Biochemical, Histological, and Transcriptomic Analyses Reveal Underlying Differences in Flesh Quality between Wild and Farmed Ricefield Eel (Monopterus albus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Analysis
2.2.1. Muscle Nutrient Composition
2.2.2. Muscle Quality Analysis
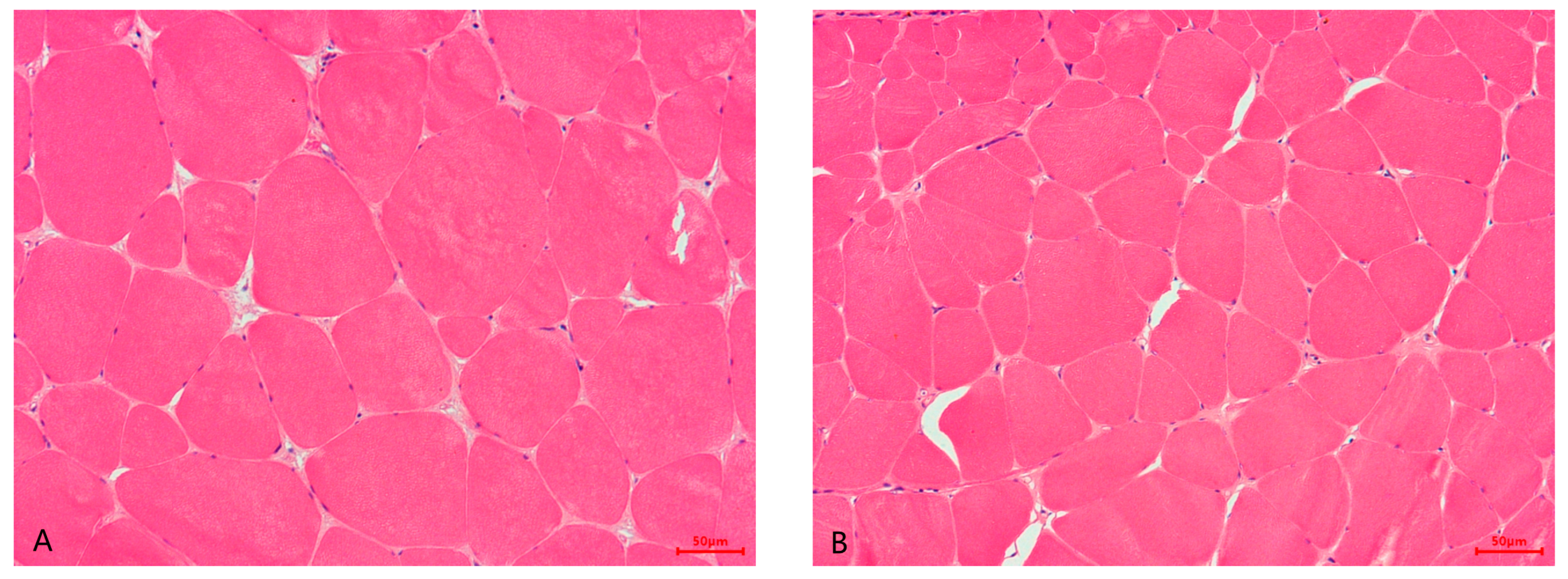
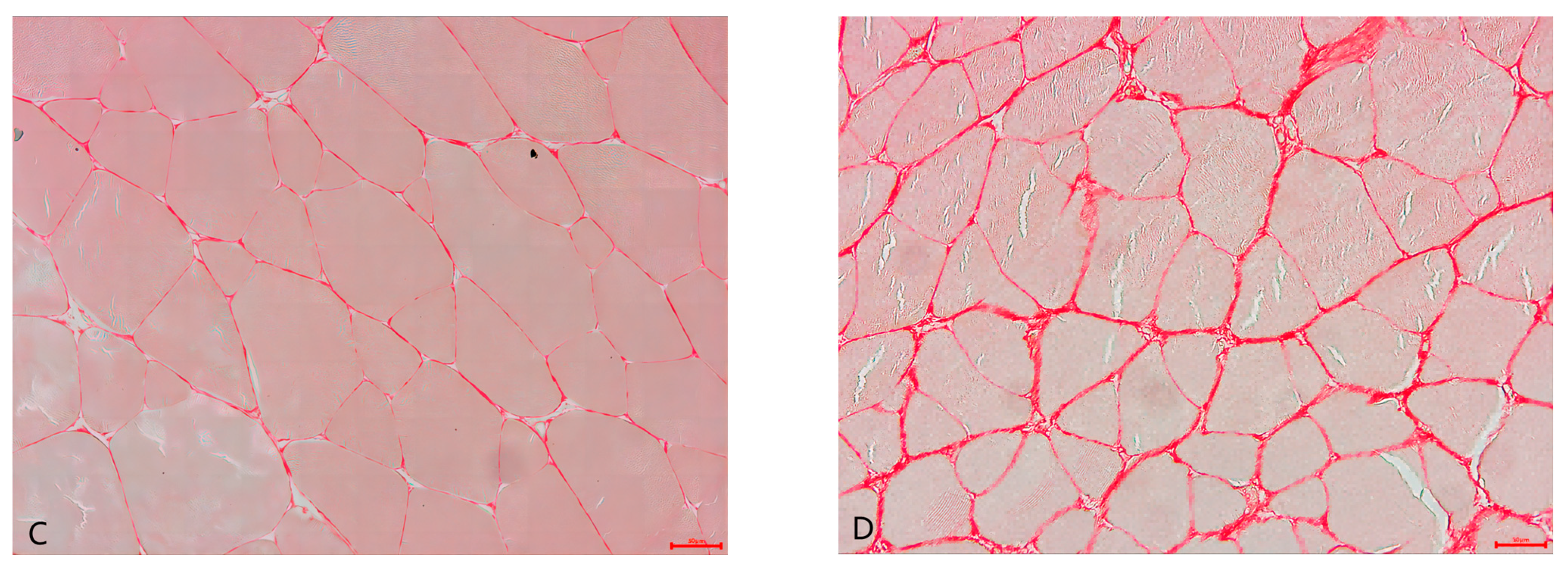
2.2.3. Muscle Tissue Morphology
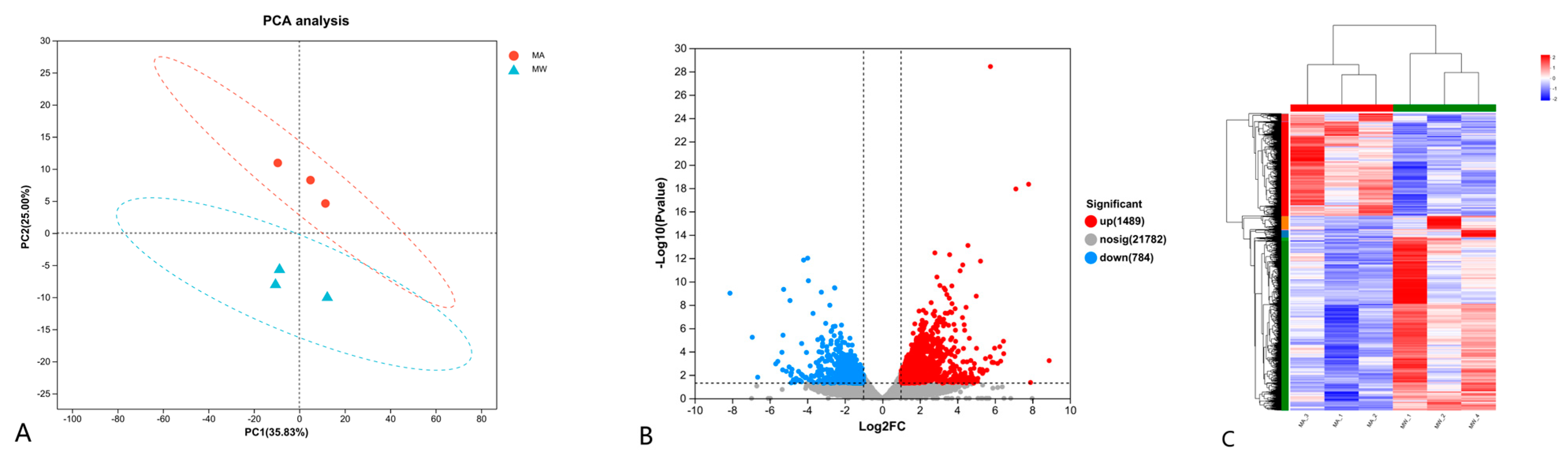
2.2.4. Transcriptome Analysis
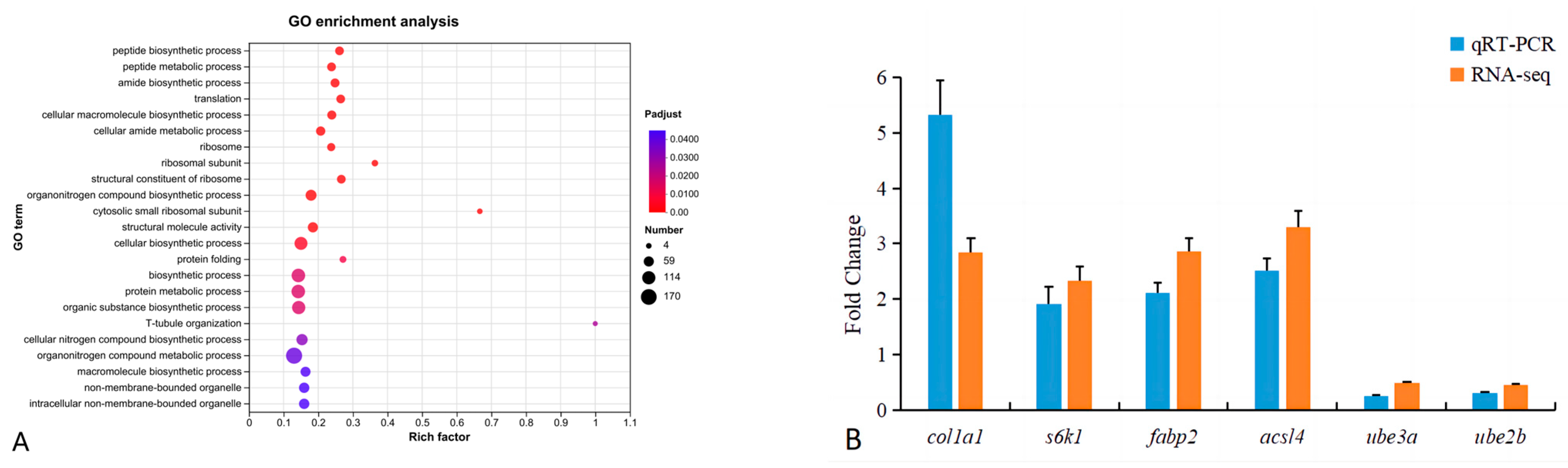
2.2.5. DEGs Verification Using qRT-PCR
2.3. Statistical Analysis
3. Results
3.1. Muscle Proximate Composition
3.2. Muscle Amino Acid Composition
3.3. Muscle Fatty Acid Composition
3.4. Muscle Water Holding Capacity, Texture Profile, and Muscle Histology
3.5. Transcriptomic Assay
3.6. qRT-PCR Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thong, N.T.; Solgaard, H.S. Consumer’s food motives and seafood consumption. Food Qual. Prefer. 2017, 56, 181–188. [Google Scholar] [CrossRef]
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The role and opportunity for aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Feed matters: Satisfying the feed demand of aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- López-Mas, L.; Claret, A.; Reinders, M.J.; Banovic, M.; Krystallis, A.; Guerrero, L. Farmed or wild fish? Segmenting European consumers based on their beliefs. Aquaculture 2021, 532, 735992. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Lorenzen, K.; Beveridge, M.C.; Mangel, M. Cultured fish: Integrative biology and management of domestication and interactions with wild fish. Biol. Rev. 2012, 87, 639–660. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological changes in fish gut in response to prebiotics and probiotics treatment to improve their health status: A Review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- China Fishery Statistical Yearbook. Ministry of Agriculture and Rural Affairs; China Agriculture Press: Beijing, China, 2023; p. 25. [Google Scholar]
- Johnston, I.A.; Li, X.; Vieira, V.L.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2006, 256, 323–336. [Google Scholar] [CrossRef]
- El-Zaeem, S.Y.; Ahmed, M.M.M.; Salama, M.S.; El-Kader, W.N.A. Flesh quality differentiation of wild and cultured Nile tilapia (Oreochromis niloticus) populations. Afr. J. Biotechnol. 2012, 11, 4085–4089. [Google Scholar]
