Abstract
The fermentation process of Chinese Baijiu’s fermented grains involves the intricate succession and metabolism of microbial communities, collectively shaping the Baijiu’s quality. Understanding the composition and succession of these living microbial communities within fermented grains is crucial for comprehending fermentation and flavor formation mechanisms. However, conducting high-throughput analysis of living microbial communities within the complex microbial system of fermented grains poses significant challenges. Thus, this study addressed this challenge by devising a high-throughput analysis framework using light-flavor Baijiu as a model. This framework combined propidium monoazide (PMA) pretreatment technology with amplicon sequencing techniques. Optimal PMA treatment parameters, including a concentration of 50 μM and incubation in darkness for 5 min followed by an exposure incubation period of 5 min, were identified. Utilizing this protocol, viable microorganism biomass ranging from 8.71 × 106 to 1.47 × 108 copies/μL was successfully detected in fermented grain samples. Subsequent amplicon sequencing analysis revealed distinct microbial community structures between untreated and PMA-treated groups, with notable differences in relative abundance compositions, particularly in dominant species such as Lactobacillus, Bacillus, Pediococcus, Saccharomycopsis, Issatchenkia and Pichia, as identified by LEfSe analysis. The results of this study confirmed the efficacy of PMA-amplicon sequencing technology for analyzing living microbial communities in fermented grains and furnished a methodological framework for investigating living microbial communities in diverse traditional fermented foods. This technical framework holds considerable significance for advancing our understanding of the fermentation mechanisms intrinsic to traditional fermented foods.
1. Introduction
The longstanding culinary legacy of China spanning millennia has engendered a varied spectrum of fermented comestibles, such as pickle, wine, vinegar and various fermented dairy products. Fermented comestibles, resulting from microbial growth metabolism and enzymatic processes, emerge as integral constituents of the dietary regimen for individuals [1].
Traditional fermented foods undergo natural fermentation within exposed fermentative settings, wherein microorganisms are domesticated over an extended period within specified fermentation parameters (temperature, pH and nutrients, etc.), thereby establishing a steadfast core microbiota [2]. The core microbial community dominates the transformation of raw materials into substances, bestowing upon fermented food its distinctive flavor profile [3]. In the case of Chinese vinegar, primarily manufactured via liquid and solid-state open fermentation methods, the enriched microorganisms involved in the fermentation process undergo metabolic processes leading to the production of various flavor compounds [4]. During vinegar fermentation, functional microorganisms such as Acetobacillus, Lactobacillus, and Bacillus produce saccharifying enzymes, amylases, and organic acids, exerting influence on the acidity and overall quality of vinegar [5]. Paocai is crafted through spontaneous anaerobic or microaerobic fermentation, with its flavor genesis intricately tied to the indigenous Lactobacillus in the raw material and the subsequent fermentation facilitated by microorganisms, such as Bacillus and Pichia, which are enriched by the fermentation [6]. In the realm of traditional fermented foods, the brewing process of Baijiu is based on solid-state fermentation, an intricate process amalgamating saccharification with spontaneous fermentation. Within this milieu, the microbial community executes its metabolic roles through complex interactions [7]. For example, during the fermentation of light-flavor Baijiu, flavor substances such as ethyl lactate and ethyl decanoate are positively correlated with the fungal genera Dipodascus, Saccharomyces, Alternaria and Cosmospora. These microorganisms interact in the direct or indirect synthesis of the mentioned esters or their respective precursor substances [8]. In summary, the various flavor substances found in fermented foods arise through the activities of functional microorganisms in the fermentation system. Precise analysis of the diversity and structure of functional microorganisms during the brewing process significantly impacts the accuracy of comprehending the fermentation mechanism.
Only the living microbial community can fulfill a distinct ecological role [9]; thus, the perpetual succession of the living microbial community drives the food fermentation process. Currently, the prevailing method for analyzing microbial communities is still based on high-throughput sequencing at the DNA level. However, since the DNA of dead cells can persist in the environment for an extended duration [10], high-throughput sequencing fails to discern between DNA signals emanating from dead and viable microorganisms. This limitation culminates in outcomes that incline toward an overestimate of the abundance and diversity of species [11]. To address the aforementioned challenges, Hu [12] used metatranscriptome technology to analyze the living microbial community during the fermentation process of Baijiu, and the results exhibited notable disparities compared to high-throughput 16S rRNA and ITS gene sequencings, for instance, Aspergillus was solely identified in amplicon sequencing results, while Amanita, Baudoinia and Naumovozyma were exclusively identified in the metatranscriptome results. This indicates that the results derived from high-throughput sequencing at the DNA level may not comprehensively and impartially portray the structure of the living microbial community and its succession during the process of food fermentation. At present, the studies of the composition and functionality of viable microorganisms during the process of food fermentation rely on traditional cultivation and metatranscriptome technology. However, the traditional cultivation method is time-consuming, as is DNA/RNA-based sequencing; in addition, the methods are not effective at isolating and culturing the majority of microorganisms, with only 0.1–1.0% of the naturally occurring microorganisms being successfully cultured [13]. Compared with the traditional cultivation method, metatranscriptome technology has the capacity to scrutinize both culturable and non-culturable microorganisms in fermented food systems. Nevertheless, the intrinsic challenges lie in the instability of RNA and the intricate nature of the environment in fermented foods, characterized by the abundance of enzymes and various metabolites, rendering the extraction of high-quality RNA a formidable task [14]. Additionally, the elevated expenses associated with sequencing pose constraints on the broader utilization of macro-transcriptomic technologies [15]. Therefore, the high-throughput analysis of the living microbial community in traditional fermented foods remains a persistent challenge.
Propyl azide bromide (PMA), as a high-affinity photoreactive DNA crosslinker, exhibits the capability to selectively associate with the DNA found within deceased cells possessing membrane permeability. Leveraging this distinctive attribute, PMA are widely used for the detection of viable microorganisms. For example, Xu [16] proposed PMA-CAMP for real-time and visual detection of active Salmonella in milk. This innovative approach enables the discernment of viable bacteria in artificially contaminated milk samples with a detection threshold of 102 CFU/mL. Yang [17] improved the PMA-qPCR methodology and successfully completed the quantitative assessment of five viable bacteria in fermented milk, including Lactiplantibacillus plantarum, Streptococcus thermophilus and Lactobacillus helveticus; this refined PMA-qPCR technique enabled the analysis completion within a mere 3 h timeframe. PMA has been successfully applied to the monitoring of living microbial communities in a variety of fields, including food, gut microbiology and the environment [18,19,20]. PMA is an ideal method for the analysis of a living microbial community due to its exceptional selectivity for the DNA of dead cells. This characteristic imparts to PMA an outstanding level of specificity and sensitivity in the discernment of nonviable cells.
In the realm of food science research, current studies focus on the combination of PMA with real-time quantitative PCR (qPCR) for the detection of viable target microorganisms [21,22,23]. In fact, the method of sample pretreatment employing PMA offers an efficacious interface for the subsequent analysis of the living microbial community structure of traditional fermented foods through amplicon sequencing. Presently, only the living microbial communities in the fermented grains of Chinese strong-flavored Baijiu have been elucidated through the aforementioned methodology [24]. However, the elucidation of how the parameters for PMA pretreatment samples were established, along with a detailed optimization scheme, remains unprovided. In light of this problem, the present study focused on the establishment of the optimization scheme and process of the PMA pretreatment samples by taking the fermented grains of light-flavor Baijiu as a case study. Employing a fusion of qPCR and amplicon sequencing techniques, it confirmed the efficacy and reliability of PMA pretreatment in analyzing the amplicon of the living microbial community within the fermented grains of light-flavor Baijiu. This study aimed to offer a framework for establishing a methodology to scrutinize the living microbial community structure in fermented foods. Therefore, we hope this study will be a significant contribution to deepening the understanding of the fermentation mechanisms in traditional fermented foods.
2. Materials and Methods
2.1. Sample Collection and Suspension Preparation of Fermented Grain Samples
Fermented grain samples were collected from a local liquor distillery in Xinghuacun Town, Fenyang City, Shanxi Province, China (111°48′45″ E, 37°18′21″ N). In the production of light-flavor Baijiu, a dual fermentation process is employed using the same batch of sorghum raw material. The fermented grains from the first fermentation are termed “Dacha”. After distillation of the fermented grains (Dacha), the remaining sorghum was cooled and reintroduced with a saccharification fermenting agent, known as “Daqu”, for the second round of fermentation. At this stage, the fermented grains are referred to as “Ercha”. The fermented grains were sampled from the two fermentation cycles of Dacha and Ercha, and 100 g of fermented grains were collected at different fermentation time points. Subsequently, 2.5 g of fresh samples were added to 10 mL of PBS buffer (1 M, pH = 7.4), supplemented with 5–8 glass beads (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) in number. After 20–30 min of vortexing at a speed of 4000 rpm and oscillating, the upper layer of the liquid was extracted, resulting in the formation of a 25% (w/v) suspension of fermented grains.
2.2. Preparation of PMA Working Solution
A total of 1 mg of PMA (SB-P4036, share-bio, Shanghai, China) was added to 98 μL of sterile distilled deionized water (ddH2O) under light-avoidant conditions. It was dissolved and thoroughly mixed to obtain a 20 mM storage solution, which was stored at −20 °C while shielded from light. The PMA working solution was diluted to 2 mM.
2.3. Preparation of Positive Control Group
Escherichia coli (E. coli), preserved in glycerol at a final concentration of 30% in the −80 °C freezer, were streaked onto Luria-Bertani (LB) agar plates and then incubated at 37 °C for 24 h. Subsequently, individual colonies were selected, inoculated into liquid LB medium, and cultured at 37 °C with agitation of 180 rpm on a shaker until reaching the logarithmic growth phase (OD600 ≈ 1.0). A total of 1 mL of E. coli culture solution was extracted in the logarithmic phase into a 1.5 mL centrifuge tube, incubated in a 95 °C water bath for 30 min to kill the bacteria and immediately placed on ice for cooling. In total, 50 μL of the heat-killed E. coli cell suspension was taken, and the suspension was plated on LB solid medium. Three replicates were set up in parallel for each group. The plates were inverted and incubated at 37 °C for 48 h, and the growth of colonies was observed. The absence of colony growth indicated the successful creation of a heat-killed bacterial suspension [25].
The positive control consisted of a suspension of heat-killed E. coli cells and suspensions formed from grains fermented for 0, 3, 7, 11, 15, 21, 24, and 28 days. In total, 400 μL of the heat-killed bacterial suspension was taken and centrifuged at 10,000 rpm for 5 min. The sediment was retained and then dissolved in 1 mL of a 25% (w/v) suspension of fermented grains to form a positive control bacterial suspension [24].
2.4. Establishment of PMA Pre-Treatment Method for Fermented Grains
The effectiveness of PMA binding to dead microorganisms was influenced by factors such as the concentration of the PMA, the duration of dark incubation, and exposure time [26]. To obtain an optimal PMA pre-treatment protocol for the samples, optimization was carried out separately for these three crucial factors. After applying factors such as PMA concentration, dark incubation time, and exposure time, there was a significant decrease in bacterial biomass, indicating the optimal treatment conditions. All experiments were conducted in triplicate.
2.4.1. Selection of Fermented Grain Samples for Optimization of the PMA Pre-Treatment Protocol
DNA was extracted from fermented grain samples collected at 0, 2, 4, 6, 10, 15 and 24 days. Subsequently, DNA was amplified by qPCR. The fermented grains harboring the highest microbial density, encompassing bacteria or fungi, were subsequently utilized for refining the PMA pretreatment methodology.
