | 502-99-8 | 0.76 ± 0.18 a | 0.79 ± 0.18 a | 0.37 ± 0.02 a | 0.55 ± 0.14 b | 0.46 ± 0.03 b | 0.20 ± 0.11 b | 0.16 ± 0.05 c | 0.30 ± 0.02 c | 0.17 ± 0.15 c | 0.10 ± 0.03 c | 0.23 ± 0.01 c | 0.14 ± 0.01 c |
| 15 | Terpinen-4-ol | C10H18O | 20126-76-5 | 0.71 ± 0.13 a | 0.60 ± 0.06 a | 0.51 ± 0.06 a | 0.56 ± 0.09 ab | 0.46 ± 0.07 b | 0.37 ± 0.01 b | 0.42 ± 0.03 bc | 0.33 ± 0.01 c | 0.26 ± 0.02 c | 0.25 ± 0.03 c | 0.25 ± 0.04 c | 0.18 ± 0.01 d |
| 16 | α-Humulene | C15H24 | 6753-98-6 | 0.70 ± 0.13 a | 0.95 ± 0.24 a | 0.55 ± 0.07 a | 0.46 ± 0.06 b | 0.62 ± 0.04 a | 0.32 ± 0.04 b | 0.35 ± 0.11 b | 0.42 ± 0.02 b | 0.30 ± 0.02 bc | 0.25 ± 0.03 b | 0.34 ± 0.04 bc | 0.27 ± 0.03 c |
| 17 | 2-Methylhexadecane | C17H36 | 1560-92-5 | 0.68 ± 0.12 a | 0.57 ± 0.04 a | 0.45 ± 0.02 a | 0.61 ± 0.09 a | 0.53 ± 0.01 b | 0.43 ± 0.02 a | 0.33 ± 0.04 b | 0.44 ± 0.02 bc | 0.38 ± 0.02 b | 0.21 ± 0.02 b | 0.32 ± 0.02 c | 0.30 ± 0.03 b |
| 18 | β-Thujone | C10H16O | 471-15-8 | 0.66 ± 0.11 a | 0.48 ± 0.04 a | 0.41 ± 0.04 a | 0.30 ± 0.08 b | 0.36 ± 0.01 b | 0.36 ± 0.02 a | 0.24 ± 0.06 b | 0.24 ± 0.01 c | 0.29 ± 0.02 b | 0.14 ± 0.03 b | 0.18 ± 0.01 d | 0.22 ± 0.01 c |
| 19 | 2-Methyltetradecane | C15H32 | 1560-95-8 | 0.65 ± 0.07 a | 0.45 ± 0.03 a | 0.41 ± 0.04 a | 0.35 ± 0.05 b | 0.34 ± 0.01 b | 0.36 ± 0.02 ab | 0.22 ± 0.03 bc | 0.30 ± 0.01 c | 0.31 ± 0.02 b | 0.18 ± 0.01 c | 0.26 ± 0.02 c | 0.24 ± 0.03 c |
| 20 | β-Elemene | C15H24 | 515-13-9 | 0.09 ± 0.02 a | 0.07 ± 0.00 a | 0.06 ± 0.00 a | 0.08 ± 0.01 a | 0.06 ± 0.00 ab | 0.06 ± 0.01 a | 0.07 ± 0.00 a | 0.05 ± 0.01 bc | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.01 c | 0.05 ± 0.00 a |
| 21 | Phenethyl acetate | C10H12O2 | 103-45-7 | 0.05 ± 0.01 a | 0.03 ± 0.01 a | 0.05 ± 0.01 a | 0.04 ± 0.01 b | 0.03 ± 0.01 b | 0.04 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.00 c | 0.03 ± 0.01 c | 0.00 ± 0.00 b | 0.01 ± 0.00 d | 0.01 ± 0.00 d |
| 22 | Tetradecane | C14H30 | 629-59-4 | 0.52 ± 0.09 a | 0.40 ± 0.01 a | 0.33 ± 0.02 a | 0.34 ± 0.05 b | 0.29 ± 0.03 b | 0.31 ± 0.02 b | 0.19 ± 0.03 c | 0.22 ± 0.03 b | 0.25 ± 0.01 b | 0.17 ± 0.01 c | 0.18 ± 0.03 c | 0.16 ± 0.01 c |
| 23 | Nerolidol | C15H26O | 40716-66-3 | 0.49 ± 0.09 a | 0.36 ± 0.03 a | 0.25 ± 0.01 a | 0.28 ± 0.04 b | 0.18 ± 0.00 b | 0.16 ± 0.01 b | 0.16 ± 0.02 b | 0.14 ± 0.01 b | 0.13 ± 0.00 bc | 0.16 ± 0.01 b | 0.13 ± 0.01 c | 0.12 ± 0.01 c |