- Tang, X.; Xu, G.; Dai, H.; Xu, P.; Zhang, C.; Gu, R. Differences in muscle cellularity and flesh quality between wild and farmed Coilia nasus (Engraulidae). J. Sci. Food Agric. 2012, 92, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Rincón, L.; Castro, P.L.; Álvarez, B.; Hernández, M.D.; Álvarez, A.; Claret, A.; Guerrero, L.; Ginés, R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Ma, R.; Meng, Y.; Zhang, W.; Mai, K. Comparative study on the organoleptic quality of wild and farmed large yellow croaker Larimichthys crocea. J. Oceanol. Limnol. 2020, 38, 260–274. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, H.; Peng, Z.; Yu, J.; Yan, L.; Wang, W.; Ren, T.; Han, Y. Comparison of nutritional quality, flesh quality, muscle cellularity, and expression of muscle growth-related genes between wild and recirculating aquaculture system (RAS)-farmed black rockfish (Sebastes schlegelii). Aquac. Int. 2023, 31, 2263–2280. [Google Scholar] [CrossRef]
- Ye, H.; Lin, Q.; Luo, H. Applications of transcriptomics and proteomics in understanding fish immunity. Fish Shellfish Immunol. 2018, 77, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chandhini, S.; Rejish Kumar, V.J. Transcriptomics in aquaculture: Current status and applications. Rev. Aquac. 2019, 11, 1379–1397. [Google Scholar] [CrossRef]
- Ali, A.; Rexroad, C.E.; Thorgaard, G.H.; Yao, J.; Salem, M. Characterization of the rainbow trout spleen transcriptome and identification of immune-related genes. Front. Genet. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Gene expression profiling reveals functional specialization along the intestinal tract of a carnivorous teleostean fish (Dicentrarchus labrax). Front. Physiol. 2016, 7, 359. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Xu, X.; Rahman, M.M.; Li, X.; Leng, X. Transcriptomic and biochemical analyses revealed the improved growth, lipid metabolism, and flesh quality of grass carp (Ctenopharyngodon idellus) by dietary Eucommia ulmoides bark and leaf supplementation. J. Anim. Sci. 2022, 100, skac250. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, C.; Liu, J.; Chen, J.; Zou, S. Transcriptome analysis provides new insights into the growth superiority of a novel backcross variety, Megalobrama amblycephala ♀ × (M. amblycephala ♀ × Culter alburnus ♂)♂. Aquaculture 2019, 512, 734317. [Google Scholar] [CrossRef]
- Zhao, Y.; Weng, M.; Zhang, Q.; Li, A.; Zhang, J. Transcriptomics analysis of the infected tissue of gibel carp (Carassius auratus gibelio) with liver myxobolosis infers the underlying defense mechanisms from the perspective of immune-metabolic interactions. Aquaculture 2021, 542, 736867. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2010. [Google Scholar]