2.4.2. Optimization of PMA Concentration
PMA working solution was introduced to 1 mL of a 25% (w/v) suspension of fermented grains, yielding final concentrations of 0, 50, 100, 150, 200 μM, accompanied by a blank control (the suspension of fermented grains devoid of PMA treatment). Following thorough mixing, the mixture was placed in darkness for incubation and subsequently exposed utilizing a photolysis device (PL-PMA, Beijing Princess Technology, Beijing, China). The mixture was centrifuged at 10,000 rpm for 10 min, retaining the sediment. Subsequently, the DNA of the mixture was extracted using the Cetyltrimethylammonium bromide (CTAB) protocol [27]. The concentration of extracted DNA was quantified using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA), and the optimal working concentration of PMA was determined based on the total bacterial biomass obtained from the qPCR results.
2.4.3. Optimization of Dark Incubation Time
In total, 1 mL of a 25% (w/v) suspension of fermented grains was mixed with a final concentration of 50 μM of PMA working solution. The aforementioned mixtures were incubated in darkness for 0, 5, 10, 15, and 20 min, and subsequently, a photolysis process was conducted inside a photolysis device with light wavelength ranging from 465 to 475 nm within a centrifuge tube (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min, with agitation occurring every 5 min during exposure. After the aforementioned steps, the mixture was centrifuged at 10,000 rpm for 10 min, resulting in the retention of the sediment. The genomic DNA of the mixture was then extracted using the CTAB method. The optimization of dark incubation time was assessed based on the total bacterial biomass obtained from qPCR results.
2.4.4. Optimization of Exposure Time
The samples were prepared following the method outlined in Section 2.4.3 with a PMA concentration of 50 μM. The mixtures were mixed, incubated in darkness for 5 min, and then exposed to the photolysis device for intervals of 0, 5, 10, 15 and 20 min. A blank control group was included, consisting of the suspension of fermented grains without exposure treatment. Subsequently, the mixture was centrifuged at 10,000 rpm for 10 min, resulting in the retention of the sediment. The genomic DNA of the mixture was extracted. The optimal exposure time was determined based on the total bacterial biomass obtained from qPCR results.
2.5. Bacterial Strains, Plasmids and Cultivation
The DNA extracted from fermented grain samples was used as a template for PCR amplification using primers specific to bacteria and fungi. The forward and reverse primers of the bacteria were P1 (5′-CCTACGGGAGGCAGCAG-3′) and P2 (5′-ATTACCGCGGCTGCTGG-3′), and the forward and reverse primers of the fungi were Y1 (5′-GCGGTAATTCCAGCTCCAATAG-3′) and Y2 (5′-GCCACAAGGACTCAAGGTTAG-3′) [28]. Subsequently, the PCR products were loaded on 1% agarose gel, the target bands of 196 and 151 bp were isolated and recovered from the gel using a gel recovery kit (cat# DP219-03; Tiangen Biotech, Beijing, China). For vector ligation, the reaction solution consisting of the pMD-19T vector (cat# 6013, Takara, Japan), DNA of fermented grains, ddH2O and Solution I (cat# 6013, Takara, Shiga, Japan) was mixed and incubated overnight at 16 °C. The ligation products were added to E. coli DH5α Competent Cells (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), followed by incubation on ice for 30 min, a 90 s incubation in a 42 °C water bath, and addition of 800 µL LB medium without antibiotics under aseptic conditions. After cultivation at 37 °C for 1 h, a bacterial suspension was plated onto a selection agar plate containing a concentration of 100 μg/mL of ampicillin (Amp). Subsequently, the plate was sealed and incubated overnight at 37 °C. Afterward, the colonies were selected, transferred to LB liquid medium supplemented with Amp, and cultured at 200 rpm at 37 °C for 12 h. The recombinant plasmid DNA was extracted using the TlANprep Mini Plasmid Kit (cat# DP103-03; Tiangen Biotech, Beijing, China), and the concentration and purity of the plasmid were determined. The integrity of the plasmid was verified by PCR amplification and 1% agarose gel electrophoresis [29,30].
2.6. Amplicon Sequencing
DNA was extracted from fermented grain samples using the CTAB method, the concentration and purity of the DNA were measured using the NanoDrop 2000, and the quality was assessed by 1% agarose gel electrophoresis. The DNA was stored at −80 °C. Universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGGTWTCTAAT-3′) were employed for amplifying the V4 variable region of the bacterial 16S rRNA gene, while primer sets ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGGTTCTTCATCGATGC-3′) were utilized to amplify the fungal internal transcribed spacer region (ITS), followed by PCR amplification [31].
The DNA was purified and analyzed by amplicon sequencing on the NovaSeq 6000 sequencing platform. According to the characteristics of the amplified 16S region, a small fragment library was constructed, and the library was subjected to paired-end sequencing on the Illumina HiSeq4000 sequencing platform (Novogene, Tianjin, China). The downstream data obtained from Illumina NovaSeq sequencing underwent sequence assembly, quality control, and chimera filtering to yield valid data suitable for subsequent analyses. Finally, the classify-sklearn in QIIME2 is used to classify amplicon sequence variation (ASVs) [32]. The classify-sklearn naive Bayes taxonomy classifier in the feature-classifier plugin was employed to compare and identify sequences against the 16S rRNA Greengenes (version 13.8) database and the fungal ITS UNITE (version 8.0) database [33].
2.7. qPCR and PCR Reaction System and Procedure
qPCR was performed using a CFX96 Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA) with a final reaction volume of 20 μL. The reaction system for qPCR consisted of 10 μL of 2 × SYBR qPCR Master Mix (Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China), 0.4 μL of forward primers, and 0.4 μL of reverse primers, 1 μL of DNA template, and 8.2 μL of ddH2O. Each reaction was set up in triplicates to ensure the robustness and reproducibility of the results. Plasmid DNA for a standard curve was extracted and diluted in a 10-fold gradient: 10−1, 10−2, 10−3, 10−4, 10−5. The qPCR amplification procedure consisted of an initial denaturation step at 94 °C for 10 s, followed by 40 cycles of denaturation at 94 °C for 5 s, annealing at 58 °C for 15 s, and extension at 72 °C for 15 s. A final extension step was performed at 72 °C for 5 min [34].
The PCR amplification program was set as follows: pre-denaturation: 95 °C for 3 min; 27 denaturation cycles: 95 °C for 30 s; annealing: 55 °C for 30 s; extension: 72 °C for 45 s; final extension: 72 °C for 5 min [35].
2.8. Statistical Analyses
Data were analyzed using SPSS 21.0, and Origin 2021 was used for graphing stacking histograms and bar charts. Differences among groups were estimated using Tukey’s test and Student’s t-test, where p < 0.05 was considered statistically significant. The use of different letters such as “a” and “b” indicates significant differences at the p < 0.05 level; “**” indicates extremely significant difference at the p < 0.01 level, “*” indicates significant difference at the p < 0.05 level, and “ns” indicates no significant difference between the two groups.
3. Results
3.1. Selection of Fermented Grains for Optimization
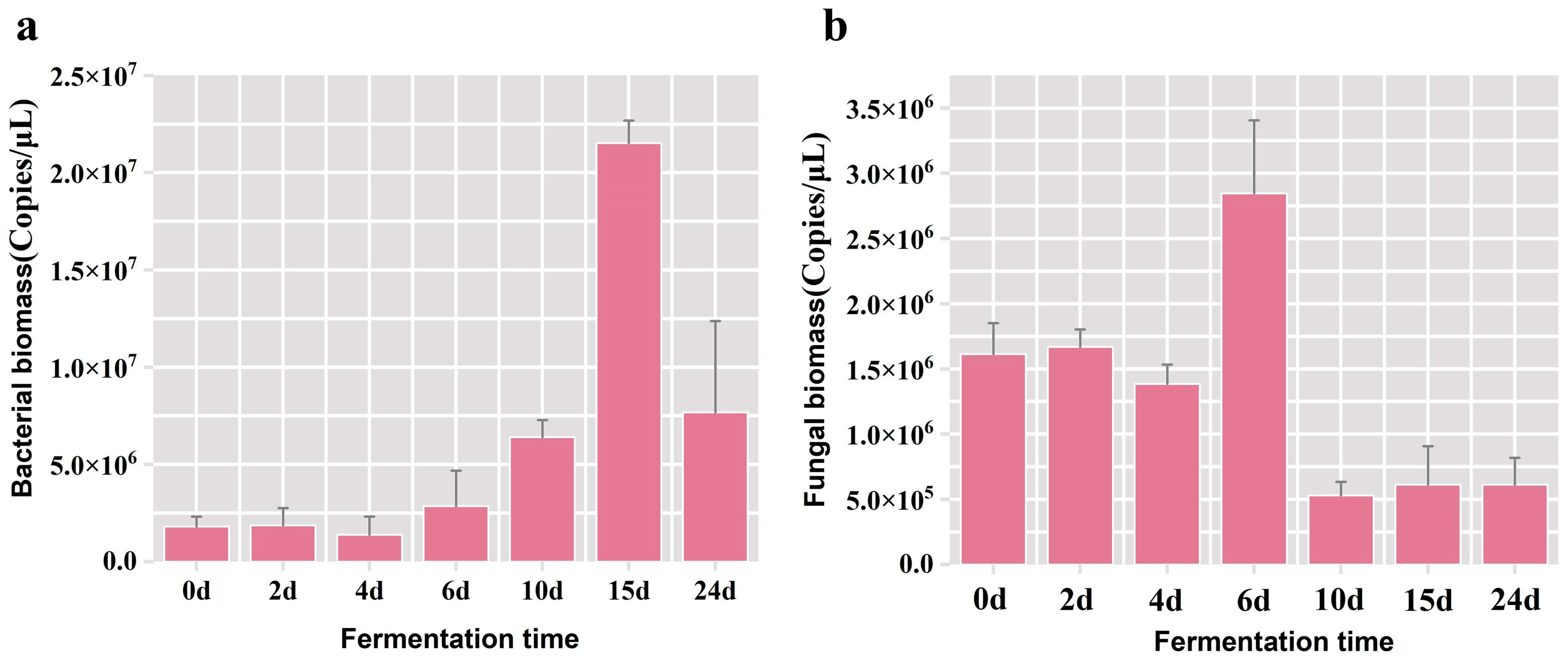
Fermented grain samples hosting the highest microbial population were meticulously chosen as the experimental subjects for refining the PMA pre-treatment protocol in the subsequent phase. Firstly, the fluctuation patterns of overall bacterial and fungal populations during the fermentation were analyzed. The results of qPCR showed a gradual elevation in the total bacterial biomass throughout the fermentation duration, peaking at 2.15 × 107 copies/μL on the 15th day, followed by a subsequent decline (Figure 1a). The maximal of total fungal biomass was observed at 2.85 × 106 copies/μL on the 6th day of fermentation (Figure 1b), and it was discerned that the fungal biomass in the fermented grains was one order of magnitude inferior to that of the bacterial population. Henceforth, in the subsequent experiments aimed at optimizing the pretreatment conditions of the PMA, we used samples of grains fermented for 15 days. The bacterial biomass served as the evaluative metric, ensuring the PMA dye was thoroughly bound to the DNA from dead cells in the fermented grain samples at any time point during the fermentation process.
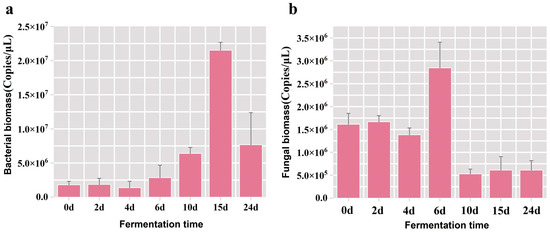
Figure 1.
