Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance
Abstract
:1. Introduction
2. Materials and Methods
2.1. KW Production
2.2. Analysis of Aroma Profile in KW
2.3. Analysis of Taste Profile in KW
2.3.1. E-Tongue Analysis
2.3.2. 1H-NMR Analysis
2.4. Statistical Analysis
3. Results
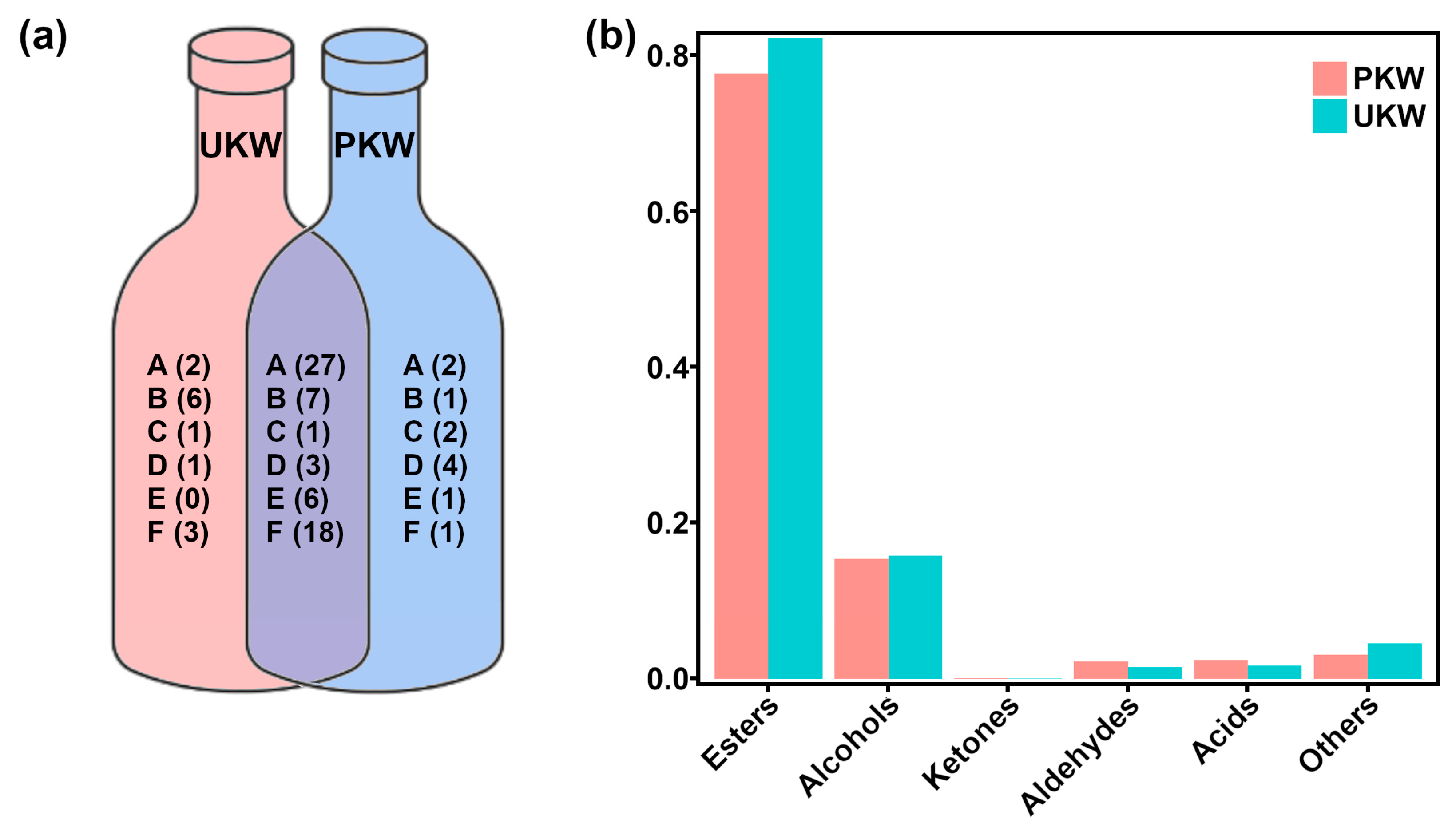
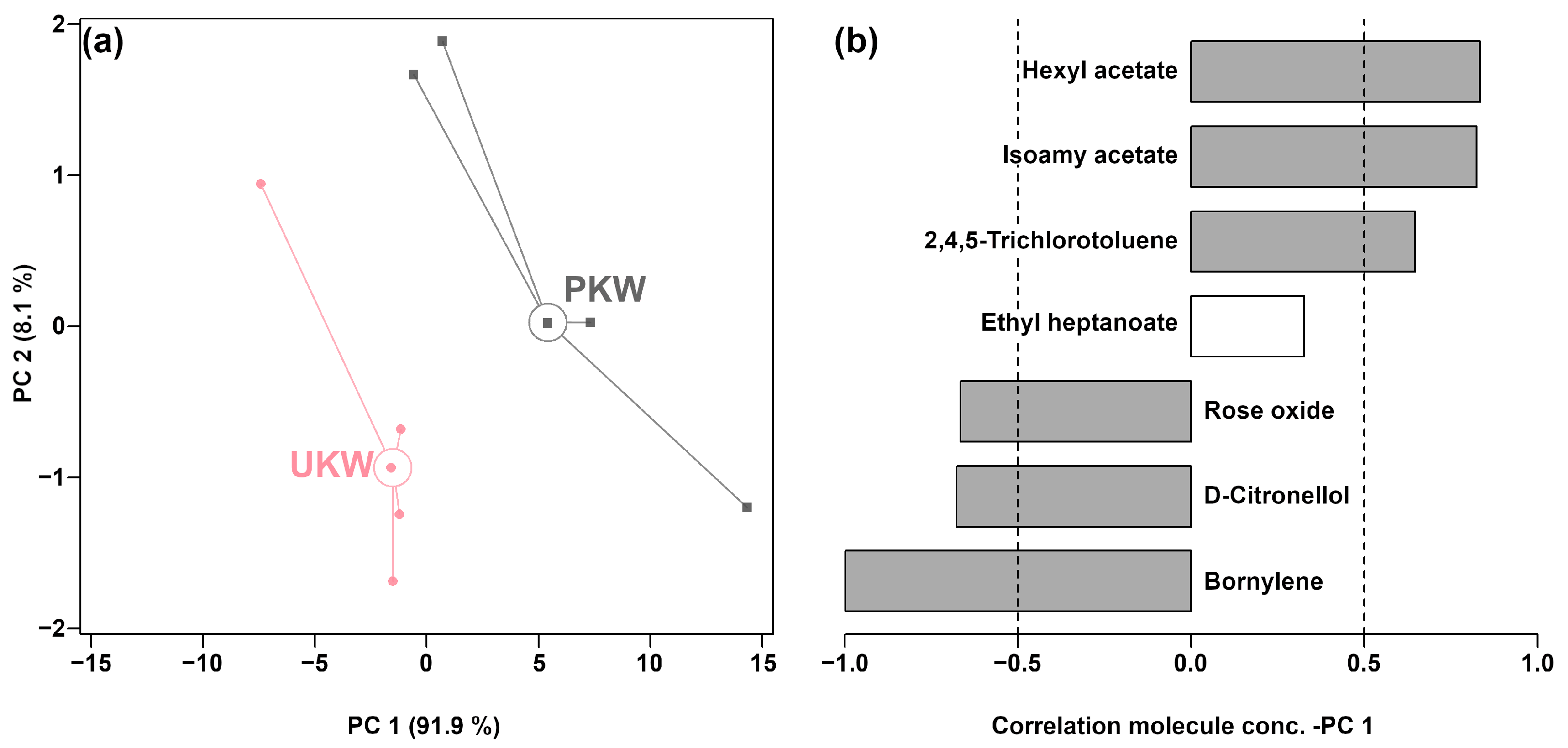
3.1. Analysis of Aroma Profile in KW
3.2. Analysis of Taste Profiles in KW
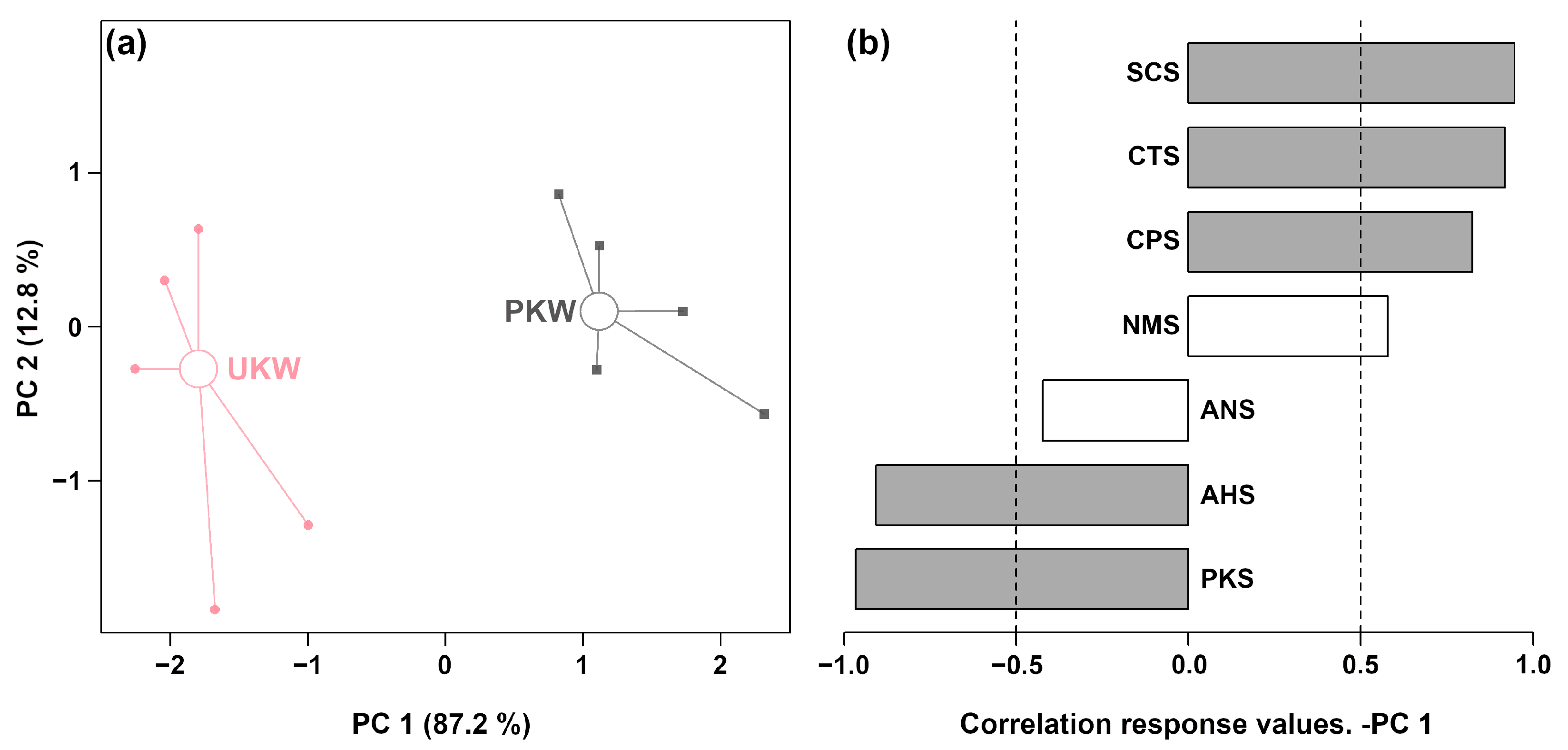
3.2.1. E-Tongue Analysis
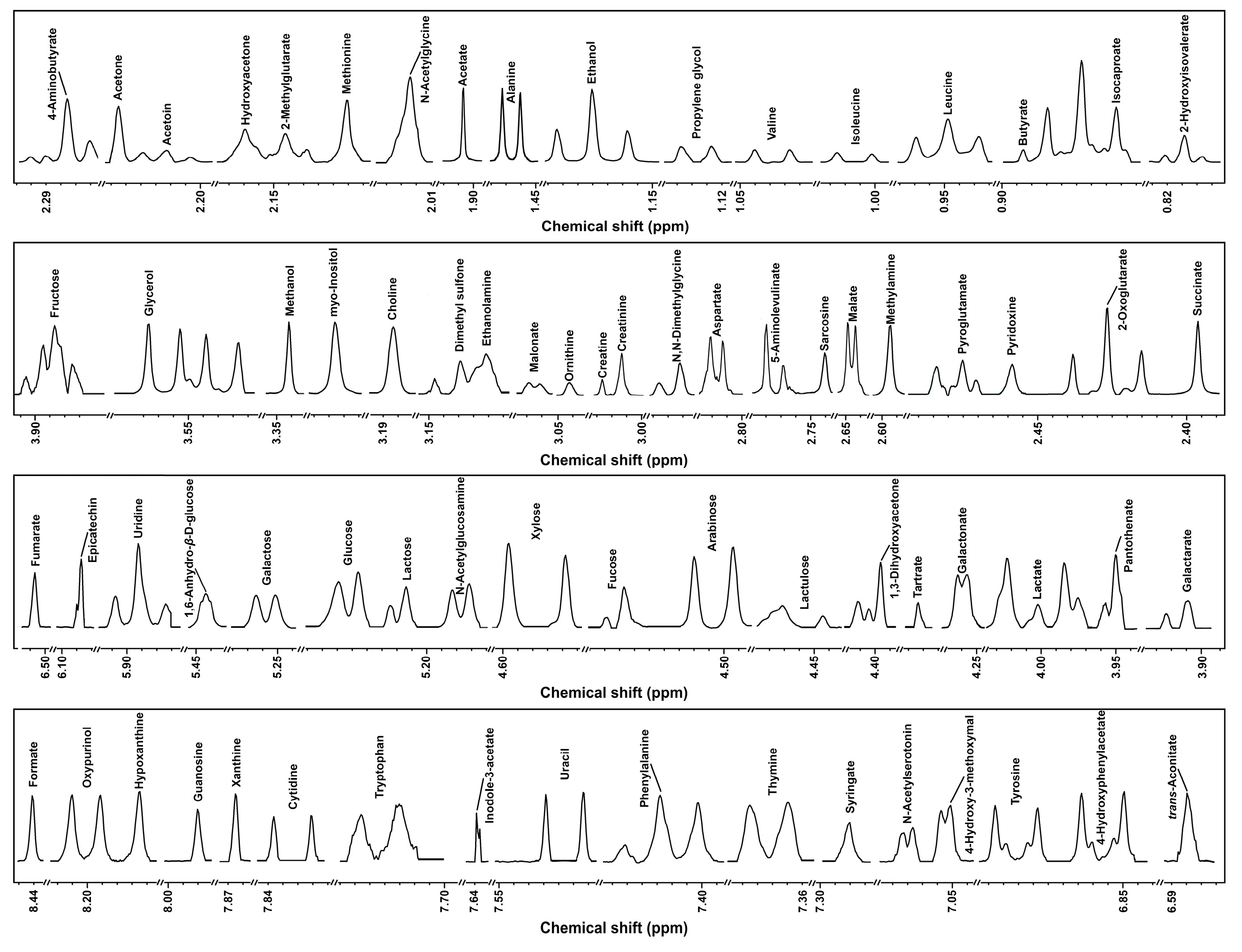
3.2.2. 1H-NMR Analysis
3.2.3. Correlation Analysis of E-Tongue with 1H-NMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, T.; Lan, T.; Ju, Y.; Cheng, G.; Que, Z.; Geng, T.; Fang, Y.; Sun, X. Comparison of the Nutritional Properties and Biological Activities of Kiwifruit (Actinidia) and Their Different Forms of Products: Towards Making Kiwifruit More Nutritious and Functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Gao, Z. Evaluation of the Quality of Fermented Kiwi Wines Made from Different Kiwifruit Cultivars. Food Biosci. 2021, 42, 101051. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Yang, L.; Zhao, J.; Zhu, X. Effect of Deacidification Treatment on the Flavor Quality of Zaosu Pear–Kiwifruit Wine. Foods 2022, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Effect of Sequential Fermentation with Four Non-Saccharomyces and Saccharomyces Cerevisiae on Nutritional Characteristics and Flavor Profiles of Kiwi Wines. J. Food Compos. Anal. 2022, 109, 104480. [Google Scholar] [CrossRef]
- Zhu, M.T.; Huang, Y.S.; Wang, Y.L.; Shi, T.; Zhang, L.L.; Chen, Y.; Xie, M.Y. Comparison of (Poly)Phenolic Compounds and Antioxidant Properties of Pomace Extracts from Kiwi and Grape Juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef]