- Yang, H.; Xu, Z.; Li, X.Q.; Tan, S.M.; Cheng, Z.; Leng, X.J. Influences of dietary Eucommia ulmoides extract on growth, flesh quality, antioxidant capacity and collagen-related genes expression in grass carp (Ctenopharyngodon idellus). Anim. Feed. Sci. Technol. 2021, 277, 114965. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Rahman, M.M.; Leng, X. Dietary supplementation of leucine improved the flesh quality of largemouth bass, Micropterus salmoides through TOR, FoxO3a and MRFs regulation. Aquaculture 2023, 566, 739237. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Poolsawat, L.; Rahman, M.M.; Xu, X.Y.; Jiang, X.R.; Li, X.Q.; Tan, H.X.; Leng, X.J. Flavonoid-enriched diets improved the growth and flesh quality of grass carp (Ctenopharyngodon idellus) based on metabolomics. Aquac. Nutr. 2021, 27, 2514–2528. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.F.; Li, X.Q.; Leng, X.J. Dietary calcium β-hydroxy-β-methylbutyrate supplementation improved the flesh quality, but did not promote the growth performance of largemouth bass (Micropterus salmoides). Anim. Feed. Sci. Technol. 2024, 307, 115829. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Zhang, C.; Bian, Y.; Yao, W.; Xu, Z.; Li, X.; Leng, X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2022, 548, 737551. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Zhang, X.; He, X.; Li, D. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): A comparison between grass and artificial feed. Front. Physiol. 2018, 9, 312148. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
- Huang, X.; Hegazy, A.M.; Zhang, X. Swimming exercise as potential measure to improve flesh quality of cultivable fish: A review. Aquac. Res. 2021, 52, 5978–5989. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Wen, H.L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Feng, L.; et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, B.; Jonsson, N. Cultured Atlantic salmon in nature: A review of their ecology and interaction with wild fish. ICES J. Mar. Sci. 2006, 63, 1162–1181. [Google Scholar] [CrossRef]
- Pankhurst, N.W. The endocrinology of stress in fish: An environmental perspective. Gen. Comp. Endocrinol. 2011, 170, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tasbozan, O.; Gökçe, M.A. Fatty acids in fish. Fat. Acids 2017, 1, 143–159. [Google Scholar]
