Quality- and Health-Promoting Compounds of Whole Wheat Bread with the Addition of Stale Bread, Cornmeal, and Apple Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Manufacture of Extrudates from Recycled Materials—Preparations P1 and P2
2.1.2. Bread Baking
2.2. Chemical Reagents
2.3. Chemical Composition
2.3.1. Proximate Composition
2.3.2. Dietary Fiber Content
2.4. Bioactive Compounds and Antioxidant Activity
2.4.1. Preparation of Extracts
2.4.2. UHPLC-DAD Analysis of Phenolic Compounds
2.4.3. UHPLC-ESI-MS Analysis of the Vitamin B Group
2.4.4. Total Phenolic Content (TPC)
2.4.5. Total Flavonoid Content
2.4.6. Antioxidant Activity Determined by ABTS and DPPH Assays
2.4.7. Ferric Ion-Reducing Antioxidant Power
2.4.8. Fe(II) Chelating Activity
2.5. Bread Quality Analysis
2.5.1. Bread Yield
2.5.2. Bread Volume
2.6. Analysis of Texture Parameters
2.7. Color Measurement
2.8. Analysis of Volatile Compounds Using an Electronic Nose
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of P1 and P2 Preparations and the Wheat Flour Type 1850
3.2. Effect of the Extruded Preparations on the Amount of Nutritional Compounds and Bioactive Compounds in Whole Wheat Bread
3.3. The Effect of Extruded Preparations on the Texture and Physical Properties of Whole Wheat Bread
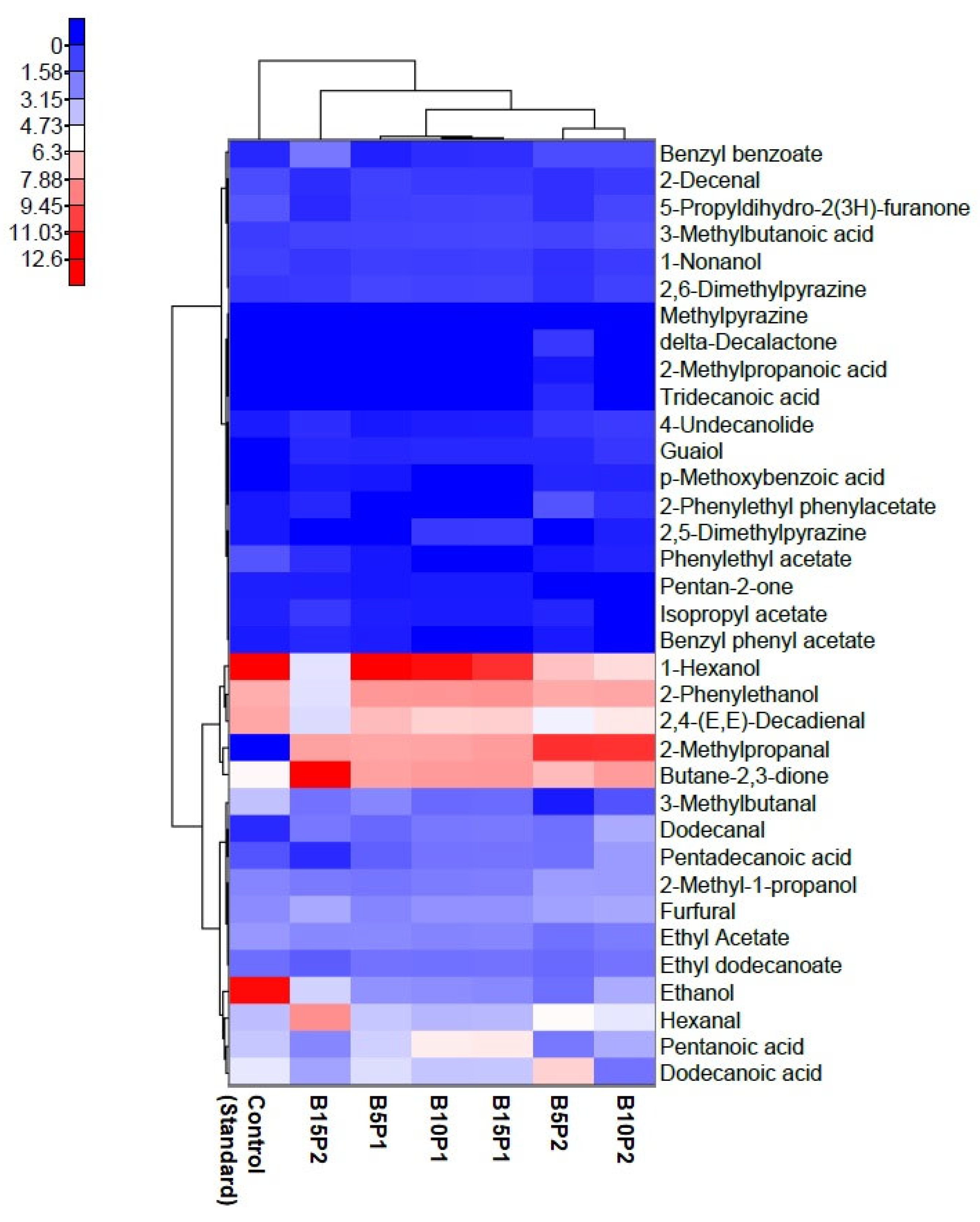
3.4. Influence of the Extruded Preparations on the Volatile Compound Profile Whole Wheat Bread Evaluated by Electronic Nose
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dymchenko, A.; Geršl, M.; Gregor, T. Trends in bread waste utilisation. Trends Food Sci. Technol. 2023, 132, 93–102. [Google Scholar] [CrossRef]
- Narisetty, V.; Cox, R.; Willoughby, N.; Aktas, E.; Tiwari, B.; Matharu, A.S.; Salonitis, K.; Kumar, V. Recycling bread waste into chemical building blocks using a circular biorefining approach. Sustain. Energy Fuels 2021, 5, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Samray, M.N.; Masatcioglu, T.M.; Koksel, H. Bread crumbs extrudates: A new approach for reducing bread waste. J. Cereal Sci. 2019, 85, 130–136. [Google Scholar] [CrossRef]
- Goryńska-Goldmann, E.; Gazdecki, M.; Rejman, K.; Kobus-Cisowska, J.; Łaba, S.; Łaba, R. How to prevent bread losses in the baking and confectionery industry? Measurement, Causes, Management and Prevention. Agriculture 2021, 11, 19. [Google Scholar] [CrossRef]
- Cox, R.; Narisetty, V.; Nagarajan, S.; Agrawal, D.; Ranade, V.V.; Salonitis, K.; Venus, J.; Kumar, V. High-Level fermentative production of Lactic acid from bread waste under non-sterile conditions with a circular biorefining approach and zero waste discharge. Fuel 2022, 313, 122976. [Google Scholar] [CrossRef]