- Latocha, P.; Łata, B.; Stasiak, A. Phenolics, Ascorbate and the Antioxidant Potential of Kiwiberry vs. Common Kiwifruit: The Effect of Cultivar and Tissue Type. J. Funct. Foods 2015, 19, 155–163. [Google Scholar] [CrossRef]
- Akbulut, M.; Çoklar, H.; Bulut, A.N.; Hosseini, S.R. Evaluation of Black Grape Pomace, a Fruit Juice by-Product, in Shalgam Juice Production: Effect on Phenolic Compounds, Anthocyanins, Resveratrol, Tannin, and in Vitro Antioxidant Activity. Food Sci. Nutr. 2024, 1–13. [Google Scholar] [CrossRef]
- Lindsay, M.A.; Granucci, N.; Greenwood, D.R.; Villas-Boas, S.G. Fermentative Production of Volatile Metabolites Using Brettanomyces Bruxellensis from Fruit and Vegetable By-Products. Fermentation 2022, 8, 457. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, Y.; Liu, D.; Zhang, J.; Qi, Y.; Xu, J.; Wei, X.; Fan, M. Free and Bound Volatile Compounds in ‘Hayward’ and ‘Hort16A’ Kiwifruit and Their Wines. Eur. Food Res. Technol. 2020, 246, 875–890. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Huang, Q.; Fu, X. Digestibility, Bioactivity and Prebiotic Potential of Phenolics Released from Whole Gold Kiwifruit and Pomace by in Vitro Gastrointestinal Digestion and Colonic Fermentation. Food Funct. 2020, 11, 9613–9623. [Google Scholar] [CrossRef]
- Liu, J.; Guan, W.; Sun, Z.; Ni, Y.; He, L.; Tian, F.; Cai, L. Application of Cyclocarya Paliurus–Kiwifruit Composite Fermented to Enhance Antioxidant Capacity, Flavor, and Sensory Characteristics of Kiwi Wine. Molecules 2024, 29, 32. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces Cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Lan, T.; Wang, J.; Yuan, Q.; Lei, Y.; Peng, W.; Zhang, M.; Li, X.; Sun, X.; Ma, T. Evaluation of the Color and Aroma Characteristics of Commercially Available Chinese Kiwi Wines via Intelligent Sensory Technologies and Gas Chromatography-Mass Spectrometry. Food Chem. X 2022, 15, 100427. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of Chemical Constitution and Aroma Properties of Kiwi Wines Obtained from Pure and Mixed Fermentation with Wickerhamomyces Anomalus and Saccharomyces Cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Z.; Lu, X.; Yi, Y.; Tian, Q.; Deng, J.; Jiang, D.; Tang, J.; Laghi, L. Effects of Saccharomyces Cerevisiae Strains on the Metabolomic Profiles of Guangan Honey Pear Cider. LWT 2023, 182, 114816. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, J.; Yang, Y.; Deng, J.; Zhu, K.; Yi, Y.; Tang, J.; Jiang, X.; Zhu, C.; Laghi, L. Effects of S. Cerevisiae Strains on the Sensory Characteristics and Flavor Profile of Kiwi Wine Based on E-Tongue, GC-IMS and 1H-NMR. LWT 2023, 185, 115193. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Ren, Y.; Wang, X.; Li, H.; Liu, Z.; Yue, T.; Gao, Z. Effect of Inoculation Method on the Quality and Nutritional Characteristics of Low-Alcohol Kiwi Wine. LWT 2022, 156, 113049. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Bi, P.; Han, J.; Li, S.; Liu, X.; Zhang, Z.; Long, F.; Guo, J. Screening and Characterization of Indigenous Non-Saccharomyces Cerevisiae with High Enzyme Activity for Kiwifruit Wine Production. Food Chem. 2024, 440, 138309. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Parri, S.; Campani, T.; Conti, V.; Cai, G.; Romi, M.; Casini, S.; Zari, R.; Caldini, F.; Marsili, L. New Olive-Pomace Fertilizer Tested with a 2-Tiers Approach: Biomarkers on Eisenia Fetida, Physiochemical Effects on Solanum Lycopersicum and Olea Europaea. J. Environ. Manag. 2024, 351, 119915. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Zhou, Z.; Ye, X. Polysaccharides of Chinese Bayberry Pomace Wine: Structural Characteristics, Antioxidant Activity and Influence on the Bayberry Wine. Food Biosci. 2022, 50, 102025. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Xin, G.; Li, B.; Tian, J. Phenolics Profile and Antioxidant Activity Analysis of Kiwi Berry (Actinidia arguta) Flesh and Peel Extracts from Four Regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- Yiman, Q.; Miaomiao, L.; Kun, Y.; Mingtao, F. Effect of Skin Maceration Treatment on Aroma Profiles of Kiwi Wines Elaborated with Actinidia deliciosa “Xuxiang” and A. chinensis “Hort16A”. J. AOAC Int. 2019, 102, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, M.Y.; Hunter, D.C.; Matich, A.J.; McAtee, P.A.; Knäbel, M.; Hamiaux, C.; Popowski, E.A.; Jaeger, S.R.; Nieuwenhuizen, N.J.; et al. Sensory-Directed Genetic and Biochemical Characterization of Volatile Terpene Production in Kiwifruit. Plant Physiol. 2020, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Du, Q.; Qu, R.; Ye, D.; Lu, Y.; Liu, Y. Analysis of Volatile Aroma Compounds and Sensory Characteristics Contributing to Regional Style of Red Wines from Hexi Corridor Based on Sixteen Grape Varieties/Clones. Fermentation 2022, 8, 501. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Pang, X. Characterization of the Color, Physicochemical Properties, Organic Acids, and Aroma Profiles of Kiwifruit Wines by Different Fermentation Patterns and Fining Stages. LWT 2024, 199, 116097. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; De Revel, G.; Barbe, J.C. Distribution and Organoleptic Impact of Ethyl 2-Hydroxy-4-Methylpentanoate Enantiomers in Wine. J. Agric. Food Chem. 2012, 60, 1503–1509. [Google Scholar] [CrossRef]