- Gladyshev, M.I.; Sushchik, N.N.; Tolomeev, A.P.; Dgebuadze, Y.Y. Meta-analysis of factors associated with omega-3 fatty acid contents of wild fish. Rev. Fish Biol. Fish. 2018, 28, 277–299. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: The disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Cahu, C.; Salen, P.; De Lorgeril, M. Farmed and wild fish in the prevention of cardiovascular diseases: Assessing possible differences in lipid nutritional values. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 34–41. [Google Scholar] [CrossRef]
- Tanamati, A.; Stevanato, F.B.; Visentainer, J.E.L.; Matsushita, M.; de Souza, N.E.; Visentainer, J.V. Fatty acid composition in wild and cultivated pacu and pintado fish. Eur. J. Lipid Sci. Technol. 2009, 111, 183–187. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Claret, A.; Guerrero, L.; Ginés, R.; Grau, A.; Hernández, M.D.; Aguirre, E.; Peleteiro, J.B.; Rodríguez-Rodríguez, C. Consumer beliefs regarding farmed versus wild fish. Appetite 2014, 79, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, W.; Rong, Y.; Lou, B. Compositions, nutritional and texture quality of wild-caught andcage-cultured small yellow croaker. J. Food Compos. Anal. 2022, 107, 104370. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, F.; Zhang, W.B.; Parisi, G.; Du, Z.Y.; Zhang, M.L. The flesh texture of teleost fish: Characteristics and interventional strategies. Rev. Aquac. 2024, 16, 508–535. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Li, X.; Xu, X.; Tan, H.; Leng, X. Dietary supplementation of kaempferol improved the growth, lipid metabolism and flesh quality of juvenile grass carp (Ctenopharyngodon idellus) based on metabolomics. Anim. Feed. Sci. Technol. 2024, 295, 115520. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, Z.Y.; Song, C.W.; Luo, S.; Yuan, X.Y.; Huang, Y.Y.; Desouky, H.E. A comparative study on growth, muscle cellularity and flesh quality of farmed and imitative ecological farming loach, Misgurnus anguillicaudatus. Aquaculture 2021, 543, 736933. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Wang, L.; Jin, M.; Zhou, Q.; Nie, G. Fillet texture, physicochemical indexes, muscle cellularity and molecular expression in muscle of Yellow River carp (Cyprinus carpio haematopterus) in response to dietary hydroxyproline supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of processing methods on nutritional and physico-chemical composition of fish: A review. MOJ Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef]
- Zhao, H.F.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q.; et al. Flesh shear force, cooking loss, muscle antioxidant status and relative expression of signaling molecules (Nrf2, Keap1, TOR, and CK2) and their target genes in young grass carp (Ctenopharyngodon idella) muscle fed with graded levels of choline. PLoS ONE 2015, 10, e0142915. [Google Scholar] [CrossRef]