- Demirci, A.S.; Palabiyik, I.; Gumus, T. Bread wastage and recycling of waste bread by producing biotechnological products. J. Biotechnol. 2016, 231, S13. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.-Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Narisetty, V.; Nagarajan, S.; Gadkari, S.; Ranade, V.V.; Zhang, J.; Patchigolla, K.; Bhatnagar, A.; Awasthi, M.K.; Pandey, A.; Kumar, V. Process optimization for recycling of bread waste into bioethanol and biomethane: A circular economy approach. Energy Convers. Manag. 2022, 266, 115784. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Pietrzak, W.; Lennartsson, P.R. High-efficiency conversion of bread residues to ethanol and edible biomass using filamentous fungi at high solids loading: A biorefinery approach. Appl. Sci. 2022, 12, 6405. [Google Scholar] [CrossRef]
- García-Hernández, Á.; Roldán-Cruz, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Stale bread waste recycling as ingredient for fresh oven-baked white bread: Effects on dough viscoelasticity, bread molecular organization, texture, and starch digestibility. J. Sci. Food Agric. 2023, 103, 4174–4183. [Google Scholar] [CrossRef]
- Meral, H.; Karaoğlu, M.M. The effect of the stale bread flour addition on flour and bread quality. Int. J. Food Eng. 2020, 16, 20190100. [Google Scholar] [CrossRef]
- Immonen, M.; Maina, N.H.; Coda, R.; Katina, K. The molecular state of gelatinized starch in surplus bread affects bread recycling potential. LWT-Food Sci. Technol. 2021, 150, 112071. [Google Scholar] [CrossRef]
- Savkina, O.; Kuznetsova, L.; Lokachuk, M.; Parakhina, O.; Pavlovskaya, E.; Lavrenteva, N. The way of old bread recycling in the bread making. E3S Web Conf. 2020, 161, 01082. [Google Scholar] [CrossRef]
- Weegels, P. Fast Return: Reusing One-Day-Old Bread by Fermentation; European Bakery Innovation Centre: Papendrecht, The Netherlands, 2010; pp. 5–18. [Google Scholar]
- Sisman, S.; Yagci, B.; Ermis, E. Physical properties of protein extract powder from stale bread and evaluation of its use in wheat bread containing chickpea flour. GIDA J. Food 2022, 47, 34–41. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- AACC. Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Kahoun, D.; Fojtíková, P.; Vácha, F.; Čížková, M.; Vodička, R.; Nováková, E.; Hypša, V. Development and validation of an LC-MS/MS method for determination of B vitamins and some its derivatives in whole blood. PLoS ONE 2022, 17, e0271444. [Google Scholar] [CrossRef] [PubMed]
- Zohora, F.-T.; Sarwar, S.; Khatun, O.; Begum, P.; Khatun, M.; Ahsan, M.; Nazrul Islam, S. Estimation of B-vitamins (B1, B2, B3 and B6) by HPLC in vegetables including ethnic selected varieties of Bangladesh. Pharm. Pharmacol. Int. J. 2020, 8, 16–23. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent). Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D. In vitro antioxidant activity and FTIR characterization of high-molecular weight melanoidin fractions from different types of cocoa beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef]