Variation in the bacterial and fungal biomass in fermented grains across various time points during the fermentation process. (a) The bacterial biomass in fermented grain samples; (b) the fungal biomass in fermented grain samples.
3.2. Optimization of PMA Pre-Treatment Conditions for Fermented Grains
3.2.1. Optimization of PMA Concentration
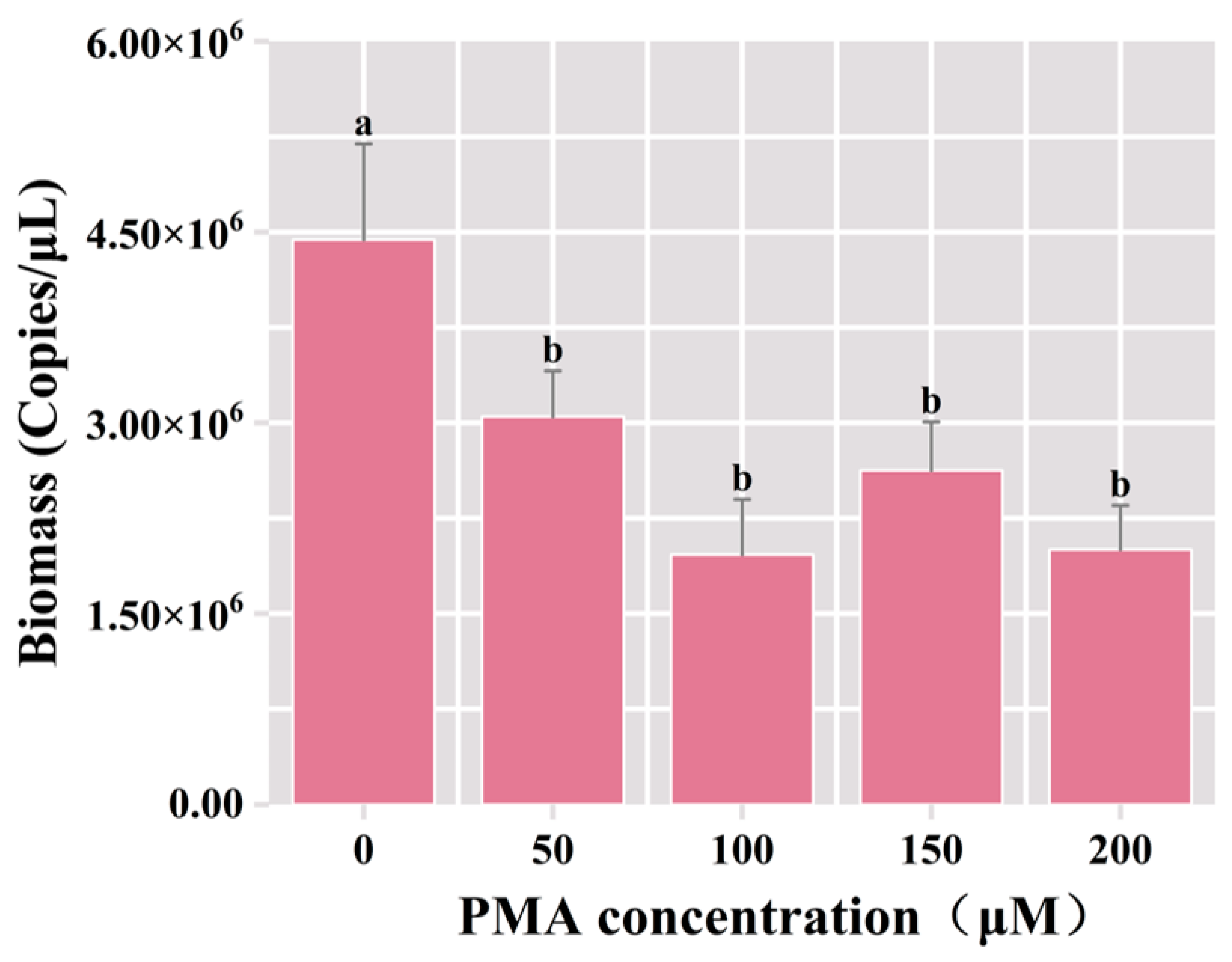
Following the incubation of samples with diverse concentrations of PMA, the DNA extraction ensued, and subsequent PCR amplification utilizing universal bacterial primers was conducted, followed by agarose gel electrophoresis. The results of qPCR showed that the total bacterial biomass was significantly reduced in the PMA-treated groups compared with the control group (p < 0.05). The total bacterial biomass decreased from 4.44 × 106 copies/μL to 3.05 × 106 copies/μL under the 50 μM PMA treatment, and the dead bacterial biomass accounted for 31.3% of the total bacterial biomass (Figure 2). In comparison to the group treated with 50 μM PMA dye, there was no significant difference in the number of viable bacteria in the PMA-treated groups employing the elevated concentration. This result indicated that 50 μM PMA effectively bound to the DNA of deceased cells in the fermented grain samples, proficiently differentiating viable microorganisms. Consequently, the optimal concentration for the PMA was determined to be 50 μM.
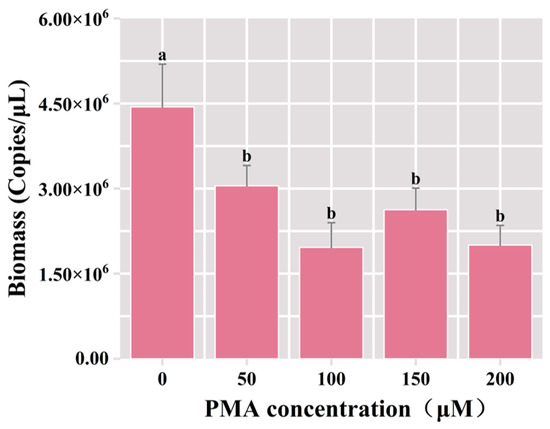
Figure 2.
Variation of bacterial biomass in the samples of fermented grains under different concentrations of PMA treatment. “a” and “b” Superscript letters indicate significant statistical differences at p ≤ 0.05.
3.2.2. Optimization of Duration for Incubation in Darkness
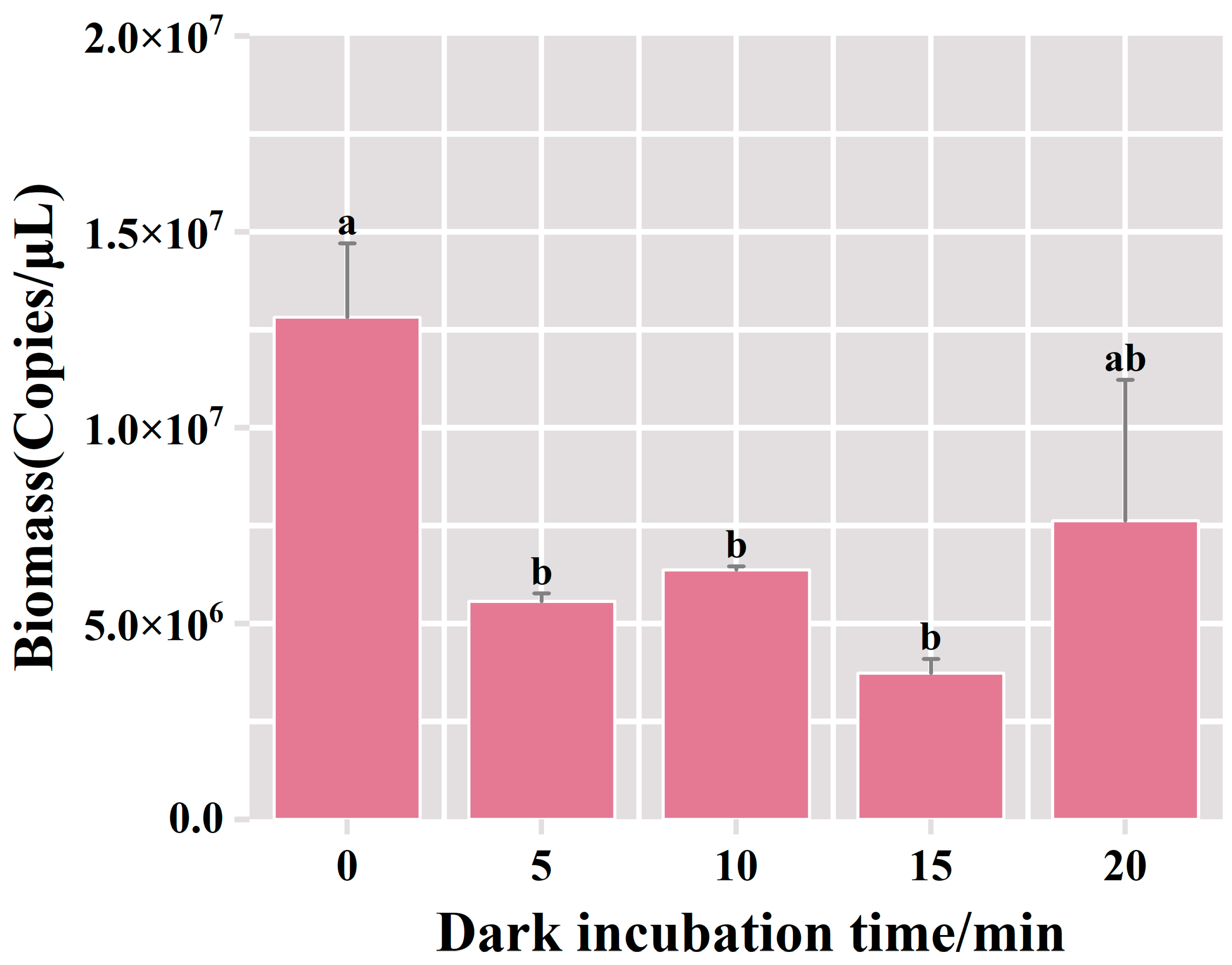
This section describes the results of 1% agarose gel electrophoresis, conducted on the DNA extracted from samples subjected to varying durations of dark incubation with the PMA dye. Within the 0 min dark incubation period in the PMA-treated group, the bacterial biomass resulted in 1.28 × 107 copies/μL, surpassing the levels observed in the alternative experimental groups (Figure 3). In the PMA-treated group of dark incubation for 5 min, there was a noteworthy reduction in bacterial biomass, reaching 5.57 × 106 copies/μL. This suggests that deceased bacteria comprised 56% of the overall bacterial content. There was no significant change in bacterial biomass with the increase in dark incubation time. This outcome suggested that the 5 min dark incubation treatment facilitated the complete binding of PMA to the DNA of deceased bacteria, effectively suppressing the amplification of dead bacterial DNA in the fermented grains. Therefore, the optimal duration for dark incubation was determined to be 5 min.
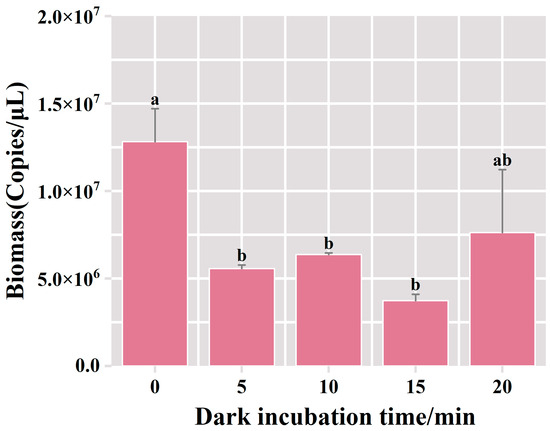
Figure 3.