| 24 | γ-Terpinene | C10H16 | 99-85-4 | 0.49 ± 0.09 a | 0.36 ± 0.03 a | 0.25 ± 0.01 a | 0.28 ± 0.04 b | 0.18 ± 0.00 b | 0.16 ± 0.01 b | 0.16 ± 0.02 bc | 0.14 ± 0.01 c | 0.13 ± 0.00 b | 0.16 ± 0.01 c | 0.13 ± 0.01 d | 0.12 ± 0.01 c |
| 25 | 8-Hetadecene | C17H34 | 2579-04-6 | 0.44 ± 0.08 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.05 b | 0.36 ± 0.01 b | 0.00 ± 0.00 a | 0.24 ± 0.03 bc | 0.29 ± 0.01 b | 0.00 ± 0.00 b | 0.16 ± 0.01 c | 0.15 ± 0.01 b | 0.00 ± 0.00 b |
| 26 | 2-Tridecanone | C13H26O | 593-08-8 | 0.42 ± 0.08 a | 0.21 ± 0.02 a | 0.17 ± 0.01 a | 0.35 ± 0.04 ab | 0.16 ± 0.00 b | 0.12 ± 0.01 b | 0.27 ± 0.05 b | 0.08 ± 0.00 c | 0.09 ± 0.00 c | 0.08 ± 0.00 c | 0.07 ± 0.00 c | 0.08 ± 0.01 c |
| 27 | 4-Thujanol | C10H18O | 546-79-2 | 0.42 ± 0.08 a | 0.21 ± 0.02 a | 0.17 ± 0.01 a | 0.35 ± 0.04 ab | 0.16 ± 0.00 a | 0.12 ± 0.01 a | 0.27 ± 0.05 bc | 0.08 ± 0.00 b | 0.09 ± 0.00 b | 0.08 ± 0.00 c | 0.07 ± 0.00 c | 0.08 ± 0.01 c |
| 28 | Citronellal | C10H18O | 106-23-0 | 0.25 ± 0.06 a | 0.24 ± 0.05 a | 0.19 ± 0.02 a | 0.09 ± 0.02 b | 0.15 ± 0.01 b | 0.14 ± 0.01 b | 0.06 ± 0.01 b | 0.05 ± 0.02 c | 0.11 ± 0.01 c | 0.03 ± 0.01 b | 0.03 ± 0.00 c | 0.03 ± 0.01 d |
| 29 | 1,7-Octadien-3-one, 2-methyl-6-methylene | C10H14O | 41702-60-7 | 0.23 ± 0.04 a | 0.15 ± 0.01 a | 0.12 ± 0.01 a | 0.08 ± 0.01 b | 0.07 ± 0.00 b | 0.08 ± 0.00 b | 0.03 ± 0.01 c | 0.04 ± 0.00 c | 0.05 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 d | 0.03 ± 0.01 d |
| 30 | Decanal | C10H20O | 112-31-2 | 0.21 ± 0.05 a | 0.17 ± 0.03 a | 0.13 ± 0.02 a | 0.13 ± 0.01 a | 0.13 ± 0.01 ab | 0.12 ± 0.01 a | 0.12 ± 0.05 ab | 0.10 ± 0.02 b | 0.10 ± 0.00 ab | 0.03 ± 0.05 b | 0.00 ± 0.00 c | 0.08 ± 0.02 b |
| 31 | Cyclohexane, 2-ethenyl-1,1-dimethyl-3-methylene- | C11H18 | 95452-08-7 | 0.19 ± 0.04 a | 0.15 ± 0.01 a | 0.11 ± 0.00 a | 0.08 ± 0.01 b | 0.07 ± 0.00 b | 0.10 ± 0.01 a | 0.05 ± 0.01 bc | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c |
| 32 | Undecanal | C11H22O | 112-44-7 | 0.18 ± 0.06 a | 0.15 ± 0.01 a | 0.14 ± 0.00 b | 0.14 ± 0.05 a | 0.06 ± 0.01 b | 0.12 ± 0.03 b | 0.16 ± 0.02 a | 0.04 ± 0.00 bc | 0.08 ± 0.01 c | 0.09 ± 0.01 a | 0.03 ± 0.01 c | 0.05 ± 0.01 c |
| 33 | γ-Elemene | C15H24 | 3242-08-8 | 0.17 ± 0.03 a | 0.12 ± 0.01 a | 0.00 ± 0.00 a | 0.11 ± 0.02 b | 0.09 ± 0.00 b | 0.00 ± 0.00 a | 0.09 ± 0.01 b | 0.08 ± 0.00 bc | 0.00 ± 0.00 b | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.00 ± 0.00 c |