- Johnston, I.A. Muscle development and growth: Potential implications for flesh quality in fish. Aquaculture 1999, 177, 99–115. [Google Scholar] [CrossRef]
- Parma, L.; Badiani, A.; Bonaldo, A.; Viroli, C.; Farabegoli, F.; Silvi, M.; Pirini, M.; Gatta, P.P. Farmed and wild common sole (Solea solea L.): Comparative assessment of morphometric parameters, processing yields, selected nutritional traits and sensory profile. Aquaculture 2019, 502, 63–71. [Google Scholar] [CrossRef]
- Smith, R.W.; Palmer, R.M.; Houlihan, D.F. RNA turnover and protein synthesis in fish cells. J. Comp. Physiol. B 2000, 170, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Ni, W.; Gao, Q.; Tian, F.; Qi, D. Insights Into miRNA-mRNA Regulatory Mechanisms of Cold Adaptation in Gymnocypris eckloni: Ubiquitin-Mediated Proteolysis Is Pivotal for Adaptive Energy Metabolism. Front. Genet. 2022, 13, 903995. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Liang, X.F.; He, S.; Tang, S.; Li, L.; Chen, X. mTOR-Mediated protein synthesis by inhibiting protein catabolism in Chinese perch (Siniperca chuatsi). Biochem. Biophys. Res. Commun. 2020, 533, 23–29. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, T.P.; Michelato, M.; Dal-Pai-Silva, M.; de Paula, T.G.; Macedo, E.A.; Peres, H.; Furuya, V.R.B.; Furuya, W.M. Growth performance, amino acid retention and mRNA levels of mTORC1 signaling pathway genes in Nile tilapia fingerlings fed protein-bound and crystalline amino acids. Aquaculture 2021, 543, 736953. [Google Scholar] [CrossRef]
- Fang, C.C.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Liu, X.A.; Zhou, X.Q. Effects of dietary methionine on growth performance, muscle nutritive deposition, muscle fibre growth and type I collagen synthesis of on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2021, 126, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiang, J.; Zhu, X.; Bao, J.; Tao, Y.; Zhu, H. Effect of Siberian ginseng water extract as a dietary additive on growth performance, blood biochemical indexes, lipid metabolism, and expression of PPARs pathway-related genes in genetically improved farmed tilapia (Oreochromis niloticus). Fishes 2022, 7, 149. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y. Key factors in mTOR regulation. Cell. Mol. Life Sci. 2010, 67, 239–253. [Google Scholar] [CrossRef]
| Index | Farmed | Wild | p-Value |