- Zhu, D.; Kou, C.; Shen, Y.; Xi, P.; Cao, X.; Liu, H.; Li, J. Effects of Different Processing Steps on the Flavor and Colloidal Properties of Cloudy Apple Juice. J. Sci. Food Agric. 2021, 101, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Wang, Y.; Wang, S.; Zou, Y.; Sun, Z.; Yuan, L. Changes on Physiochemical Properties and Volatile Compounds of Chinese Kombucha during Fermentation. Food Biosci. 2023, 55, 103029. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; He, F.; Pan, Z.; Du, Y.; Chen, Z.; He, Y.; Sun, Y.; Li, M. Effect of Microbial Communities on Flavor Profile of Hakka Rice Wine throughout Production. Food Chem. X 2024, 21, 101121. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Schieberle, P. Changes in the Major Odorants of Grape Juice during Manufacturing of Dornfelder Red Wine. J. Agric. Food Chem. 2022, 70, 13979–13986. [Google Scholar] [CrossRef]
- Baniţă, C.; Antoce, O.A.; Cojocaru, G.A. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors 2023, 11, 98. [Google Scholar] [CrossRef]
- Lan, T.; Gao, C.; Yuan, Q.; Wang, J.; Zhang, H.; Sun, X.; Lei, Y.; Ma, T. Analysis of the Aroma Chemical Composition of Commonly Planted Kiwifruit Cultivars in China. Foods 2021, 10, 1645. [Google Scholar] [CrossRef]
- Fan, S.; Tang, K.; Xu, Y.; Chen, S. Characterization of the Potent Odorants in Tibetan Qingke Jiu by Sensory Analysis, Aroma Extract Dilution Analysis, Quantitative Analysis and Odor Activity Values. Food Res. Int. 2020, 137, 109349. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ren, X.; Zhong, Y.; Liu, Y.; Jiang, J.; Song, Y.; Xin, L.; Lu, Y.; Qin, Y. Effect of Multi-Step Chaptalisation on Physicochemical Properties, Potentially Harmful Alcohols, Nutritional Composition and Volatile Profiles of Kiwi Wine. Int. J. Food Sci. Technol. 2023, 58, 3715–3726. [Google Scholar] [CrossRef]