| 34 | Geranyl acetate | C12H20O2 | 105-87-3 | 0.17 ± 0.03 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.14 ± 0.02 b | 0.00 ± 0.00 ab | 0.00 ± 0.00 b | 0.06 ± 0.01 b | 0.08 ± 0.01 b | 0.07 ± 0.00 c | 0.05 ± 0.01 b | 0.05 ± 0.01 c | 0.05 ± 0.00 c |
| 35 | Terpinolene | C10H16 | 586-62-9 | 0.15 ± 0.03 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.06 ± 0.01 b | 0.10 ± 0.00 b | 0.09 ± 0.01 b | 0.06 ± 0.01 b | 0.06 ± 0.01 c | 0.04 ± 0.00 c | 0.03 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 d |
| 36 | Neryl acetate | C12H20O2 | 141-12-8 | 0.13 ± 0.02 a | 0.09 ± 0.01 a | 0.09 ± 0.00 a | 0.06 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.04 ± 0.01 b | 0.07 ± 0.00 b | 0.06 ± 0.01 b | 0.04 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.01 b |
| 37 | Dodecane, 2-methyl- | C13H28 | 1560-97-0 | 0.11 ± 0.02 a | 0.07 ± 0.01 a | 0.06 ± 0.00 a | 0.05 ± 0.01 b | 0.04 ± 0.00 b | 0.05 ± 0.00 b | 0.04 ± 0.00 b | 0.04 ± 0.01 c | 0.04 ± 0.01 b | 0.04 ± 0.00 b | 0.03 ± 0.00 d | 0.04 ± 0.01 b |
| 38 | 2-Cyclohexen-1-one,4-(1-methylethyl)- | C9H14O | 500-02-7 | 0.09 ± 0.02 a | 0.07 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 b | 0.05 ± 0.00 b | 0.03 ± 0.00 b | 0.02 ± 0.01 c | 0.03 ± 0.00 c | 0.02 ± 0.00 b | 0.02 ± 0.00 c | 0.02 ± 0.01 c | 0.02 ± 0.00 c |
| 39 | Cryptomeridiol | C15H28O2 | 4666-84-6 | 0.08 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 b | 0.04 ± 0.00 b | 0.05 ± 0.01 a | 0.00 ± 0.00 c | 0.04 ± 0.00 c | 0.04 ± 0.00 b | 0.00 ± 0.00 c | 0.02 ± 0.01 d | 0.02 ± 0.00 c |
| 40 | (-)-Germacrene-D | C15H24 | 317819-80-0 | 0.08 ± 0.02 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.07 ± 0.01 b | 0.05 ± 0.01 ab | 0.05 ± 0.00 a | 0.04 ± 0.00 bc | 0.04 ± 0.01 b | 0.04 ± 0.01 a | 0.03 ± 0.00 c | 0.03 ± 0.00 b | 0.03 ± 0.00 a |
| 41 | α-Elemol | C15H26O | 639-99-6 | 0.06 ± 0.01 a | 0.05 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 ab | 0.04 ± 0.00 b | 0.03 ± 0.00 a | 0.04 ± 0.01 bc | 0.04 ± 0.00 c | 0.03 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 d | 0.03 ± 0.00 c |
| Number | Name | CAS Number | Threshold Value (μg/g) | OAV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 10 | Day 15 | ||||||||||||
| PPO | SS | SG | PPO | SS | SG | PPO | SS | SG | PPO | SS | SG | ||||
| 1 | 2,4-Hexadienal | 142-83-6 | 0.06 | 14.33 ± 2.26 a | 10.77 ± 1.17 a | 7.75 ± 1.11 a | 6.60 ± 1.74 b | 6.22 ± 0.05 b | 3.72 ± 1.06 b | 3.45 ± 2.06 bc | 4.97 ± 0.29 b | 2.61 ± 0.42 b | 2.01 ± 0.97 c | 2.66 ± 0.45 c | 2.17 ± 0.56 b |