|---|---|---|---|
| Moisture | 709.1 ± 6.1 a | 684.5 ± 7.6 b | 0.030 * |
| Crude protein | 253.6 ± 5.5 b | 283.1 ± 4.8 a | 0.002 ** |
| Crude lipid | 14.7 ± 0.5 a | 13.1 ± 0.2 b | 0.013 * |
| Crude ash | 20.1 ± 0.3 a | 16.3 ± 0.2 b | 0.010 * |
| Collagen | 9.39 ± 0.11 b | 13.12 ± 0.15 a | 0.005 ** |
| Index | Farmed | Wild | p-Value |
|---|---|---|---|
| Arginine | 44.45 ± 0.4 | 47.05 ± 1.7 | 0.136 |
| Histidine | 42.58 ± 0.4 b | 48.33 ± 1.6 a | 0.019 * |
| Isoleucine | 18.42 ± 1.1 | 20.41 ± 1.4 | 0.193 |
| Leucine | 11.15 ± 1.5 | 13.04 ± 1.3 | 0.219 |
| Lysine | 43.91 ± 0.3 b | 50.44 ± 1.4 a | 0.008 ** |
| Threonine | 124.36 ± 2.5 | 124.67 ± 5.3 | 0.967 |
| Valine | 0.91 ± 0.0 | 1.04 ± 0.1 | 0.055 |
| Methionine | 15.81 ± 0.1 b | 17.78 ± 0.7 a | 0.031 * |
| Phenylalanine | 17.62 ± 0.1 | 18.94 ± 1.1 | 0.210 |
| EAAs | 319.21 ± 3.9 | 341.71 ± 10.9 | 0.168 |
| Aspartate | 66.25 ± 0.5 b | 75.62 ± 1.8 a | 0.006 ** |
| Serine | 48.75 ± 2.6 a | 31.89 ± 1.4 b | 0.002 ** |
| Glycine | 5.21 ± 0.1 b | 5.86 ± 0.1 a | 0.004 ** |
| Alanine | 18.32 ± 0.1 b | 20.61 ± 0.8 a | 0.036 * |
| Tyrosine | 103.15 ± 0.2 b | 114.64 ± 4.4 a | 0.039 * |
| Cysteine | 21.23 ± 1.1 b | 25.70 ± 1.7 a | 0.041 * |
| Glutamate | 112.82 ± 0.4 b | 131.49 ± 3.5 a | 0.006 ** |
| Proline | 59.22 ± 2.9 b | 67.58 ± 3.2 a | 0.035 * |
| NEAAs | 434.02 ± 9.0 | 473.39 ± 10.8 | 0.100 |
| TAAs | 754.17 ± 5.0 b | 815.10 ± 16.9 a | 0.045 * |
| Index | Farmed | Wild | p-Value |
|---|---|---|---|
| Undecanoic acid (C11:0) | — | 0.64 ± 0.04 | 0.000 ** |
| Lauric acid (C12:0) | 1.21 ± 0.02 b | 1.52 ± 0.12 a | 0.044 * |
| Tridecanoic acid (C13:0) | — | 0.32 ± 0.03 | 0.001 ** |
| Myristic acid (C14:0) | 3.51 ± 0.02 a | 2.61 ± 0.20 b | 0.010 * |
| Pentadecanoic acid (C15:0) | — | 1.22 ± 0.06 | 0.000 ** |
| Palmitic acid (C16:0) | 23.42 ± 1.94 b | 33.66 ± 0.99 a | 0.003 ** |
| Stearic acid (C18:0) | 11.85 ± 1.23 b | 14.89 ± 0.06 a | 0.018 * |
| Tricosanoic acid (C23:0) | 4.85 ± 0.25 | 5.52 ± 0.34 | 0.105 |
| Total saturated fatty acids (SFAs) | 44.84 ± 0.99 b | 60.39 ± 0.39 a | 0.000 ** |
| Palmitoleic acid (C16:1) | 8.51 ± 0.27 | 7.48 ± 0.47 | 0.071 |
| Cis-10-Heptadecenoic acid (C17:1) | 1.18 ± 0.02 | — | 0.000 ** |
| Oleic acid (C18:1) | 14.45 ± 1.14 a | 7.08 ± 0.09 b | 0.001 ** |
| Nervonic acid (C24:1) | 1.12 ± 0.2 b | 1.59 ± 0.04 a | 0.024 * |
| Total monounsaturated fatty acids (MUFAs) | 25.25 ± 0.61 a | 16.16 ± 0.61 b | 0.001 ** |
| Linoleic acid (C18:2) | 21.9 ± 0.66 a | 8.8 ± 0.2 b | 0.000 ** |
| Arachidonic acid (C20:4) | 3.32 ± 0.69 b | 6.81 ± 0.01 a | 0.000 ** |