- Kowalski, S.; Oracz, J.; Skotnicka, M.; Mikulec, A.; Gumul, D.; Mickowska, B.; Mazurek, A.; Sabat, R.; Wywrocka-Gurgul, A.; Żyżelewicz, D. Chemical composition, nutritional value, and acceptance of nut bars with the addition of edible insect powder. Molecules 2022, 27, 8472. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbeym, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin−Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects and Applications; Technomic Publishing Company: Lancaster, UK, 1995. [Google Scholar]
- Zieliński, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2007, 107, 1217–1224. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; He, Z.; Wang, D.; Liu, A.; Zhang, Y. Determination of phenolic acid concentrations in wheat flours produced at different extraction rates. J. Cereal Sci. 2013, 57, 67–72. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.B.; Andreasen, M.; Nielsen, M.; Larsen, L.; Knudsen, B.K.; Meyer, A.; Christensen, L.; Hansen, A. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Foss, F.W.; Schug, K.A. Thermally accelerated oxidative degradation of quercetin using continuous flow kinetic electrospray-ion trap-time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.-F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H. Low molecular weight antioxidants and other biologically active components of buckwheat seeds. Eur. J. Plant Sci. Biotechnol. 2009, 3, 29–38. [Google Scholar]
- Şensoy, Í.; Rosen, R.T.; Ho, C.-T.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, A.; Ziobro, R. Extruded preparations with sour cherry pomace influence quality and increase the level of bioactive components in gluten-free breads. Int. J. Food Sci. 2020, 2020, 8024398. [Google Scholar] [CrossRef] [PubMed]
- Gadow, A.; Joubert, E.; Hansmann, C. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem. 1997, 60, 73–77. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska, A.; Pegg, R.B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 14, 165–169. [Google Scholar]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Andjelković, M.; Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Z.; Wang, W.; Mu, J.; Liu, Y.; Wang, J.; Stipkovits, L.; Hui, X.; Wu, G.; Sun, J. The effects of enzymatic modification on the functional ingredient—Dietary fiber extracted from potato residue. LWT-Food Sci. Technol. 2022, 153, 112511. [Google Scholar] [CrossRef]
- Ghoshal, G.; Shivhare, U.S.; Banerjee, U.C. Rheological properties and microstructure of xylanase containing whole wheat bread dough. J. Food Sci. Technol. 2017, 54, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Zeng, F.; Yang, Y.; Liu, Q.; Yang, J.; Jin, Z.; Jiao, A. Effect of fermentation methods on properties of dough and whole wheat bread. J. Sci. Food Agric. 2023, 103, 4876–4886. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Gutkoski, L.C.; Bravo-Núñez, Á. Understanding whole-wheat flour and its effect in breads: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3241–3265. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Oliveira, P.; Fernández-Peláez, J.; Gallego, C.; Gómez, M. Effects of particle size in wasted bread flour properties. Int. J. Food Sci. Technol. 2022, 57, 4782–4791. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Ma, Y.-S. Optimization of the extrusion process for preparation of soluble dietary fiber-enriched calamondin pomace and its influence on the properties of bread. J. Food Sci. Technol. 2019, 56, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Villasante, J.; Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Heredia-Olea, E.; Metón, I.; Almajano, M.P. Evaluation of non-extruded and extruded pecan (Carya illinoinensis) shell powder as functional ingredient in bread and wheat tortilla. LWT-Food Sci. Technol. 2022, 160, 113299. [Google Scholar] [CrossRef]
- Kaack, K.; Pedersen, L.; Laerke, H.N.; Meyer, A. New potato fibre for improvement of texture and colour of wheat bread. Eur. Food Res. Technol. 2006, 224, 199–207. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Sanou, I.; Turgeon, S.L.; Canizares, D.; Khalloufi, S. Natural plant fibers obtained from agricultural residue used as an ingredient in food matrixes or packaging materials: A review. Compr. Rev. Food Sci. Food Saf. 2021, 21, 371–415. [Google Scholar] [CrossRef]
- Francis, F.J.; Clydesdale, F.M. Food Colorimetry: Theory and Applications; AVI Publishing Co. Inc.: Westport, CN, USA, 1975. [Google Scholar]
- Cantero, L.; Salmerón, J.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.D.P.; Bustamante, M.Á.; Matias, S.; Navarro, V.; Simón, E.; Martínez, O. Performance of apple pomace for gluten-free bread manufacture: Effect on physicochemical characteristics and nutritional value. Appl. Sci. 2022, 12, 5934. [Google Scholar] [CrossRef]
- Protonotariou, S.; Stergiou, P.; Christaki, M.; Mandala, I.G. Physical properties and sensory evaluation of bread containing micronized whole wheat flour. Food Chem. 2020, 318, 126497. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H. Characterization of the key aroma compounds in white bread by aroma extract dilution analysis, quantitation, and sensory evaluation experiments. J. Food Process. Preserv. 2019, 43, e13933. [Google Scholar] [CrossRef]

| Control * (Standard) | B5P1 | B10P1 | B15P1 | B5P2 | B10P2 | B15P2 | |
|---|---|---|---|---|---|---|---|
| Wheat flour [g] | 1000 | 950 | 900 | 850 | 950 | 900 | 850 |
| Preparation P1 [g] | 0 | 50 | 100 | 150 | 0 | 0 | 0 |
| Preparation P2 [g] | 0 | 0 | 0 | 0 | 50 | 100 | 150 |
| Water [mL] | 688 | 688 | 688 | 688 | 688 | 688 | 688 |
| Salt [g] | 20.00 | 19.59 | 19.19 | 18.78 | 19.44 | 18.91 | 18.34 |