- Delcros, L.; Collas, S.; Hervé, M.; Blondin, B.; Roland, A. Evolution of Markers Involved in the Fresh Mushroom Off-Flavor in Wine During Alcoholic Fermentation. J. Agric. Food Chem. 2023, 71, 14687–14696. [Google Scholar] [CrossRef]
- Chen, X.; Peng, M.; Wu, D.; Cai, G.; Yang, H.; Lu, J. Physicochemical Indicators and Sensory Quality Analysis of Kiwi Wines Fermented with Different Saccharomyces Cerevisiae. J. Food Process Preserv. 2022, 46, e17132. [Google Scholar] [CrossRef]
- Yang, B.; Liu, S.; Zang, H.; Dai, Y.; Zhang, S.; Lin, X.; Liang, H.; Chen, Y. Flavor Profile and Quality of Strawberry Wine Are Improved through Sequential Fermentation with Indigenous Non-Saccharomyces Yeasts and Saccharomyces Cerevisiae. Food Biosci. 2024, 59, 104021. [Google Scholar] [CrossRef]
- Nan, L.; Liu, L.; Li, Y.; Huang, J.; Wang, Y.; Wang, C.; Wang, Z.; Xu, C. Comparison of Aroma Compounds in Cabernet Sauvignon Red Wines from Five Growing Regions in Xinjiang in China. J. Food Qual. 2021, 2021, 5562518. [Google Scholar] [CrossRef]
- Shan, T.; Wei, J.; Wang, Y.; Zhao, X.; Zhao, Y.; Ge, Q.; Yuan, Y.; Yue, T. Effects of Different Pesticides Treatments on the Nutritional Quality of Kiwifruit. J. Food Sci. 2021, 86, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, T.; Yang, H.; Li, E. Isolation and Selection of Non-Saccharomyces Yeasts Being Capable of Degrading Citric Acid and Evaluation Its Effect on Kiwifruit Wine Fermentation. Fermentation 2020, 6, 25. [Google Scholar] [CrossRef]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated Biorefinery Approach to Valorize Winery Waste: A Review from Waste to Energy Perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef] [PubMed]