Variation of bacterial biomass in the samples of fermented grains under different dark incubation time treatments. “a” and “b” Superscript letters indicate significant statistical differences at p ≤ 0.05.
3.2.3. Optimization of Exposure Time
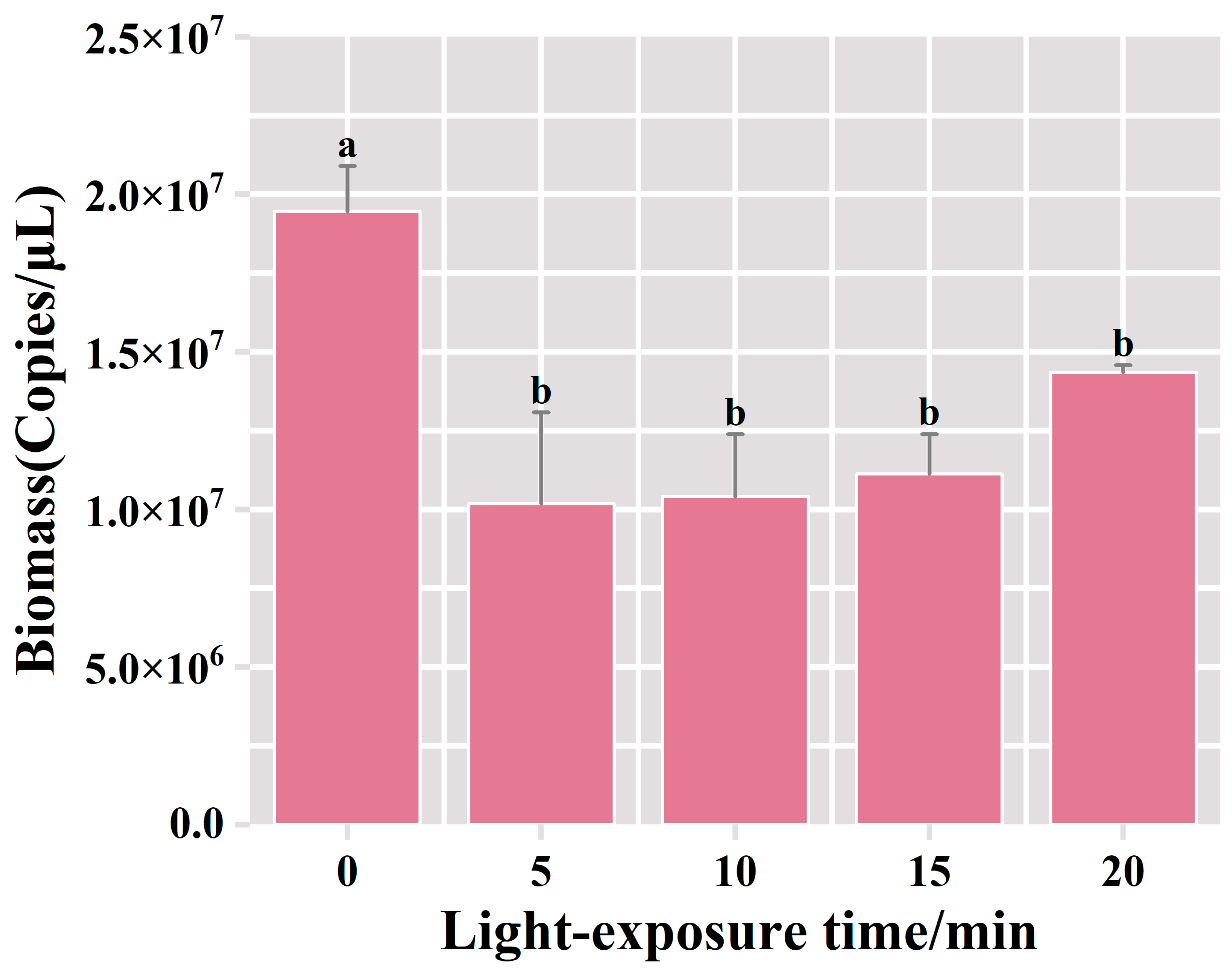
The qPCR results showed that the bacterial biomass reached 1.95 × 107 copies/μL in the PMA-treated group without exposure. Compared with the unexposed control group, after 5 mins of exposure, the bacterial biomass in the PMA-treated group experienced a decrease to 1.02 × 107 copies/μL, and deceased bacteria accounted for 48% of the total bacterial biomass in the suspension of fermented grains (Figure 4). No statistically significant difference was observed in the biomass of viable bacteria among the treatment groups with extended exposure durations. These results indicated that a 5 min exposure treatment was effective in fully binding PMA dye to the DNA of deceased bacteria, leading to the complete inhibition of dead bacterial DNA amplification. Furthermore, the prolongation of the exposure time did not result in a significant change in the detected viable bacterial biomass. Therefore, the optimal exposure time for this procedure appeared to be 5 min.
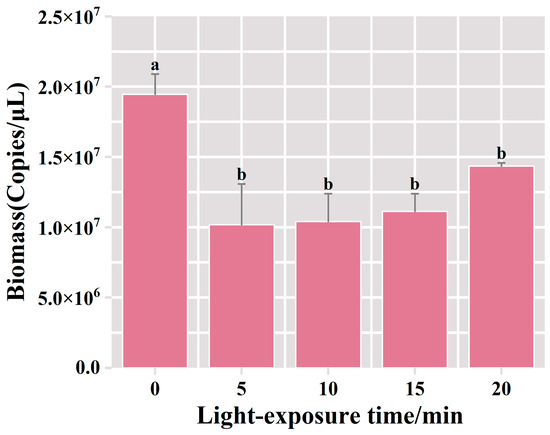
Figure 4.
Variation of bacterial biomass in the samples of fermented grains under different exposure time treatments. “a” and “b” Superscript letters indicate significant statistical differences at p ≤ 0.05.
3.3. Assessment of the Binding Efficiency of PMA with the DNA of Deceased Bacteria in Fermented Grains under Optimal Treatment Conditions
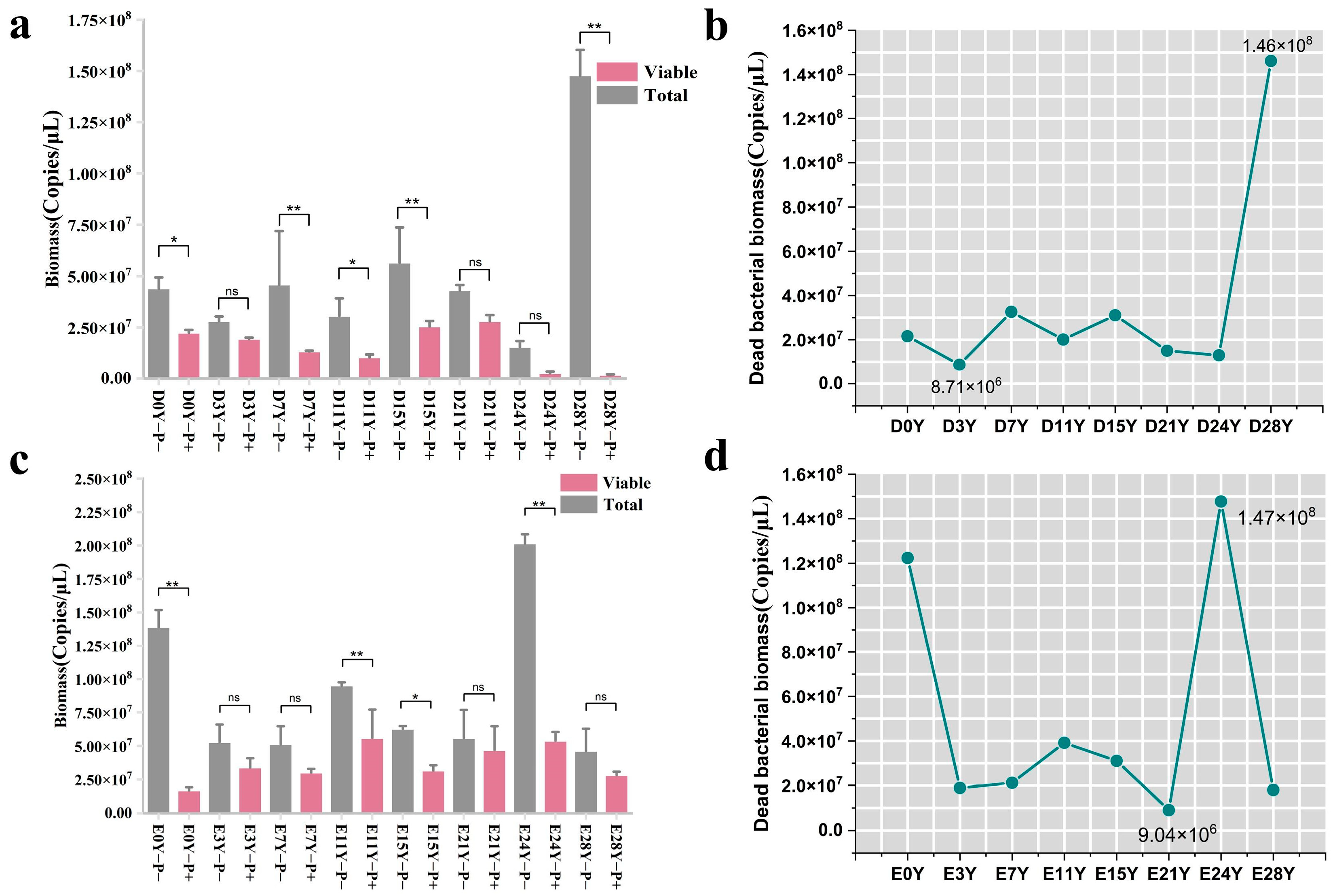
In order to assess the binding efficiency of the PMA dye with the DNA of dead cells in fermented grains during the fermentation process, we used a mixture of heat-killed E. coli and suspensions of fermented grains obtained at different fermentation times as the positive control group. The upper threshold of PMA binding to the DNA of dead cells in fermented grains was determined via the positive control. Compared with the positive control group without PMA treatment, the total bacterial biomass of the positive control group, experiencing diverse fermentation durations, exhibited a decrease following treatment with the optimal PMA conditions (50 μM PMA, dark incubation for 5 min, exposure treatment for 5 min) (p < 0.01) (Figure 5a,c).
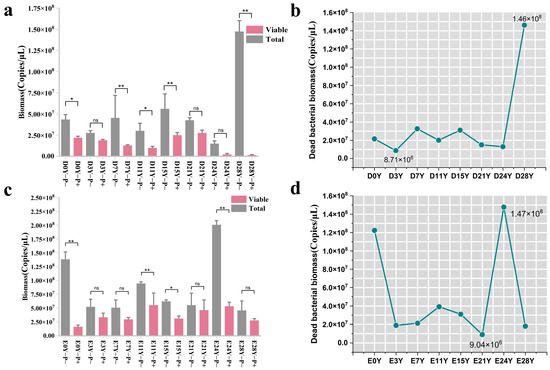
Figure 5.
Effectiveness analysis of optimal PMA conditions for combining DNA from dead cells in fermented grains. (a,c) Comparison of changes of bacterial biomass before and after PMA treatment in the positive control group of fermented grains in Dacha and Ercha fermented grains; (b,d) curves of dead bacteria in the positive control group of fermented grains of Dacha and Ercha. “**” indicate significant statistical differences at p ≤ 0.01, and “*” indicate significant statistical differences at p ≤ 0.05. “ns” indicates no significant difference between the two groups.