| Dry yeast [g] | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Sample | Chemical Composition (g/100 g d.m.) | |||||
|---|---|---|---|---|---|---|
| Protein | Fat | Ash | IDF | SDF | TDF | |
| P1 | 9.53 ± 0.10 b | 1.17 ± 0.01 b | 0.82 ± 0.03 b | 3.60 ± 0.15 b | 1.38 ± 0.2 b | 4.98 ± 0.07 b |
| P2 | 9.28 ± 0.07 a | 1.02 ± 0.02 a | 0.70 ± 0.01 a | 3.15 ± 0.17 a | 1.10 ± 0.07 a | 4.25 ± 0.04 a |
| Phenolic compounds and antioxidant activities | ||||||
| TPC (mg catechin/100 g d.m.) | Flavonoids (mg rutin/100 g d.m. | ABTS (mgTx/g d.m.) | DPPH (mgTx/g d.m.) | FRAP (µMTx/g d.m.) | Chel. Fe(II) (mg EDTA/g d.m.) | |
| P1 | 261.31 ± 3.6 b | 49.02 ± 0.01 b | 15.15 ± 0.07 b | 1.89 ± 0.02 b | 8.91 ± 0.03 b | 1.21 ± 0.02 a |
| P2 | 213.72 ± 11.6 a | 39.31 ± 0.27 a | 14.51 ± 0.02 a | 1.78 ± 0.01 a | 6.57 ± 0.02 a | 1.23 ± 0.01 a |
| Sample | Content (mg/100 g d.m.) | ||||||
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic Acid | |||||||
| GA | VA | PA | SYA | DA | PAL | EA | |
| P1 | 31.43 ± 0.13 b * | 0.26 ± 0.01 a | 12.26 ± 0.05 b | 0.49 ± 0.01 a | nd | nd | 2.47 ± 0.06 a |
| P2 | 17.32 ± 0.1 a | nd | 6.12 ± 0.03 a | 0.47 ± 0.03 a | nd | 0.14 ± 0.01 a | 2.39 ± 0.02 a |
| Hydroxycinnaminic acid | |||||||
| CA | FA | PCA | CLA | SIA | |||
| P1 | nd | 0.22 ± 0.02 a | 0.19 ± 0.01 a | 3.01 ± 0.08 b | nd | ||
| P2 | nd | 0.29 ± 0.03 b | 0.16 ± 0.03 a | 2.58 ± 0.07 a | nd | ||
| Flavonols and flavanols | |||||||
| Q3Gal | Q3Glu | Q3R | Q | E | |||
| P1 | 1.02 ± 0.01 a | nd | 1.64 ± 0.02 a | 1.74 ± 0.02 b | 2.56 ± 0.07 a | ||
| P2 | 1.29 ± 0.02 b | nd | 3.24 ± 0.04 b | 0.91 ± 0.01 a | 3.23 ± 0.03 b | ||
| B vitamins | |||||||
| B1 | B2 | B3 | B6 | Total | |||
| P1 | 0.15 ± 0.01 a * | 1.73 ± 0.01 b | 0.50 ± 0.01 a | 0.12 ± 0.01 a | 2.50 ± 0.04 b | ||
| P2 | 0.19 ± 0.01 b | 1.34 ± 0.01 a | 0.80 ± 0.01 b | 0.10 ± 0.01 a | 2.43 ± 0.04 a | ||
| Sample | Chemical Composition (g/100 g d.m.) | |||||
|---|---|---|---|---|---|---|
| Protein | Fat | Ash | IDF | SDF | TDF | |
| Control (standard) | 13.46 ±0.01 a * | 1.89 ± 0.1 b | 3.57 ± 0.01 b | 9.72 ± 0.2 b | 2.06 ±0.01 b | 11.78 ± 0.01 d |
| B5P1 | 13.51 ± 0.10 a | 1.63 ± 0.07 a | 3.32 ± 0.02 a | 9.65 ± 0.15 b | 1.69 ±0.17 a | 11.34 ± 0.12 b |
| B10P1 | 13.36 ± 0.12 a | 1.59 ± 0.01 a | 3.28 ± 0.03 a | 10.60 ± 0.01 c | 1.43 ±0.1 a | 12.03 ± 0.3 e |
| B15P1 | 13.15 ± 0.01 a | 1.49 ± 0.12 a | 3.28 ± 0.01 a | 9.76 ± 0.01 b | 1.53 ± 0.16 a | 11.28 ± 0.01 bc |
| B5P2 | 13.44 ± 0.02 a | 1.68 ± 0.01 a | 3.17 ± 0.03 a | 9.05 ± 0.17 a | 1.58 ± 0.02 a | 10.62 ± 0.1 a |
| B10P2 | 13.22 ± 0.1 a | 1.62 ± 0.13 a | 3.22 ± 0.05 a | 9.50± 0.2 b | 1.62 ± 0.01 a | 11.11 ± 0.12 b |
| B15P2 | 13.03 ± 0.2 a | 1.59 ± 0.12 a | 3.16 ± 0.07 a | 9.68 ± 0.19 b | 1.91 ± 0.01 a | 11.60 ± 0.3 d |
| Phenolic compounds and antioxidant activities | ||||||
| TPC (mg catechin/100 g d.m.) | Flavonoids (mg rutin/100 g d.m.) | ABTS (mgTx/g d.m.) | DPPH (mgTx/g d.m.) | FRAP (µMTx/g d.m.) | Chel. Fe(II) (mg EDTA/g d.m.) | |