| 2 | Citronellal | 106-23-0 | 0.10 | 0.25 ± 0.06 a | 0.24 ± 0.05 a | 0.19 ± 0.02 a | 0.09 ± 0.02 b | 0.15 ± 0.01 b | 0.14 ± 0.01 b | 0.06 ± 0.01 b | 0.05 ± 0.02 c | 0.11 ± 0.01 c | 0.03 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 d |
| 3 | Decanal | 112-31-2 | 0.003 | 70.39 ± 15.66 a | 56.37 ± 10.63 a | 43.91 ± 6.24 a | 44.08 ± 2.63 a | 44.05 ± 1.12 ab | 38.43 ± 4.42 a | 40.91 ± 15.42 ab | 32.08 ± 7.28 b | 33.21 ± 0.43 ab | 11.15 ± 15.77 b | 0.00 ± 0.00 c | 26.27 ± 6.08 b |
| 4 | Undecanal | 112-44-7 | 0.0125 | 14.78 ± 5.03 a | 12.31 ± 0.98 a | 11.05 ± 0.38 b | 11.02 ± 3.84 a | 5.01 ± 0.99 b | 9.77 ± 2.20 b | 12.58 ± 1.40 a | 3.54 ± 0.18 bc | 6.34 ± 0.95 c | 7.51 ± 0.92 b | 2.54 ± 0.55 c | 3.64 ± 0.97 c |
| 5 | 4-Thujanol | 546-79-2 | 0.59 | 0.22 ± 0.04 a | 0.13 ± 0.01 a | 0.23 ± 0.05 a | 0.25 ± 0.04 ab | 0.21 ± 0.00 a | 0.19 ± 0.01 a | 0.18 ± 0.02 bc | 0.17 ± 0.01 b | 0.17 ± 0.01 b | 0.14 ± 0.01 c | 0.12 ± 0.01 c | 0.12 ± 0.01 c |
| 6 | Linalool | 78-70-6 | 0.006 | 2418.58 ± 565.92 a | 2210.00 ± 368.84 a | 2136.67 ± 187.64 a | 2021.67 ± 326.70 a | 1949.46 ± 178.75 a | 1815.33 ± 203.00 b | 1731.34 ± 274.53 ab | 1758.67 ± 305.27 ab | 1466.62 ± 110.18 bc | 1443.3 ± 86.66 b | 1671.98 ± 36.83 b | 1639.33 ± 156.08 c |
| 7 | Terpinen-4-ol | 20126-76-5 | 1.2 | 0.59 ± 0.11 a | 0.50 ± 0.05 a | 0.43 ± 0.05 a | 0.47 ± 0.07 ab | 0.38 ± 0.05 b | 0.31 ± 0.01 b | 0.35 ± 0.03 bc | 0.28 ± 0.03 c | 0.22 ± 0.02 c | 0.21 ± 0.02 c | 0.21 ± 0.04 c | 0.15 ± 0.01 d |
| 8 | α-Terpineol | 98-55-5 | 1.2 | 1.44 ± 0.14 a | 1.13 ± 0.21 a | 0.85 ± 0.07 a | 1.14 ± 0.28 a | 0.98 ± 0.10 a | 0.73 ± 0.07 a | 0.52 ± 0.15 b | 0.63 ± 0.06 b | 0.58 ± 0.04 b | 0.23 ± 0.03 b | 0.30 ± 0.08 c | 0.26 ± 0.04 c |
| 9 | α-Elemol | 639-99-6 | 0.10 | 0.85 ± 0.19 a | 0.60 ± 0.04 a | 0.48 ± 0.03 a | 0.68 ± 0.10 ab | 0.53 ± 0.02 b | 0.47 ± 0.02 a | 0.43 ± 0.04 bc | 0.39 ± 0.02 c | 0.41 ± 0.01 b | 0.34 ± 0.02 c | 0.29 ± 0.01 d | 0.26 ± 0.02 c |
| 10 | Nerolidol | 40716-66-3 | 0.25 | 0.25 ± 0.05 a | 0.20 ± 0.01 a | 0.15 ± 0.01 a | 0.18 ± 0.03 b | 0.17 ± 0.01 b | 0.14 ± 0.01 b | 0.17 ± 0.02 b | 0.16 ± 0.01 b | 0.13 ± 0.00 b | 0.13 ± 0.01 b | 0.11 ± 0.00 c | 0.11 ± 0.01 c |
| 11 | Tetradecane | 629-59-4 | 1.00 | 0.52 ± 0.09 a | 0.40 ± 0.01 a | 0.33 ± 0.02 a | 0.34 ± 0.05 b | 0.29 ± 0.03 b | 0.32 ± 0.03 a | 0.19 ± 0.03 c | 0.22 ± 0.03 c | 0.26 ± 0.01 b | 0.17 ± 0.01 c | 0.18 ± 0.03 c | 0.15 ± 0.01 c |