- Dabare, P.R.; Reilly, T.; Mierczynski, P.; Bindon, K.; Vasilev, K.; Mierczynska-Vasilev, A. A Novel Solution to Tartrate Instability in White Wines. Food Chem. 2023, 422, 136159. [Google Scholar] [CrossRef]
- Prusova, B.; Licek, J.; Kumsta, M.; Baron, M.; Sochor, J. Effect of New Methods for Inhibiting Malolactic Fermentation on the Analytical and Sensory Parameters of Wines. Fermentation 2024, 10, 122. [Google Scholar] [CrossRef]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Rossi, C.O.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of New Baking (+)-Catechin Based Leavening Agents: Effects on Rheology, Sensory and Antioxidant Features during Muffin Preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef]
- Núñez, L.; Serratosa, M.P.; Godoy, A.; Fariña, L.; Dellacassa, E.; Moyano, L. Comparison of Physicochemical Properties, Amino Acids, Mineral Elements, Total Phenolic Compounds, and Antioxidant Capacity of Cuban Fruit and Rice Wines. Food Sci. Nutr. 2021, 9, 3673–3682. [Google Scholar] [CrossRef]
- Huang, D.; Zhong, Y.; Liu, Y.; Song, Y.; Zhao, X.; Qin, Y. Reducing Higher Alcohols by Integrating Indigenous Saccharomyces Cerevisiae, Nitrogen Compensation, and Chaptalization Methods during Fermentation of Kiwifruit Wine. LWT 2023, 184, 115059. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of Boiling, Ultra-High Temperature and High Hydrostatic Pressure on Free Amino Acids, Flavor Characteristics and Sensory Profiles in Chinese Rice Wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
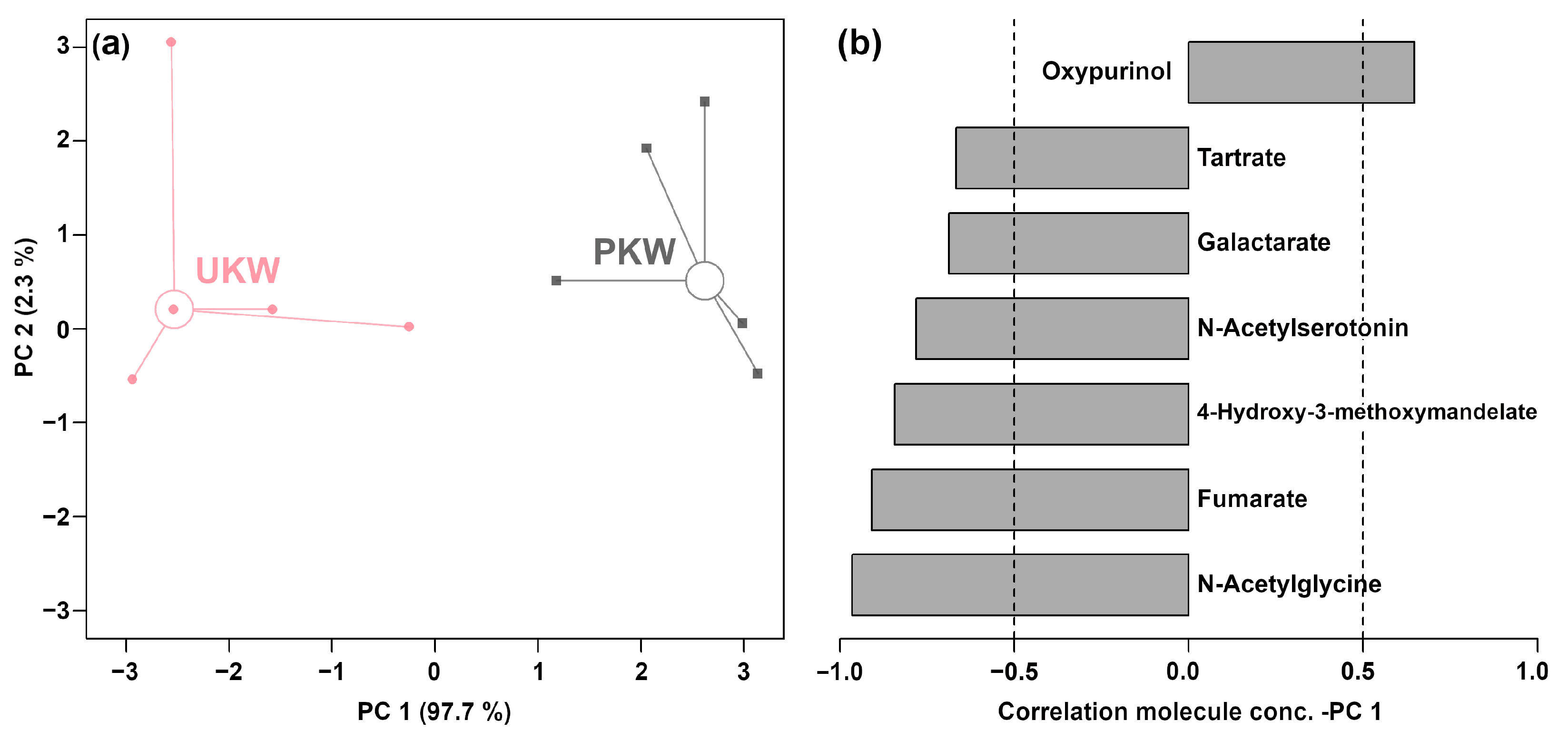
| Compound Name | Relative Concentration | p-Value | |
|---|---|---|---|
| PKW | UKW | ||
| Ethyl heptanoate | 1.23 × 10−3 ± 2.28 × 10−4 | 1.99 × 10−3 ± 3.52 × 10−4 | 0.005 |
| Isoamyl acetate | 5.09 × 10−2 ± 8.79 × 10−3 | 4.07 × 10−2 ± 5.60 × 10−3 | 0.002 |