| Control (standard) | 79.69 ± 1.55 b | 8.83 ± 0.05 a | 11.83 ± 0.14 a | 1.52 ± 0.02 b | 2.69 ± 0.01 b | 1.22 ± 0.03 c |
| B5P1 | 62.18 ± 1.56 a | 18.60 ±1.8 c | 12.87 ± 0.11 b | 1.47 ± 0.01 a | 2.54 ± 0.03 a | 1.14 ± 0.02 b |
| B10P1 | 88.44 ± 1.50 c | 19.66 ± 2.1 c | 12.75 ± 0.08 b | 1.62 ± 0.05 c | 2.98 ± 0.02 d | 1.24 ± 0.01 c |
| B15P1 | 111.97 ± 2.32 e | 25.31 ± 1.84 d | 17.15 ± 0.71 f | 1.97 ± 0.04 f | 3.42 ± 0.02 f | 1.31 ± 0.02 d |
| B5P2 | 87.90 ± 2.28 c | 12.68 ± 1.79 b | 13.62 ± 0.21 c | 1.61 ± 0.01 c | 2.83 ± 0.01 c | 1.05 ± 0.01 a |
| B10P2 | 99.38 ± 1.52 d | 13.955 ± 1.2 b | 14.78 ± 0.11 d | 1.81 ± 0.03 e | 3.25 ± 0.03 e | 1.30 ± 0.02 d |
| B15P2 | 118.53 ± 0.77 f | 16.25 ± 0.87 c | 15.51 ± 0.33 e | 1.74 ± 0.02 d | 3.40 ± 0.04 f | 1.37 ± 0.03 e |
| Sample | Content (mg/100 g d.m) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acid | |||||||||||
| GA | VA | PA | SYA | DA | PAL | EA | |||||
| Control | 0.28 ± 0.01 a * | nd | nd | 0.35 ± 0.01 d | 1.19 ± 0.01 d | 6.30 ± 0.03 e | nd | ||||
| B5P1 | 2.10 ± 0.04 c | nd | nd | 0.28 ± 0.01 c | 1.03 ± 0.01 c | 6.00 ± 0.02 cd | 0.28 ± 0.01 a | ||||
| B10P1 | 2.50 ± 0.01 d | nd | nd | 0.21 ± 0.02 b | 1.15 ± 0.04 d | 5.55 ± 0.02 a | 0.48 ± 0.01 c | ||||
| B15P1 | 2.93 ± 0.07 e | nd | 0.46 ± 0.01 b | 0.09 ± 0.01 a | 1.07 ± 0.02 c | 5.99 ± 0.03 c | 0.36 ± 0.03 b | ||||
| B5P2 | 1.25 ± 0.08 b | nd | nd | nd | 1.00 ± 0.03 c | 5.99 ± 0.02 c | 0.54 ± 0.04 d | ||||
| B10P2 | 1.35 ± 0.03 b | nd | nd | nd | 0.75 ± 0.02 a | 6.06 ± 0.02 d | 0.32 ± 0.02 b | ||||
| B15P2 | 2.57 ± 0.06 d | nd | 0.34 ± 0.01 a | 0.22 ± 0.03 b | 0.88 ± 0.01 b | 5.75 ± 0.03 b | 0.40 ± 0.04 cd | ||||
| Hydroxycinnaminic acid | |||||||||||
| CA | FA | PCA | CLA | SIA | |||||||
| Control | nd | 1.35 ± 0.1 c | nd | nd | 0.70 ± 0.01 c | ||||||
| B5P1 | nd | 1.18 ± 0.08 b | nd | nd | 0.56 ± 0.04 a | ||||||
| B10P1 | nd | 1.05 ± 0.04 a | 0.07 ± 0.01 a | nd | 0.55 ± 0.02 a | ||||||
| B15P1 | 0.25 ± 0.02 b | 1.05 ± 0.08 a | 0.15 ± 0.02 c | nd | 0.64 ± 0.02 b | ||||||
| B5P2 | nd | 1.30 ± 0.03 c | nd | nd | 0.55 ± 0.04 a | ||||||
| B10P2 | nd | 1.19 ± 0.03 b | nd | nd | 0.52 ± 0.04 a | ||||||
| B15P2 | 0.15 ± 0.01 a | 1.02 ± 0.06 a | 0.10 ± 0.01 b | nd | 0.52± 0.05 a | ||||||
| Flavonols and flavanols | |||||||||||
| Q3Gal | Q3Glu | Q3R | Q | E | |||||||
| Control | nd | nd | nd | nd | nd | ||||||
| B5P1 | 0.03 ± 0.01 a | nd | 0.08 ± 0.01 a | nd | nd | ||||||
| B10P1 | 0.03 ± 0.01 a | nd | 0.15 ± 0.02 b | 0.15 ± 0.02 a | nd | ||||||
| B15P1 | 0.04 ± 0.01 a | nd | 0.15 ± 0.01 b | 0.26 ± 0.03 c | nd | ||||||
| B5P2 | nd | nd | 0.06 ± 0.02 a | 0.16 ± 0.01 a | nd | ||||||
| B10P2 | nd | nd | 0.04 ± 0.01 a | 0.20 ± 0.01 b | nd | ||||||
| B15P2 | 0.04 ± 0.01 a | nd | 0.16 ± 0.01 b | 0.17 ± 0.01 a | nd | ||||||
| Sample | B1 | B2 | B3 | B6 | Total |