In the positive control group of Dacha fermented grains, the fluctuation range of dead bacterial biomass was detected using the PMA pretreatment method, amounting to 8.71 × 106–1.46 × 108 copies/μL, with dead bacteria constituting 31–99% of the total bacteria. In the positive control group of Ercha fermented grains, this range was found to be 9.04 × 106–1.47 × 108 copies/μL, with dead bacteria comprising 16% to 88% of the total bacteria (Figure 5b,d). The maximum total bacterial biomass in the fermented grain samples was 2.15 × 107 copies/μL (Figure 1a). Nevertheless, the aforementioned findings suggested that, within the positive control cohort, subsequent to PMA treatment, the ascertained biomass of deceased bacteria surpassed the overall bacterial biomass observed in typical fermented grain samples. This implied that the refined pretreatment conditions facilitated proficient association of the PMA dye with the entirety of DNA derived from deceased cells in the fermented grain samples, thereby furnishing precise and dependable outcomes concerning the composition of the viable bacterial community.
3.4. Evaluation of the Efficacy in Deciphering the Living Microbial Community Profile in Fermented Grains through the Coupling of PMA Pretreatment Technology with Amplicon Sequencing
3.4.1. The α-Diversity of the Active Bacterial Community in Fermented Grains Exhibited a Markedly Reduced Level Compared to the Total Bacterial Community
To investigate the feasibility of employing a PMA-coupled amplicon sequencing analysis method in the fermented grains, the 15-day fermented grains treated with PMA were chosen as the sequencing object. This experimental group, designated DV15, provided insight into the composition of the viable microbial community. In parallel, 15-day fermented grains without prior PMA treatment served as the sequencing control group (DT15), offering an overview of the entire microbial community structure. The rarefaction curves for bacterial and fungal communities attained saturation, implying that the quantity of sequenced data was judicious (Figure S3). Further data acquisition would not exert a substantial influence on the α-diversity index.
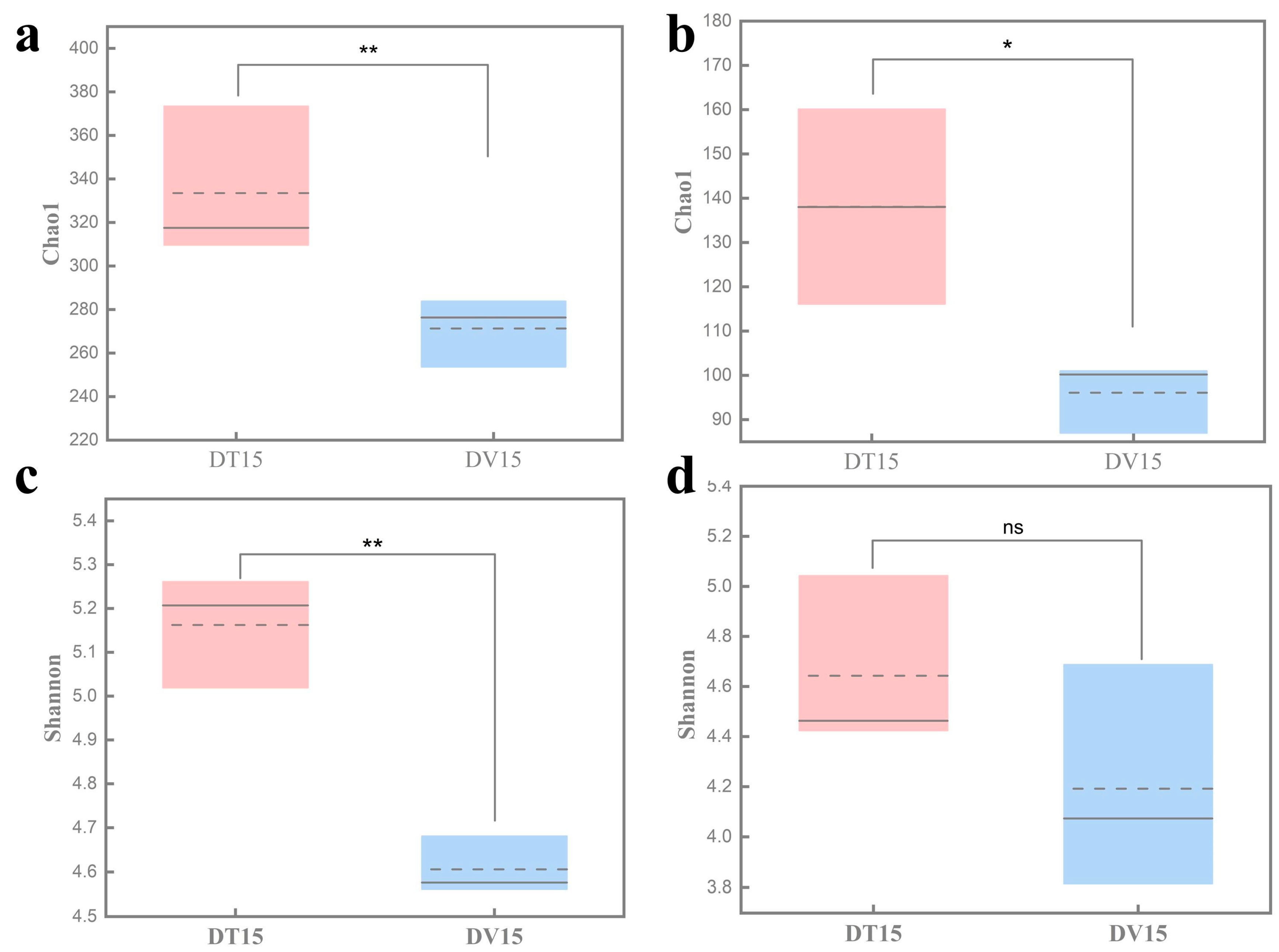
The α-diversity serves as a comprehensive indicator of the structure of the microbial community, elucidating both the diversity and evenness within the sample’s microbial composition [36]. Compared with the untreated group DT15, the Chao1 and Shannon index of the bacterial community in the PMA-treated group DV15 was significantly reduced (p < 0.01), and no statistical difference was observed in Shannon indices of the fungal community between the DT15 group and DV15 group (p = 0.239). At the same time, we found that the Chao1 and Shannon index of the fungal community was lower than that of bacterial communities, indicating that the diversity of the viable bacterial community was reduced compared with the total microbial community, while the diversity of the viable fungal community was not significantly changed (Figure 6).
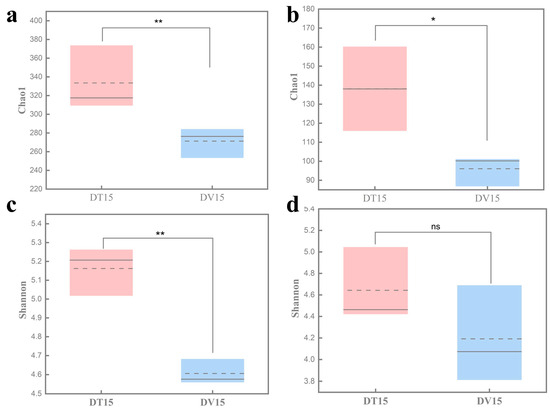
Figure 6.
Comparative analysis of the α-diversity of total microbial community and viable microbial community in fermented grains. (a) Bacterial Chao1 index; (b) fungal Chao1 index; (c) bacterial Shannon index; (d) fungal Shannon index. “**” indicate significant statistical differences at p ≤ 0.01, and “*” indicate significant statistical differences at p ≤ 0.05. “ns” indicates no significant difference between the two groups.
3.4.2. Differences in Bacterial and Fungal Microbial Structure Composition of the DT and DV Groups
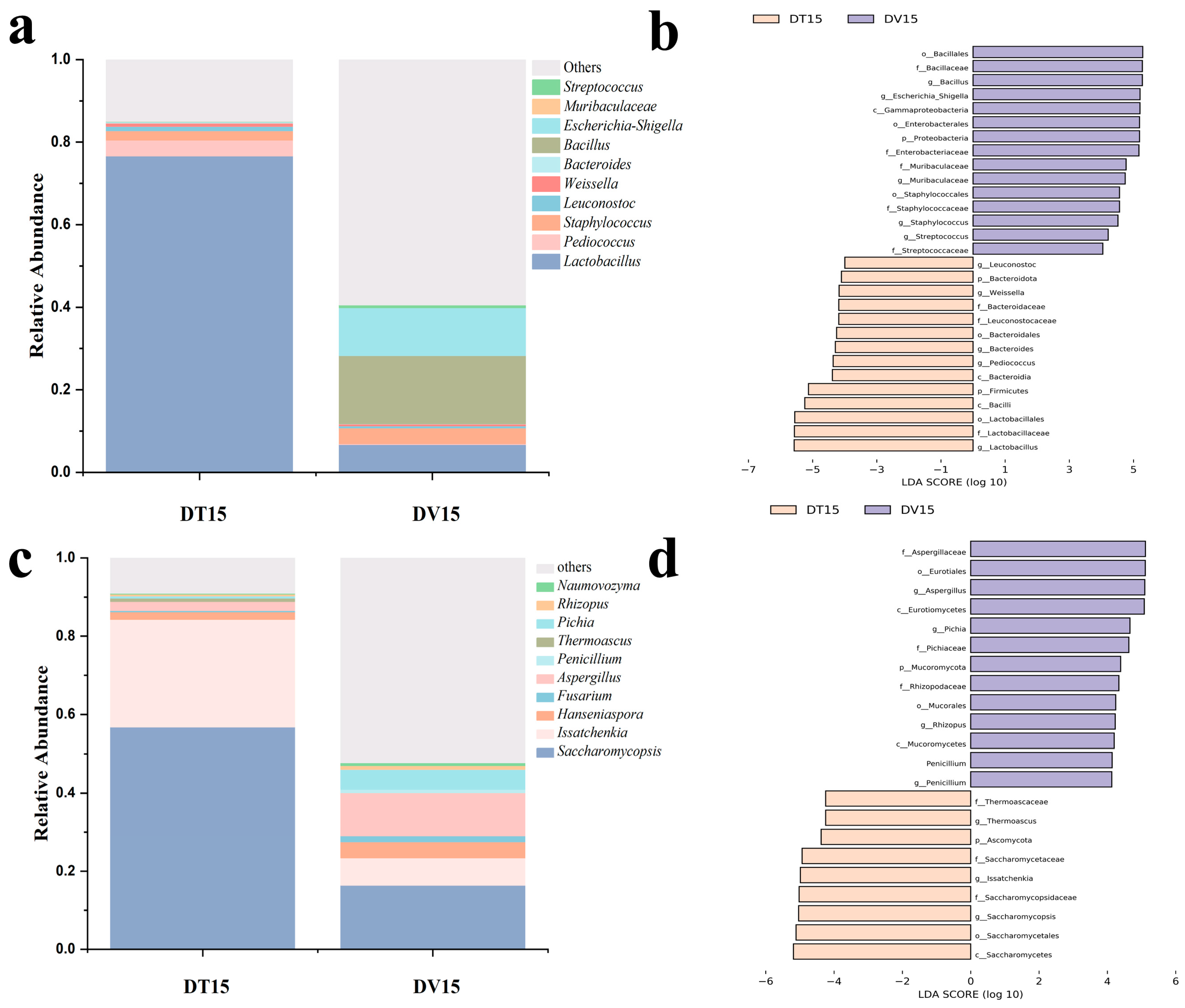
Dominant genera were characterized by possessing an average abundance exceeding 1.0%. Within both experimental cohorts, Lactobacillus and Staphylococcus were discerned as predominant bacterial genera. Pediococcus and Leuconostoc were only identified as dominant bacterial genera in the DT15 group, while Bacillus (abundance accounted for 12.16% of viable bacterial) were only identified as dominant bacterial genera in the DV15 group. Compared to the DT15 group, the DV15 group exhibited a heightened relative abundance of Bacillus, Staphylococcus and Streptococcus, alongside a diminished relative abundance of Lactobacillus, Streptococcus, and Bacteroidetes (Figure 7a). It is noteworthy that the relative abundance of Weissella and Leuconostoc in the DT15 group was 1.31% and 0.75%, respectively; however, these two genera were not detected in the DV15 group.