| 12 | α-Pinene | 80-56-8 | 0.041 | 26.51 ± 5.71 a | 19.03 ± 0.96 a | 16.47 ± 1.54 a | 15.64 ± 1.97 b | 14.91 ± 3.12 b | 13.94 ± 1.73 a | 7.36 ± 1.72 c | 7.70 ± 0.96 c | 7.23 ± 0.91 b | 3.12 ± 2.04 c | 3.91 ± 0.66 c | 4.26 ± 1.27 b |
| 13 | Sabinene | 3387-41-5 | 0.016 | 436.59 ± 90.56 a | 378.23 ± 21.40 a | 307.87 ± 15.30 a | 212.16 ± 51.45 b | 208.16 ± 1.85 b | 162.92 ± 9.18 b | 105.89 ± 29.62 c | 110.84 ± 6.11 c | 102.80 ± 3.50 c | 43.83 ± 4.62 c | 73.68 ± 3.54 d | 61.06 ± 14.21 d |
| 14 | β-Myrcene | 123-35-3 | 0.0049 | 843.87 ± 158.49 a | 627.50 ± 46.47 a | 575.10 ± 30.81 a | 493.49 ± 72.56 b | 439.96 ± 6.99 b | 420.91 ± 48.23 b | 270.07 ± 35.42 c | 298.58 ± 14.16 c | 277.53 ± 20.45 bc | 256.03 ± 18.70 c | 278.67 ± 47.60 c | 276.98 ± 43.16 c |
| 15 | γ-Terpinene | 99-85-4 | 1.00 | 0.49 ± 0.09 a | 0.36 ± 0.03 a | 0.25 ± 0.01 a | 0.28 ± 0.04 b | 0.18 ± 0.00 b | 0.16 ± 0.01 b | 0.16 ± 0.02 b | 0.14 ± 0.01 c | 0.13 ± 0.00 c | 0.14 ± 0.04 b | 0.13 ± 0.01 d | 0.12 ± 0.01 d |
| 16 | Terinolene | 586-62-9 | 0.20 | 0.76 ± 0.14 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.05 b | 0.48 ± 0.01 b | 0.43 ± 0.03 b | 0.31 ± 0.04 bc | 0.30 ± 0.02 c | 0.18 ± 0.01 c | 0.15 ± 0.01 c | 0.16 ± 0.01 c | 0.16 ± 0.01 c |
| 17 | β-Ocimene | 13877-91-3 | 0.034 | 26.52 ± 7.22 a | 20.71 ± 2.14 a | 18.64 ± 1.63 a | 11.9 ± 2.16 b | 15.40 ± 0.37 b | 15.12 ± 1.47 b | 6.59 ± 1.13 b | 8.59 ± 0.41 c | 8.71 ± 0.25 b | 3.85 ± 0.30 b | 4.97 ± 1.06 d | 4.93 ± 0.77 c |
| 18 | D-Limonene | 5989-27-5 | 0.034 | 334.77 ± 49.40 a | 301.15 ± 15.27 a | 227.86 ± 15.42 a | 212.16 ± 51.36 b | 208.16 ± 31.09 ab | 162.92 ± 21.45 b | 105.89 ± 7.19 c | 110.84 ± 36.66 bc | 102.80 ± 39.74 b | 43.83 ± 1.49 c | 73.68 ± 55.29 c | 61.06 ± 8.45 c |
| 19 | β-Caryophyllene | 87-44-5 | 0.064 | 23.87 ± 3.70 a | 19.14 ± 1.98 a | 16.90 ± 1.14 a | 19.49 ± 2.28 a | 20.17 ± 0.94 a | 16.76 ± 2.01 a | 18.36 ± 2.13 a | 17.41 ± 1.03 ab | 15.77 ± 0.88 a | 16.98 ± 2.44 a | 14.77 ± 1.21 b | 15.07 ± 1.49 a |
| 20 | α-Humulene | 6753-98-6 | 0.16 | 4.38 ± 0.79 a | 5.91 ± 1.53 a | 3.44 ± 0.43 a | 2.88 ± 0.34 b | 3.87 ± 0.23 b | 2.03 ± 0.24 b | 2.18 ± 0.68 bc | 2.62 ± 0.12 b | 1.88 ± 0.13 b | 1.56 ± 0.21 c | 2.13 ± 0.27 b | 1.7 ± 0.19 b |
| 21 | Caryophylene oxide | 1139-30-6 | 0.41 | 1.28 ± 0.24 a | 0.90 ± 0.24 a | 0.65 ± 0.03 a | 0.84 ± 0.12 b | 0.54 ± 0.11 b | 0.49 ± 0.14 a | 0.48 ± 0.05 b | 0.51 ± 0.02 b | 0.35 ± 0.01 a | 0.03 ± 0.00 b | 0.37 ± 0.08 b | 0.34 ± 0.03 a |