| n-6 polyunsaturated fatty acids (n-6 PUFAs) | 25.22 ± 0.4 | 15.61 ± 0.21 | 0.000 ** |
| Linolenic acid (C18:3) | 2.28 ± 0.01 a | 1.62 ± 0.04 b | 0.000 ** |
| Eicosapentaenoic acid (C20:5) | 1.20 ± 0.04 b | 2.21 ± 0.11 a | 0.001 ** |
| Docosahexaenoic acid (C22:6) | — | 1.80 ± 0.03 | 0.000 ** |
| n-3 polyunsaturated fatty acids (n-3 PUFAs) | 3.48 ± 0.01 b | 5.63 ± 0.08 a | 0.000 ** |
| Total polyunsaturated fatty acids (PUFAs) | 28.7 ± 0.39 a | 21.24 ± 0.22 b | 0.000 ** |
| n-3/n-6 | 0.14 ± 0.00 b | 0.36 ± 0.01 a | 0.000 ** |
| Index | Farmed | Wild | p-Value |
|---|---|---|---|
| Water holding capacity | |||
| Thawing loss (%) | 15.44 ± 1.27 a | 9.03 ± 1.79 b | 0.007 ** |
| Drip loss (%) | 20.84 ± 2.15 a | 14.84 ± 3.02 b | 0.047 * |
| Steaming loss (%) | 18.05 ± 1.77 a | 14.97 ± 0.92 b | 0.029 ** |
| Boiling loss (%) | 14.44 ± 1.56 a | 7.84 ± 2.08 b | 0.006 * |
| Texture profile | |||
| Hardness (gf) | 236.20 ± 31.46 b | 339.65 ± 36.28 a | 0.010 * |
| Springiness | 0.68 ± 0.02 b | 0.93 ± 0.03 a | 0.001 ** |
| Cohesiveness | 0.42 ± 0.04 b | 0.53 ± 0.04 a | 0.001 ** |
| Gumminess (gf) | 89.63 ± 11.88 b | 146.69 ± 13.61 a | 0.001 ** |
| Chewiness (gf) | 67.84 ± 12.94 b | 106.01 ± 11.55 a | 0.010 * |
| Resilience | 0.18 ± 0.02 b | 0.24 ± 0.02 a | 0.001 ** |
| Muscle morphology | |||
| Muscle fiber diameter (μm) | 85.41 ± 5.10 a | 59.10 ± 3.11 b | <0.001 ** |
| Muscle fiber density (number of fibers/mm2) | 246.14 ± 18.33 b | 358.02 ± 22.22 a | <0.001 ** |
| Pathway ID | Description | Number | p-Value |
|---|---|---|---|
| map04141 | Protein processing in endoplasmic reticulum | 61 | 1.30 × 10−13 |
| map03010 | Ribosome | 42 | 5.86 × 10−12 |
| map05171 | Coronavirus disease—COVID-19 | 65 | 2.06 × 10−8 |
| map05415 | Diabetic cardiomyopathy | 46 | 1.04 × 10−4 |
| map05012 | Parkinson’s disease | 53 | 7.33 × 10−4 |
| map03250 | Viral life cycle—HIV-1 | 18 | 8.38 × 10−4 |
| map03013 | Nucleocytoplasmic transport | 24 | 1.07 × 10−3 |
| map00260 | Glycine, serine, and threonine metabolism | 13 | 1.66 × 10−3 |
| map00513 | Various types of N-glycan biosynthesis | 13 | 1.99 × 10−3 |
| map04621 | NOD-like receptor signaling pathway | 34 | 2.16 × 10−3 |
| map04217 | Necroptosis | 33 | 2.71 × 10−3 |
| map04216 | Ferroptosis | 15 | 3.20 × 10−3 |
| map05014 | Amyotrophic lateral sclerosis | 64 | 3.56 × 10−3 |
| map03320 | PPAR signaling pathway | 18 | 4.25 × 10−3 |
| map05020 | Prion disease | 53 | 4.41 × 10−3 |
| map05022 | Pathways of neurodegeneration—multiple diseases | 84 | 4.84 × 10−3 |