|---|---|---|---|---|---|
| Control | 0.07 ± 0.01 a * | 1.39 ± 0.01 a | 0.70 ± 0.01 b | nd | 2.16 ± 0.03 a |
| B5P1 | 0.13 ± 0.01 b | 1.62 ± 0.01 d | 0.45 ± 0.01 a | 0.11 ± 0.01 b | 2.31 ± 0.04 b |
| B10P1 | 0.14 ± 0.01 b | 1.88 ± 0.03 e | 0.80 ± 0.05 c | 0.13 ± 0.03 b | 2.95 ± 0.11 e |
| B15P1 | 0.15 ± 0.03 b | 2.21 ± 0.01 f | 0.98 ± 0.01 d | 0.14 ± 0.01 b | 3.48 ± 0.06 f |
| B5P2 | 0.13 ± 0.01 b | 1.90 ± 0.01 e | 0.46 ± 0.01 a | 0.11 ± 0.02 b | 2.60 ± 0.05 c |
| B10P2 | 0.14 ± 0.01 b | 1.57 ± 0.01 c | 0.82 ± 0.02 c | 0.12 ± 0.03 b | 2.65 ± 0.07 c |
| B15P2 | 0.16 ± 0.02 b | 1.46 ± 0.01 b | 0.99 ± 0.01 d | 0.13 ± 0.01 b | 2.74 ± 0.05 d |
| Sample | Specific Volume [g/cm3] | Bread Yield [%] | Crumb Moisture [%] | Hardness [N] | Cohesiveness | |||
|---|---|---|---|---|---|---|---|---|
| Day of Baking | 48 h after Baking | Day of Baking | 48 h after Baking | Day of Baking | 48 h after Baking | |||
| Control | 2.37 ± 0.06 c | 122.0 ± 0.8 a | 44.2 ± 0.1 ab | 44.1 ± 0.7 a | 10.0 ± 0.0 a | 16.0 ± 0.3 a | 0.732 ± 0.004 a | 0.607 ± 0.024 a |
| B5P1 | 2.34 ± 0.19 c | 123.1 ± 0.8 b | 44.6 ± 0.6 b | 44.6 ± 0.3 a | 10.5 ± 0.6 a | 15.4 ± 0.3 a | 0.749 ± 0.011 abc | 0.566 ± 0.037 a |
| B10P1 | 2.11 ± 0.10 b | 123.3 ± 0.3 b | 43.5 ± 0.4 a | 44.2 ± 0.7 a | 14.3 ± 0.3 cd | 23.4 ±1.1 b | 0.749 ± 0.008 abc | 0.563 ± 0.043 a |
| B15P1 | 1.98 ± 0.08 a | 123.2 ± 0.3 b | 43.7 ± 0.8 a | 44.4 ± 0.2 a | 15.5 ± 1.4 d | 26.1 ± 0.0 c | 0.742 ± 0.003 ab | 0.579 ± 0.033 a |
| B5P2 | 2.22 ± 0.04 b | 123.5 ± 0.8 b | 44.6 ± 0.5 b | 44.7 ± 0.5 a | 10.2 ± 0.5 a | 17.2 ± 1.8 a | 0.767 ± 0.004 c | 0.554 ± 0.030 a |
| B10P2 | 2.13 ± 0.15 b | 123.6 ± 0.8 b | 43.9 ± 0.4 ab | 43.9 ± 0.7 a | 11.5 ± 0.5 ab | 22.8 ± 1.0 b | 0.761 ± 0.012 bc | 0.557 ± 0.040 a |
| B15P2 | 1.97 ± 0.17 a | 124.5 ± 0.0.6 c | 44.1 ± 0.5 ab | 43.9 ± 0.4 a | 13.2 ± 1.2 bc | 23.2 ± 0.9 b | 0.765 ± 0.016 bc | 0.561 ± 0.027 a |
| Sample | L* (D65) | a* (D65) | b* (D65) | ΔE |
|---|---|---|---|---|
| Control (Standard) | 52.42 ± 1.16 c | 8.94 ± 0.17 c | 22.13 ± 0.21 c | - |
| B5P1 | 49.39 ± 1.20 ab | 8.84 ± 0.13 bc | 21.56 ± 0.31 b | 3.10 ± 1.17 bc |
| B10P1 | 48.19 ± 0.63 a | 8.71 ± 0.16 b | 21.39 ± 0.25 b | 4.30 ± 0.66 d |
| B15P1 | 48.44 ± 1.03 a | 8.63 ± 0.22 b | 21.51 ± 0.09 b | 4.04 ± 1.01 cd |
| B5P2 | 51.53 ± 1.47 c | 8.41 ± 0.09 a | 21.58 ± 0.32 b | 1.76 ± 0.32 a |
| B10P2 | 50.10 ± 0.45 b | 8.30 ± 0.11 a | 21.07 ± 0.15 a | 2.63 ± 0.43 ab |
| B15P2 | 49.45 ± 0.51 ab | 8.34 ± 0.11 a | 21.36 ± 0.26 ab | 3.13 ± 0.52 bc |
| Compound | Compound Class | P1 | P2 |
|---|---|---|---|
| Ethanol | Alcohols | 10.19 ± 0.09 a | 12.48 ± 0.06 b |
| 2-Methyl-1-propanol | 7.59 ± 0.01 b | 4.33 ± 0.01 a | |
| 1-Hexanol | 7.14 ± 0.01 b | 6.04 ± 0.10 a | |
| 2-Phenylethanol | 3.73 ± 0.05 a | 3.72 ± 0.01 a | |
| 1-Nonanol | 3.69 ± 0.01 a | 4.35 ± 0.01 b | |