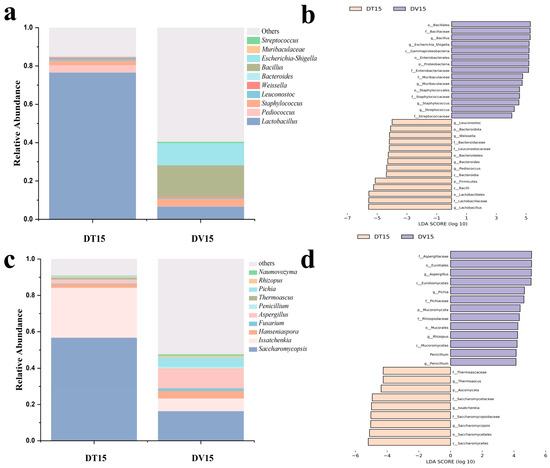
Figure 7.
Composition and differences in the dominant microbial community at the genus level. (a,c) Top 10 bacterial and fungal community composition at the genus level in both experimental groups; (b,d) LEfSe identified the differentially abundant species of bacterial and fungal between the DT and DV group at the genus level.
In the fungal community, the dominant genera in both the DT15 and DV15 groups encompassed Saccharomycopsis, Issacchenkia, Hanseniaspora and Aspergillus. Compared to the DT15 group, the DV15 group exhibited an elevation in the relative abundance of Hanseniaspora, Fusarium, Aspergillus, Penicillium, Thermoascus, Pichia and Rhizopus, whereas the relative abundance of Saccharomycopsis, Issachenkia and Thermoascus experienced a decline. The relative abundance of Fusarium within the DV15 group diminished approximately 2.4-fold, from 56% to 23.05%. Concurrently, the relative abundance of Issacchenkia experienced a reduction of approximately 3.9-fold, descending from 27.4% to 7.04%. Meanwhile, Thermoascus was exclusively identified in the DT15 group (Figure 7c). These results indicated that the combination of PMA pretreatment and amplicon sequencing facilitated the monitoring and analysis of the changes in the living microbial community, and it was observed that disparities exist in the composition of the living microbial community compared to the total microbial community, whether considering the bacterial or fungal community.
The results of Linear discriminant analysis Effect Size (LEfSe) revealed distinctive bacterial genera, namely Lactobacillus, Bacillus, Pediococcus and Staphylococcus, distinguishing between the two experimental groups. Notably, the DT15 group exhibited elevated relative abundances of Lactobacillus and Pediococcus (Figure 7b). Regarding fungi, the discernible genera variance encompassed Saccharomycopsis, Issatchenkia, Aspergillus and Pichia. DV15 exhibited increased relative abundances of Aspergillus and Pichia (Figure 7d).
3.4.3. β-Diversity of the Bacterial and Fungal Community Revealed a Clear Separation between the DT and DV Groups
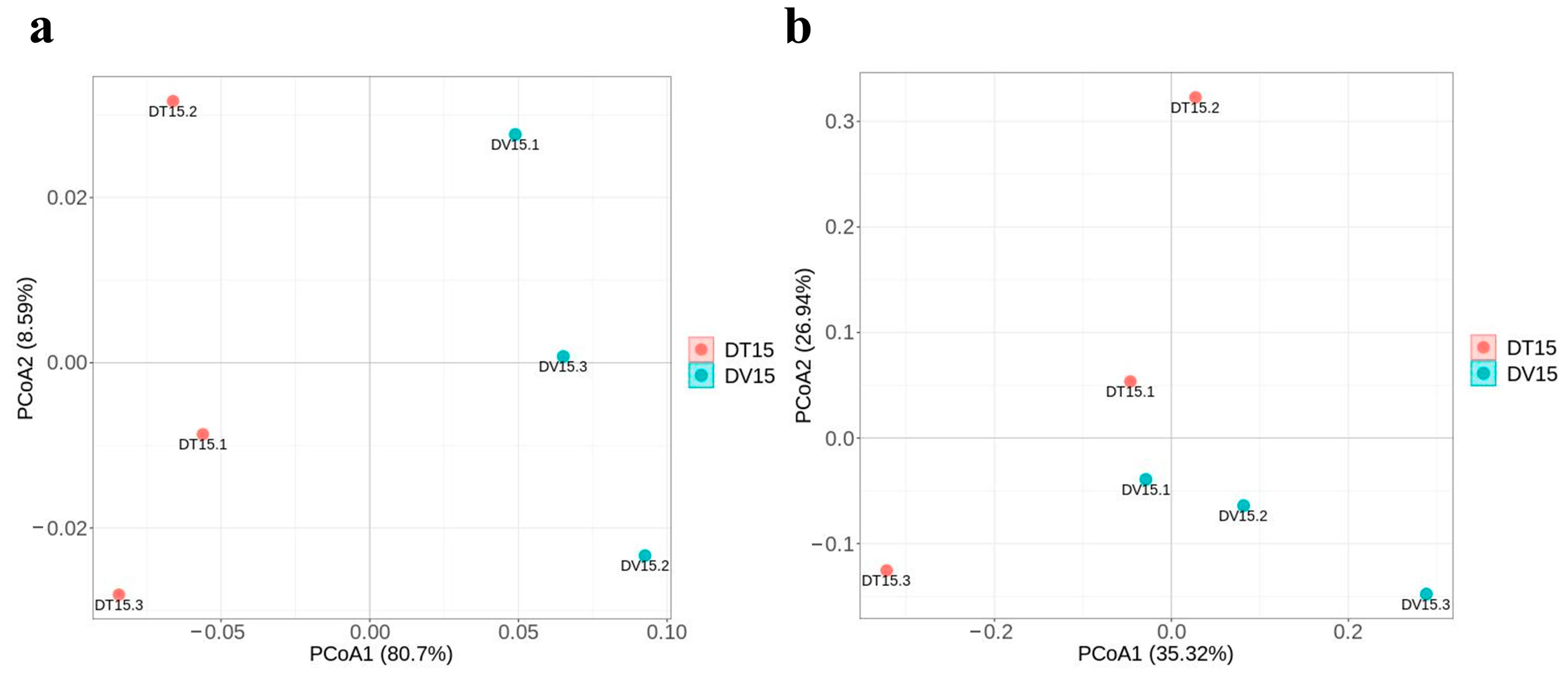
β-diversity was used to study the structural variation of microbial communities between samples and was visualized by Principal Coordinate Analysis (PCoA) [37]. Principal Coordinate Analysis (PCoA) was employed to evaluate the alterations in the structure of the living microbial community subsequent to PMA treatment in comparison to the overall microbial community structure within fermented grains. The results of PCoA showed distinctive clustering patterns in the microbial community structure between the DV15 and DT15 groups, discrepancies between the total microbial community structure and the living microbial community structure were evident (Figure 8a,b).
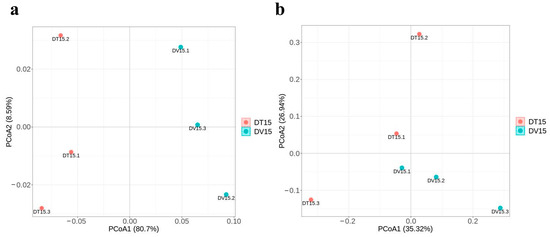
Figure 8.
Comparative analysis of microbial β-diversity in fermented grains between groups DT15 and DV15. (a,b) PCoA of bacterial and fungal community composition based on DT15 and DV15 groups.
4. Discussion
In the course of Baijiu brewing, fermented grains, serving as the substrates for Baijiu distillation, harbor a diverse array of microorganisms. The microbial composition in fermented grains employed in Baijiu fermentation is notably intricate, mainly including bacteria, mold and yeast [38]. As high-throughput technology advances, the study of a Baijiu brewing mechanism from a microbial standpoint has gained increased depth, thereby profoundly enriching our comprehension of Baijiu fermentation principles. However, we observed a lacuna in the study of the fermentation mechanism of fermented grains, wherein microorganisms served as the subject of investigation without discrimination between deceased and viable microorganisms [24]. In fact, during the fermentation process of fermented grains, only the living microbial communities possess the capacity to incessantly metabolize nutrients, namely carbohydrates and proteins inherent in the raw materials. The metabolic activity results in the generation of diverse enzymes, alcohols, esters and flavor precursors, concurrently facilitating the degradation of some potentially detrimental substances present in the raw materials [39,40,41]. Therefore, the monitoring of the living microbial community stands as a means to complement and enhance the comprehension of the Baijiu fermentation mechanism.
PMA is a compound extensively employed in the surveillance of viable microorganisms [42]. At present, PMA combined with amplicon sequencing technology has been successfully applied to the analysis of the structure of living microbial communities in soil, sewage and human gut [20,43,44]. This study established the optimal pre-treatment protocol for PMA in microbes from fermented grain samples. The integration of PMA with amplicon sequencing was employed for the analysis of the living microbial communities, thereby confirming the viability and applicability of PMA in discerning living microorganisms within fermented grains. The pre-treatment parameters of the PMA dye exhibit variability across diverse sample types. For example, the optimal pre-treatment protocol for quantifying Saccharomyces cerevisiae and Lactobacillus plantarum during the brewing process of Hong Qu glutinous rice wine involved using a PMA concentration of 25 μM. The sample was incubated in the dark for 20 min, followed by an exposure treatment for 10 min. This protocol ensured that only viable microorganisms were quantified in the subsequent analysis [45]. In the studies of the microbial community structure in samples of strong-flavored fermented grains, the pre-treatment scheme for the samples involved a dark incubation step with a 50 μM PMA for 5 min, followed by an exposure treatment for 5 min [24]. The pretreatment conditions for fermented grain samples using PMA in the aforementioned studies were congruent with the findings of this study, thereby indirectly substantiating the validity of the results obtained in this study from an alternative perspective. Despite the utilization of distinct types of fermented grains in the two studies, these findings implied the potential applicability of this set of PMA pre-treatment parameters to diverse fermented grain samples. This result provided significant reference values for selecting PMA pre-treatment parameters in studies investigating the living microbial community structure of fermented grains in other flavored Baijiu.
In this study, no discernible variance was observed in the total and viable fungal communities. However, it was determined that the diversity of living bacterial communities in the DV15 group was significantly reduced compared to the total bacterial communities in the DT15 group. These results implied the prevalence of deceased cells within bacterial communities, juxtaposed with fewer deceased cells within fungal communities. The reasons for these differences may stem from the binding of the PMA to the DNA of dead cells in the fermented grains, thereby influencing the structural composition of the microbial community, as depicted in the sequencing outcomes. Consequently, this change affected the relative abundance proportion of the microbial community [46]. Alternatively, dead cells may arise from the accumulation of dead bacterial entities generated during the initial phase of fermentation in the fermented grains. Furthermore, owing to the adaptive capacity of certain microorganisms to thrive within the fermentation milieu of fermented grains, their presence in the living microbial community was augmented by the relatively limited occurrence of deceased cells within the fermented grains [47].