| Hexyl acetate | 3.84 × 10−3 ± 7.99 × 10−4 | 2.40 × 10−3 ± 7.42 × 10−4 | 0.005 |
| D-Citronellol | 4.31 × 10−4 ± 1.78 × 10−4 | 1.37 × 10−3 ± 2.69 × 10−4 | 0.000 |
| 2,4,5-Trichlorotoluene | 9.08 × 10−4 ± 2.10 × 10−4 | 6.02 × 10−4 ± 9.21 × 10−5 | 0.002 |
| Rose oxide | 2.68 × 10−4 ± 6.75 × 10−5 | 8.51 × 10−4 ± 2.64 × 10−4 | 0.000 |
| Bornylene | 3.59 × 10−4 ± 5.73 × 10−5 | 6.11 × 10−4 ± 1.10 × 10−4 | 0.002 |
| Compound Name | Molecular Concentration | p-Value | |
|---|---|---|---|
| PKW | UKW | ||
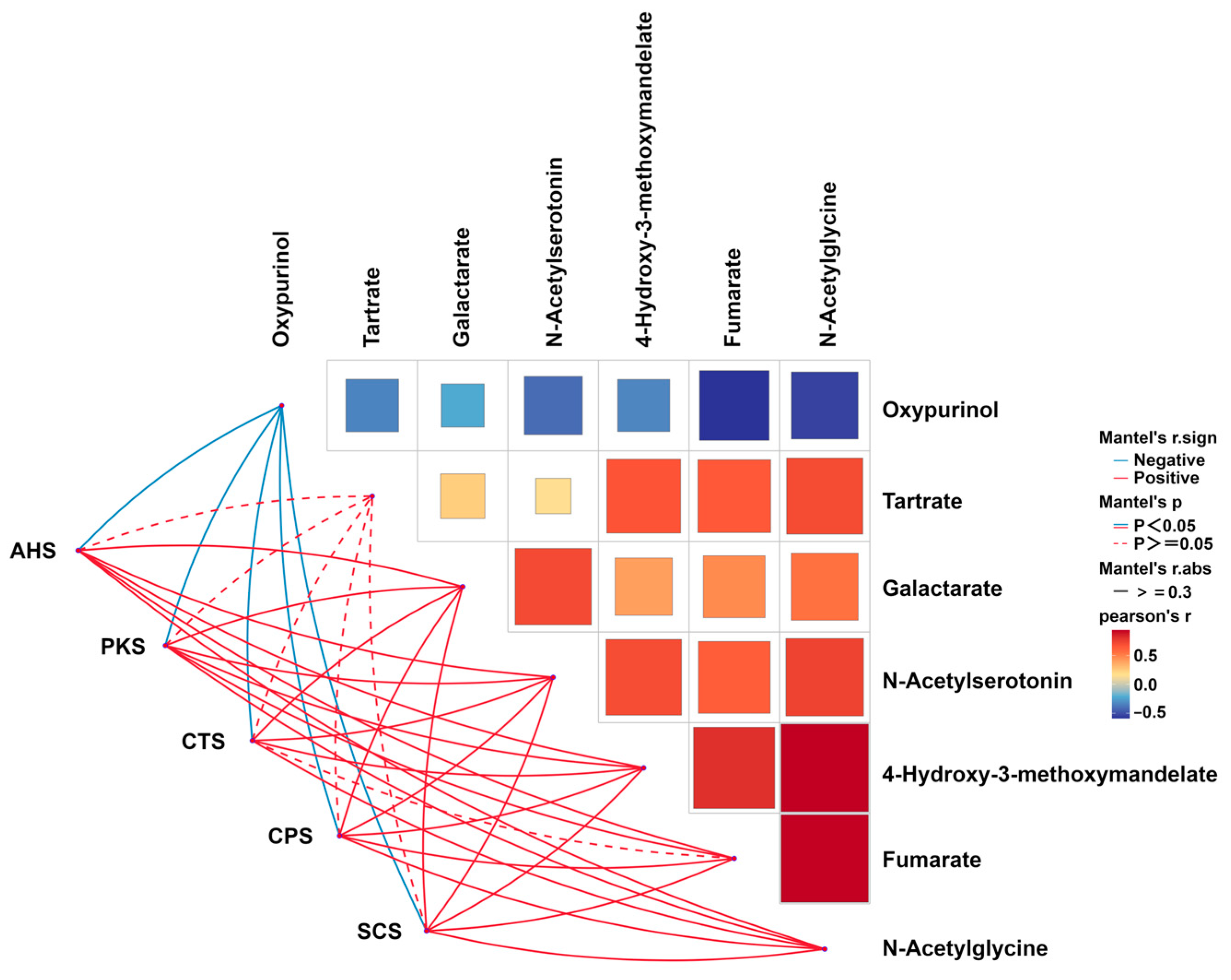
| Oxypurinol | 4.39 × 10−2 ± 1.16 × 10−2 | 2.93 × 10−2 ± 6.52 × 10−3 | 0.035 |
| Fumarate | 1.24 × 10−2 ± 1.53 × 10−3 | 1.68 × 10−2 ± 2.20 × 10−3 | 0.010 |
| Tartrate | 3.49 × 10−2 ± 5.32 × 10−3 | 4.40 × 10−2 ± 5.63 × 10−3 | 0.035 |
| N-Acetylserotonin | 2.71 × 10−2 ± 6.00 × 10−3 | 3.97 × 10−2 ± 9.00 × 10−3 | 0.031 |
| 4-Hydroxy-3-methoxymandelate | 5.65 × 10−2 ± 7.17 × 10−3 | 7.98 × 10−2 ± 1.10 × 10−2 | 0.005 |
| N-Acetylglycine | 1.70 ± 9.69 × 10−2 | 2.17 ± 0.15 | 0.001 |
| Galactarate | 0.17 ± 6.93 × 10−3 | 0.22 ± 5.05 × 10−2 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Liu, X.; Lan, Q.; Wan, F.; Yang, Z.; Nie, X.; Cai, Z.; Hu, B.; Tang, J.; Zhu, C.; et al. Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance. Foods 2024, 13, 1729. https://doi.org/10.3390/foods13111729
Zhou B, Liu X, Lan Q, Wan F, Yang Z, Nie X, Cai Z, Hu B, Tang J, Zhu C, et al. Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance. Foods. 2024; 13(11):1729. https://doi.org/10.3390/foods13111729
Chicago/Turabian StyleZhou, Bingde, Xiaochen Liu, Qiuyu Lan, Fang Wan, Zhibo Yang, Xin Nie, Zijian Cai, Bin Hu, Junni Tang, Chenglin Zhu, and et al. 2024. "Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance" Foods 13, no. 11: 1729. https://doi.org/10.3390/foods13111729