| map04512 | ECM–receptor interaction | 22 | 5.13 × 10−3 |
| map04612 | Antigen processing and presentation | 18 | 6.01 × 10−3 |
| map03060 | Protein export | 7 | 7.16 × 10−3 |
| map04714 | Thermogenesis | 43 | 7.21 × 10−3 |
| map00510 | N-Glycan biosynthesis | 13 | 9.31 × 10−3 |
| map04974 | Protein digestion and absorption | 28 | 9.73 × 10−3 |
| map05134 | Legionellosis | 18 | 1.37 × 10−2 |
| map04120 | Ubiquitin-mediated proteolysis | 27 | 1.50 × 10−2 |
| map00561 | Glycerolipid metabolism | 16 | 1.52 × 10−2 |
| map05010 | Alzheimer’s disease | 68 | 1.62 × 10−2 |
| map04614 | Renin–angiotensin system | 7 | 2.08 × 10−2 |
| map04114 | Oocyte meiosis | 24 | 2.18 × 10−2 |
| map04150 | mTOR signaling pathway | 31 | 2.49 × 10−2 |
| map04711 | Circadian rhythm—fly | 5 | 2.74 × 10−2 |
| map04910 | Insulin signaling pathway | 29 | 3.10 × 10−2 |
| map05417 | Lipid and atherosclerosis | 40 | 3.36 × 10−2 |
| map05162 | Measles | 27 | 3.44 × 10−2 |
| map04137 | Mitophagy—animal | 16 | 3.46 × 10−2 |
| map04212 | Longevity regulating pathway—worm | 16 | 3.46 × 10−2 |
| map04622 | RIG-I-like receptor signaling pathway | 14 | 4.01 × 10−2 |
| map03015 | mRNA surveillance pathway | 17 | 4.37 × 10−2 |
| map04146 | Peroxisome | 17 | 4.37 × 10−2 |
| map05131 | Shigellosis | 46 | 4.72 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Yuan, Q.; Rahman, M.M.; Lv, W.; Huang, W.; Hu, W.; Zhou, W. Biochemical, Histological, and Transcriptomic Analyses Reveal Underlying Differences in Flesh Quality between Wild and Farmed Ricefield Eel (Monopterus albus). Foods 2024, 13, 1751. https://doi.org/10.3390/foods13111751
Yang H, Yuan Q, Rahman MM, Lv W, Huang W, Hu W, Zhou W. Biochemical, Histological, and Transcriptomic Analyses Reveal Underlying Differences in Flesh Quality between Wild and Farmed Ricefield Eel (Monopterus albus). Foods. 2024; 13(11):1751. https://doi.org/10.3390/foods13111751
Chicago/Turabian StyleYang, Hang, Quan Yuan, Mohammad Mizanur Rahman, Weiwei Lv, Weiwei Huang, Wei Hu, and Wenzong Zhou. 2024. "Biochemical, Histological, and Transcriptomic Analyses Reveal Underlying Differences in Flesh Quality between Wild and Farmed Ricefield Eel (Monopterus albus)" Foods 13, no. 11: 1751. https://doi.org/10.3390/foods13111751
APA StyleYang, H., Yuan, Q., Rahman, M. M., Lv, W., Huang, W., Hu, W., & Zhou, W. (2024). Biochemical, Histological, and Transcriptomic Analyses Reveal Underlying Differences in Flesh Quality between Wild and Farmed Ricefield Eel (Monopterus albus). Foods, 13(11), 1751. https://doi.org/10.3390/foods13111751