In the sample of grains fermented for 15 days, the dominant microbial composition in both the total microorganisms and the viable microorganisms was similar, with disparities discernible solely in relative abundance. The dominant genera of viable bacteria in the fermented grains were mainly Lactobacillus, Staphylococcus and Bacillus. Meanwhile, the dominant genera of viable fungi identified were Saccharomycopsis, Issatchenkia, Hanseniaspora and Aspergillus. The study by Wang [48] employed macro-transcriptomics to investigate Luzhou-flavor fermented grains, revealing transcriptional activity in Lactobacillus, Saccharomyces, Bacillus and Staphylococcus, all of which occupied dominant positions. This finding aligned with the structure and composition of the predominant viable bacteria observed in the present study. These findings indicated the rationality of the sequencing outcomes of our investigation, further suggesting the significant involvement of the aforementioned living microorganisms in shaping the diverse flavor profiles of Baijiu.
The main differential bacterial genera between total and viable microorganisms were Lactobacillus, Bacillus and Pediococcus. During the fermentation process, Lactobacillus and Pediococcus stood out as the predominant lactic acid bacteria inhabiting the fermented grains. Their metabolic activities were notably influenced by factors within the fermentation milieu, such as oxygen availability and ethanol concentration. Meanwhile, they enzymatically generate lactic acid, thereby reducing the pH of the fermentation milieu and imposing acid stress on other microorganisms [49]. In our study, we observed a higher relative abundance of Lactobacillus and Pediococcus in the DT15 group compared to the DV15 group. This disparity may be attributed to the ethanol stress exerted by Saccharomyces cerevisiae, which produced alcohols within the identical fermentation milieu. This ethanol stress adversely impacts the metabolic processes of lactic acid bacteria, consequently hindering the growth of both Lactobacillus and Pediococcus [50]. Bacillus can utilize enzymes to efficiently hydrolyze starch into reducing sugars. This crucial process provides a nutrient-rich substrate that facilitates subsequent fermentation processes [51]. The relative abundance of Bacillus in the DV15 group surpassed that of the DT15 group, potentially attributed to Bacillus possessing traits of thermal and acid resilience, enabling proliferation even in harsh environments [52,53,54]. Furthermore, Bacillus can synthesize lipopeptides, surface proteins, rheumatic acid, fengmycin, and other antibacterial compounds to suppress the proliferation of other bacteria [55]. Therefore, it constituted a significant portion of the living bacterial community in fermented grains.
The main differential fungal genera were Saccharomycosis, Issatchenkia, Aspergillus and Pichia. Saccharomycopsis can generate a diverse array of compounds including esters, lactones, alcohols, acids, and aldehydes during the fermentation process of fermented grains; Issachenkia, characterized by its acid and heat resistance traits, predominantly originated from low-temperature Daqu and exhibited proficient alcohol production capabilities [56,57,58]. Saccharomycosis, Issatchenkia and Pichia are all categorized as non-Saccharomyces yeasts. It has been reported that Saccharomyces cerevisiae is capable of impeding the proliferation of other yeasts through cell–cell interactions during mixed-yeast fermentation [59]. Moreover, in another investigation concerning Baijiu, it was observed that the suppression of Pichia growth by S. cerevisiae primarily took place during the initial fermentation phase. Subsequently, as fermentation advances, Pichia was no longer subject to inhibition and gradually emerged as the dominant genus [60]. In the current study, it was noted that the relative abundance of Pichia was greater in the DV15 group in comparison to the DT15 group, while the relative abundance of the other two non-Saccharomyces yeast species was diminished. This outcome aligned perfectly with the findings of the aforementioned study, which elucidated the dynamics of yeast interactions during fermentation. The samples utilized in this study were derived from the mid-fermentation stage of fermented grains. At this stage, Pichia was no longer under the inhibitory effect of S. cerevisiae. However, it was plausible that the growth of Saccharomycosis and Issatchenkia was suppressed. Consequently, Pichia thrived, leading to a proportional increase, while the other two non-Saccharomyces yeast species experienced a decline within the living microbial community compared to their relative abundance in the total microbial community. The findings from the DV15 group portrayed the structure of the living microbial community, potentially mirroring the authentic microbial composition during Baijiu fermentation. This outcome further underscored the efficacy of the PMA-amplicon sequencing methodology.
5. Conclusions
This study extensively detailed the optimization process of the PMA dye for sample pretreatment in fermented grains and assessed the effectiveness of viable microorganism evaluation. Leveraging PMA pretreatment technology and amplicon sequencing, the researchers successfully devised an efficient method for high-throughput analysis of the living microbial community in Baijiu fermented grains. This method enables the analysis of changes within the living microbial community, thereby offering a novel approach to investigating and enhancing the comprehension of Baijiu fermentation mechanisms. Most conventional fermented foods, akin to Chinese Baijiu, undergo natural fermentation processes, wherein the fermentation cascade encompasses the proliferation, metabolic activities, and ecological succession of a diverse array of microbial consortia. Therefore, this study’s findings offer methodological references for analyzing living microbial communities in such foods. This contribution holds significant importance in deepening our understanding of the fermentation mechanisms underlying traditional fermented foods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13111782/s1, Figure S1: Gel images of total bacterial PCR products obtained from samples treated with different PMA concentrations. 1~5: 0, 50, 100, 150, 200 μM PMA treatment; Figure S2: Gel images of total bacterial PCR products obtained from samples treated with different dark incubation treatments. 1~5: 0, 5, 10, 15, 20 min dark incubation treatments; Figure S3: The melting curve and standard curve for quantitative analysis of bacteria and fungi during the fermentation process; Figure S4: The standard curve for quantifying bacteria under different concentrations of PMA treatment; Figure S5: The standard curve for quantifying bacteria under different dark incubation time treatment; Figure S6: The standard curve for quantifying bacteria under different exposure time treatments; Figure S7: Rarefaction curves of amplicon sequencing.
Author Contributions
T.B.: formal analysis, writing—original draft, writing—review and editing, visualization, funding acquisition. J.Z. (Jiaojiao Zhang): formal analysis, writing—original draft, writing—review and editing, visualization. E.Z.: writing—review and editing. N.L.: formal analysis, software. B.B.: methodology. Y.Y.: methodology, supervision. J.Z. (Jinhua Zhang): project administration, funding acquisition. S.F.: conceptualization, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Project Program of Xinghuacun College of Shanxi University (Shanxi Institute of Brewing Technology and Industry) (No. XCSXU-KF-202317); and Research and development project (No. 01130122090116).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Tao Bo is a postdoctoral fellow who underwent collaborative training from Shanxi University and the National Technology Center of Xinghuacun Fenjiu Distillery Co., Ltd. The role of the company was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Du, R.B.; Ren, C.; Wu, Q.; Xu, Y. The ecological fermentation technology: Principle and its applications. Food Ferment. Ind. 2021, 47, 266–275. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Zhu, Q.; Zhang, J. Research Status and Application Prospect of Frontier Technology of Traditional Fermented Food in China. J. Food Sci. Technol. 2021, 39, 1–7. [Google Scholar]
- Liu, A.; Ou, Y.; Shu, H.; Mou, T.; Li, Q.; Li, J.; Hu, K.; Chen, S.; He, L.; Zhou, J.; et al. Exploring the role of Sichuan Baoning vinegar microbiota and the association with volatile flavor compounds at different fermentation depths. Front. Microbiol. 2023, 14, 1135912. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Kim, S.H.; Jeong, W.S.; Kim, S.Y.; Yeo, S.H. Microbiome Analysis of Traditional Grain Vinegar Produced under Different Fermentation Conditions in Various Regions in Korea. Foods 2022, 11, 3573. [Google Scholar] [CrossRef]
- Jiang, L.; Xian, S.; Liu, X.; Shen, G.; Zhang, Z.; Hou, X.; Chen, A. Metagenomic Study on Chinese Homemade Paocai: The Effects of Raw Materials and Fermentation Periods on the Microbial Ecology and Volatile Components. Foods 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Du, H.; Zhang, H.; Fang, C.; Jin, G.; Chen, S.; Xu, Y. Geographically Associated Fungus-Bacterium Interactions Contribute to the Formation of Geography-Dependent Flavor during High-Complexity Spontaneous Fermentation. Microbiol. Spectr. 2022, 10, e0184422. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Yang, N.; Yang, J.; Hao, J.; Zhao, J.; Shi, S.; Hu, B. Effects of microbial interspecies relationships and physicochemical parameters on volatile flavors in sorghum-based fermented grains during the fermentation of Shanxi light-flavored liquor. Food Sci. Nutr. 2022, 11, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Kong, L.H. Analysis on Active Microorganisms in Air-Dried Agricultural Soils Based on Propidium Monoazid Incorporating High-Throughput sequencing. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar]
- Roth, P.; Hill-Spanik, K.M.; McCurry, C.; Plante, C. Propidium monoazide-denaturing gradient gel electrophoresis (PMA-DGGE) assay for the characterization of viable diatoms in marine sediments. Diatom Res. 2017, 32, 341–350. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Chen, M.; Fan, J.; Han, S.; Hou, J.; Chi, L.; Liu, Y.; Wei, T. Profiling the composition and metabolic activities of microbial community in fermented grain for the Chinese strong-flavor Baijiu production by using the metatranscriptome, high-throughput 16S rRNA and ITS gene sequencings. Food Res. Int. 2020, 138, 109765. [Google Scholar] [CrossRef] [PubMed]
- Solden, L.; Lloyd, K.; Wrighton, K. The bright side of microbial dark matter: Lessons learned from the uncultivated majority. Curr. Opin. Microbiol. 2016, 31, 217–226. [Google Scholar] [CrossRef] [PubMed]
- An, J.X.; Wu, S.H.; Zeng, L.J.; Hunag, J.X.; Feng, P.X.; Liang, T.L.; Yi, Y. Comparison of different methods to extract total RNA from Saccharomycescerevisiae. China Brew. 2021, 40, 82–86. [Google Scholar]
- Ojala, T.; Häkkinen, A.E.; Kankuri, E.; Kankainen, M. Current concepts, advances, and challenges in deciphering the human microbiota with metatranscriptomics. Trends Genet. 2023, 39, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Ma, Y. Real-time and visual detection of viable Salmonella in milk by a competitive annealing mediated isothermal amplification (CAMP) combined with propidium monoazide (PMA). Anal. Methods 2022, 14, 3773–3779. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Shu, Y.; Xia, W.; Xu, R.; Chen, Y. Modified PMA-qPCR Method for Rapid Quantification of Viable Lactobacillus spp. in Fermented Dairy Products. Food Anal. Methods 2021, 14, 1908–1918. [Google Scholar] [CrossRef]