| 22 | Linalyl acetate | 115-95-7 | 1.00 | 4.96 ± 0.92 a | 3.80 ± 0.30 a | 3.24 ± 0.23 a | 3.00 ± 0.45 b | 2.56 ± 0.06 b | 2.41 ± 0.33 b | 2.13 ± 0.28 bc | 2.09 ± 0.09 c | 1.74 ± 0.43 bc | 1.24 ± 0.10 c | 1.34 ± 0.23 d | 1.01 ± 0.29 c |
| 23 | Phenethyl acetate | 103-45-7 | 0.24959 | 0.22 ± 0.04 a | 0.13 ± 0.01 a | 0.20 ± 0.01 a | 0.14 ± 0.02 b | 0.12 ± 0.01 a | 0.14 ± 0.01 b | 0.03 ± 0.00 c | 0.06 ± 0.01 b | 0.12 ± 0.01 c | 0.00 ± 0.00 c | 0.05 ± 0.00 b | 0.05 ± 0.01 d |
| 24 | Neryl acetate | 141-12-8 | 0.042 | 3.01 ± 0.53 a | 2.08 ± 0.17 a | 2.04 ± 0.11 a | 1.44 ± 0.22 b | 1.83 ± 0.19 ab | 1.83 ± 0.10 a | 0.93 ± 0.15 b | 1.69 ± 0.08 b | 1.41 ± 0.10 b | 0.95 ± 0.06 b | 1.28 ± 0.06 c | 1.18 ± 0.12 b |
| 25 | Geranyl acetate | 105-87-3 | 0.15 | 1.13 ± 0.20 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.95 ± 0.14 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.40 ± 0.03 b | 0.32 ± 0.02 c | 0.38 ± 0.01 c | 0.36 ± 0.03 b | 0.30 ± 0.01 c | 0.34 ± 0.03 c |
| 26 | β-Thujone | 471-15-8 | 0.04 | 16.48 ± 2.63 a | 11.92 ± 0.90 a | 10.17 ± 1.07 a | 7.46 ± 2.03 b | 9.05 ± 0.34 b | 8.88 ± 0.51 a | 6.00 ± 1.59 b | 6.10 ± 0.25 c | 7.29 ± 0.42 b | 3.41 ± 0.85 b | 4.60 ± 0.37 d | 5.52 ± 0.20 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Z.; Li, X.; Zhou, S.; Liu, M.; Li, S.; Liu, H.; Gao, H.; Zhao, A.; Zhang, Y.; et al. Preparation and Characterization of Prickly Ash Peel Oleoresin Microcapsules and Flavor Retention Analysis. Foods 2024, 13, 1726. https://doi.org/10.3390/foods13111726
Zhang Z, Zhang Z, Li X, Zhou S, Liu M, Li S, Liu H, Gao H, Zhao A, Zhang Y, et al. Preparation and Characterization of Prickly Ash Peel Oleoresin Microcapsules and Flavor Retention Analysis. Foods. 2024; 13(11):1726. https://doi.org/10.3390/foods13111726
Chicago/Turabian StyleZhang, Zhiran, Ziyan Zhang, Xichao Li, Sen Zhou, Mengkai Liu, Shengxin Li, He Liu, Hui Gao, Aiyun Zhao, Yongchang Zhang, and et al. 2024. "Preparation and Characterization of Prickly Ash Peel Oleoresin Microcapsules and Flavor Retention Analysis" Foods 13, no. 11: 1726. https://doi.org/10.3390/foods13111726
APA StyleZhang, Z., Zhang, Z., Li, X., Zhou, S., Liu, M., Li, S., Liu, H., Gao, H., Zhao, A., Zhang, Y., Huang, L., & Sun, J. (2024). Preparation and Characterization of Prickly Ash Peel Oleoresin Microcapsules and Flavor Retention Analysis. Foods, 13(11), 1726. https://doi.org/10.3390/foods13111726