| 2-Methylpropanal | Aldehydes | nd | 0.77 ± 0.01 a |
| 3-Methylbutanal | nd | 0.90 ± 0.01 a | |
| Hexanal | 0.72 ± 0.01 a | 1.17 ± 0.01 b | |
| Furfural | nd | nd | |
| 2-Decenal | 5.38 ± 0.01 b | 4.43 ± 0.01 a | |
| 2.4-(E.E)-Decadienal | 3.79 ± 0.01 a | 4.65 ± 0.01 b | |
| Dodecanal | 3.80 ± 0.02 b | 1.51 ± 0.01 a | |
| 2-Methylpropanoic acid | Carboxylic acids | 1.75 ± 0.01 a | 1.95 ± 0.01 b |
| 3-Methylbutanoic acid | 6.61 ± 0.01 b | 5.20 ± 0.02 a | |
| Pentanoic acid | 3.80 ± 0.01 b | 3.47 ± 0.01 a | |
| p-Methoxybenzoic acid | nd | 0.59 ± 0.01 a | |
| Dodecanoic acid | 1.81 ± 0.01 b | 1.66 ± 0.01 a | |
| Tridecanoic acid | 8.04 ± 0.01 b | 7.34 ± 0.02 a | |
| Pentadecanoic acid | 1.41 ± 0.01 a | 1.91 ± 0.01 b | |
| Ethyl acetate | Esters | 1.51 ± 0.01 a | 1.63 ± 0.01 b |
| Isopropyl acetate | nd | 0.65 ± 0.01 a | |
| Phenylethyl acetate | 1.22 ± 0.01 a | 1.35 ± 0.01 ab | |
| Ethyl dodecanoate | 6.52 ± 0.07 a | 6.63 ± 0.07 b | |
| Benzyl phenyl acetate | 1.03 ± 0.01 b | 0.60 ± 0.01 a | |
| Benzyl benzoate | 3.92 ± 0.01 a | 5.27 ± 0.02 b | |
| 2-Phenylethyl phenylacetate | 0.72 ± 0.01 a | nd | |
| Butane-2.3-dione | Ketones | 3.45 ± 0.01 b | 3.21 ± 0.01 a |
| Pentan-2-one | 1.38 ± 0.01 b | 1.21 ± 0.01 a | |
| 5-Propyldihydro-2(3H)-furanone | Lactones | 2.69 ± 0.03 a | 2.75 ± 0.01 b |
| delta-Decalactone | 0.81 ± 0.01 a | 1.87 ± 0.01 b | |
| 4-Undecanolide | nd | 0.63 ± 0.01 a | |
| Guaiol | Phenols | 1.10 ± 0.01 a | nd |
| Methylpyrazine | Pyrazines | 3.71 ± 0.04 b | 1.97 ± 0.01 a |
| 2.5-Dimethylpyrazine | 1.38 ± 0.03 a | 3.94 ± 0.01 b | |
| 2.6-Dimethylpyrazine | 1.12 ± 0.02 a | 1.82 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Oracz, J.; Litwinek, D.; Żyżelewicz, D.; Zięba, T.; Sabat, R.; Wywrocka-Gurgul, A.; Ziobro, R. Quality- and Health-Promoting Compounds of Whole Wheat Bread with the Addition of Stale Bread, Cornmeal, and Apple Pomace. Foods 2024, 13, 1767. https://doi.org/10.3390/foods13111767
Gumul D, Oracz J, Litwinek D, Żyżelewicz D, Zięba T, Sabat R, Wywrocka-Gurgul A, Ziobro R. Quality- and Health-Promoting Compounds of Whole Wheat Bread with the Addition of Stale Bread, Cornmeal, and Apple Pomace. Foods. 2024; 13(11):1767. https://doi.org/10.3390/foods13111767
Chicago/Turabian StyleGumul, Dorota, Joanna Oracz, Dorota Litwinek, Dorota Żyżelewicz, Tomasz Zięba, Renata Sabat, Anna Wywrocka-Gurgul, and Rafał Ziobro. 2024. "Quality- and Health-Promoting Compounds of Whole Wheat Bread with the Addition of Stale Bread, Cornmeal, and Apple Pomace" Foods 13, no. 11: 1767. https://doi.org/10.3390/foods13111767
APA StyleGumul, D., Oracz, J., Litwinek, D., Żyżelewicz, D., Zięba, T., Sabat, R., Wywrocka-Gurgul, A., & Ziobro, R. (2024). Quality- and Health-Promoting Compounds of Whole Wheat Bread with the Addition of Stale Bread, Cornmeal, and Apple Pomace. Foods, 13(11), 1767. https://doi.org/10.3390/foods13111767