- Ayaka, O.; Mizuki, T.; Kazuha, A.; Ayaka, Y.; Matiur, R.M.; Yasuo, I. Research Note: Detection of Campylobacter spp. in chicken meat using culture methods and quantitative PCR with propidium monoazide. Poult. Sci. 2023, 102, 102883. [Google Scholar]
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Koriki, S. Suitability of pepper mild mottle virus as a human enteric virus surrogate for assessing the efficacy of thermal or free-chlorine disinfection processes by using infectivity assays and enhanced viability PCR. Water Res. 2020, 186, 116409. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ji, J.; Li, Y.Y.; Kubota, K. Propidium monoazide-polymerase chain reaction reveals viable microbial community shifts in anaerobic membrane bioreactors treating domestic sewage at low temperature. Bioresour. Technol. 2023, 387, 129564. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, X.; Fan, X.; Xu, J.; Liu, Q.; Wu, Z.; Pan, D. PMA-qPCR method for the selective quantitation of viable lactic acid bacteria in fermented milk. Front. Microbiol. 2022, 13, 984506. [Google Scholar] [CrossRef]
- Guo, J.; Wang, W.; Zhao, H.; Luo, Y.; Wan, M.; Li, Y. A new PMA-qPCR method for rapid and accurate detection of viable bacteria and spores of marine-derived Bacillus velezensis B-9987. J. Microbiol. Methods 2022, 199, 106537. [Google Scholar] [CrossRef] [PubMed]
- Ángeles, R.M.d.L.; Anabel, R.R.; Luma, R.R.; Fabiano, D.S.C.; Mariana, C.; Valeria, M.M.; Marcelo, C.; Ramón, V.S. High-pressure processing treatment of beef burgers: Effect on Escherichia coli O157 inactivation evaluated by plate count and PMA-qPCR. J. Food Sci. 2022, 87, 2324–2336. [Google Scholar]
- Liu, H.; Tan, G.; Chen, Q.; Dong, W.; Chen, P.; Cai, K.; Hu, Y.; Zhang, W.; Peng, N.; Liang, Y.; et al. Detection of viable and total fungal community in zaopei of Chinese strong-flavor baijiu using PMA combined with qPCR and HTS based on ITS2 region. BMC Microbiol. 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.; Barretta, C.; Ossai, S.O.; Silva, H.S.d.; Kist, A.; Vieira, C.R.W.; Parveen, S. Optimization of a propidium monoazide-qPCR method for Escherichia coli quantification in raw seafood. Int. J. Food Microbiol. 2020, 318, 108467. [Google Scholar] [CrossRef] [PubMed]
- Kibbee, R.J.; Örmeci, B. Development of a sensitive and false-positive free PMA-qPCR viability assay to quantify VBNC Escherichia coli and evaluate disinfection performance in wastewater effluent. J. Microbiol. Methods 2017, 132, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, X.; Yang, S.; Chen, F. Screening of Bacillus strains from Luzhou-flavor liquor making for high-yield ethyl hexanoate and low-yield propanol. LWT 2017, 77, 60–66. [Google Scholar] [CrossRef]
- Huang, T. Functional Analysis of Microbial Community of Zhenjiang Aromatic Vinegar and Construction of a Defined Starter for Acitic Acid Production. Master’s Thesis, Jiangnan University, Wuxi, China, 2022. [Google Scholar]
- Xing, Y.; Chen, Y.; Feng, C.; Bao, J.; Li, X.; Jiang, H. Establishment and Application of Real-Time Fluorescence Quantitative PCR Detection Technology for Metschnikowia bicuspidata Disease in Eriocheir sinensis. J. Fungi 2023, 9, 791. [Google Scholar] [CrossRef]
- Hong, B.M. Investigation of Suppressing Anopheles Stephensi Reproduction with RNAi. In Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Oxford, UK, 7 November 2022. [Google Scholar]
- Villasante, A.; Ramírez, C.; Rodríguez, H.; Dantagnan, P.; Hernández, A.; Figueroa, E.; Romero, J. Dietary carbohydrate-to-protein ratio influences growth performance, hepatic health and dynamic of gut microbiota in atlantic salmon (Salmo salar). Anim. Nutr. 2022, 10, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Zong, E.; Bo, T.; Dang, L.; Zhang, J.; Li, H.; Lv, N.; He, Y.; Bai, B.; Zhang, J.; Fan, S. Different functions can be provided by low temperature Daqu with different appearance features due to variations in the microbial community structure during fermentation. LWT 2024, 193, 115763. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Mu, Y.; Zhu, Z.; Wu, Y.; Qi, Q.; Mu, Y.; Su, W. Exploring the heterogeneity of community and function and correspondence of “species-enzymes” among three types of Daqu with different fermentation peak-temperature via high-throughput sequencing and metagenomics. Food Res. Int. 2024, 176, 113805. [Google Scholar] [CrossRef]
- Tao, J.L.; LU ZM, W.Z. Detection of the variation of microorganisms in acetic acid fermentation of Zhenjiang aromatic vinegar through real-time quantitative PCR. Food Ferment. Ind. 2013, 39, 156–160. [Google Scholar] [CrossRef]
- Wang, J.; Hao, S.; Ren, Q. Analysis of Bacterial Diversity in Fermented Grains of Baijiu Based on Culturomics and Amplicon Sequencing. Fermentation 2023, 9, 260. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Zhu, H.; Wang, H.; Lu, H.; Zhang, C.; Li, X.; Xu, Y.; Li, W.; Wang, Y. Turning over fermented grains elevating heap temperature and driving microbial community succession during the heap fermentation of sauce-flavor baijiu. LWT 2022, 172, 114173. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, Q.; Xia, X.; Liu, Q.; Chen, X.; Zhu, B. Dynamics of microbial community structure and enzyme activities during the solid-state fermentation of Forgood Daqu: A starter of Chinese strong flavour Baijiu. Arch. Microbiol. 2022, 204, 577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Du, G.; Chen, J.; Ren, T.; Wang, J.; Zhao, X. The Influence of Seasons on the Composition of Microbial Communities and the Content of Lactic Acid during the Fermentation of Fen-Flavor Baijiu. Fermentation 2022, 8, 740. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Huang, D.; Luo, H. Contribution of microorganisms from pit mud to volatile flavor compound synthesis in fermented grains for Nongxiangxing Baijiu brewing. J. Sci. Food Agric. 2023, 104, 778–787. [Google Scholar] [CrossRef]
- Liu, S.; Ren, D.; Qin, H.; Yin, Q.; Yang, Y.; Liu, T.; Mao, J. Exploring major variable factors influencing flavor and microbial characteristics of upper jiupei. Food Res. Int. 2023, 172, 113057. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, M.; Zhao, D.; Zheng, J.; Dai, M.; Li, X.; Sun, B. Simulated Fermentation of Strong-Flavor Baijiu through Functional Microbial Combination to Realize the Stable Synthesis of Important Flavor Chemicals. Foods 2023, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Long, Y.; Li, Q.; Yang, L.; Huang, Y.; Yu, D.; Song, W.; Zhou, M.; Xu, G.; Huang, C.; et al. Propidium Monoazide Combined With RT-qPCR Detects Infectivity of Porcine Epidemic Diarrhea Virus. Front. Vet. Sci. 2022, 9, 931392. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Ge, Y. Relic DNA effects on the estimates of bacterial community composition and taxa dynamics in soil. Appl. Microbiol. Biotechnol. 2023, 107, 4109–4117. [Google Scholar] [CrossRef]
- Liu, F.; Lu, H.; Dong, B.; Huang, X.; Cheng, H.; Qu, R.; Xu, Z.Z. Systematic Evaluation of the Viable Microbiome in the Human Oral and Gut Samples with Spike-in Gram+/- Bacteria. Msystems 2023, 8, e0073822. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-C.; Li, Y.; Qiu, W.-W.; Wu, X.-Q.; Xu, B.-X.; Liang, Y.-T.; Liu, B.; Chen, S.-J.; Rao, P.-F.; Ni, L. Development of propidium monoazide combined with real-time quantitative PCR (PMA-qPCR) assays to quantify viable dominant microorganisms responsible for the traditional brewing of Hong Qu glutinous rice wine. Food Control 2016, 66, 69–78. [Google Scholar] [CrossRef]
- Xue, Y.; Abdullah Al, M.; Chen, H.; Xiao, P.; Zhang, H.; Jeppesen, E.; Yang, J. Relic DNA obscures DNA-based profiling of multiple microbial taxonomic groups in a river-reservoir ecosystem. Mol. Ecol. 2023, 32, 4940–4952. [Google Scholar] [CrossRef] [PubMed]
- Guangxun, T. Research on Composition, Source and Dynamic Change Regularity of Microorganisms Related to Chinese Strong Flavor Liquor Brewing Based on Viable Microorganisms Data. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Kangli, W. Study of the Diversity and Metabolic Characteristics of Microbial Community Harbored in Fermented Grains for the Chinese Strong-Flavor Baijiu Production Based on Metatranscriptome. Master’s Thesis, Zhengzhou University of Light Industry, Zhengzhou, China, 2021. [Google Scholar]
- Li, X.; Tan, G.; Chen, P.; Cai, K.; Dong, W.; Peng, N.; Zhao, S. Uncovering acid resistance genes in lactic acid bacteria and impact of non-viable bacteria on bacterial community during Chinese strong-flavor baijiu fermentation. Food Res. Int. 2023, 167, 112741. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Du, H.; Zhang, Y.; Xu, Y. Unraveling Core Functional Microbiota in Traditional Solid-State Fermentation by High-Throughput Amplicons and Metatranscriptomics Sequencing. Front. Microbiol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Dan, H.; Song, X.; Xiang, G.; Song, C.; Dai, H.; Shao, Y.; Huang, D.; Luo, H. The response pattern of the microbial community structure and metabolic profile of jiupei to Bacillus subtilis JP1 addition during baijiu fermentation. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Qiu, S.; Zeng, C.; Zhang, Y.; Cui, D.; Wu, H.; Ban, S. Microbial community and interaction between lactic acid bacteria andmicroorganisms in liquid fermented food: A review. Microbiol. China 2021, 48, 960–973. [Google Scholar] [CrossRef]
- Zeng, C.; Qiu, S.; Hu, B.; Chen, M.; Xu, J.; Wang, X. Research progress of anaerobic microorganisms in liquor brewing system. China Brew. 2015, 34, 24–27. [Google Scholar]
- Zhu, Z.Y.; Huang, Y.G. Structure and Diversity Analysis of Mold Community in Main Maotai-flavor Baijiu Brewing Areas of Maotai Town Using High-throughput Sequencing. Food Sci. 2021, 42, 150–156. [Google Scholar]
- Wu, X.; Jiang, Q.; Wang, Z.; Xu, Y.; Chen, W.; Sun, J.; Liu, Y. Diversity, enzyme production and antibacterial activity of Bacillus strains isolated from sesame-flavored liquor Daqu. Arch. Microbiol. 2021, 203, 5831–5839. [Google Scholar] [CrossRef]
- Sun, S.J.; Zhai, L.; Yu, X.J.; Xu, L.; Yao, S. Influences of Saccharomycopsis fibuligera ClCC 33077 on microbial community and functional characteristics of high temperature Daqu in sesame flavor Baijiu. Food Ferment. Ind. 2023, 49, 99–105. [Google Scholar] [CrossRef]
- Niutian, Y. Study on the Microbial Community Structure of Light-Flavor Baijiu Brewing and Its Correlation with Physicochemical Indicators and Flavor Components. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2022. [Google Scholar]
- Cui, S.; Dai, Y. Change Rules of Microorganisms and Fermented Grains during Fermentation of Jiangxiang Baijiu. Liquor-Mak. Sci. Technol. 2021, 6, 65–68. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Wang, D. Interaction mechanism of non-Saccharomyces yeast and Saccharomyces cerevisiae in mixed fermentation of Mijiu. Food Ferment. Ind. 2024, 50, 41–47. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.Y.; Xu, Y. Effect of mixed culture of Saccharomyces cerevisiae and Pichia anomala on fermentation efficiency and flavor compounds in Chinese Liquor. Microbiol. China 2012, 39, 921–930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).