Improving the Nutritional Quality of Protein and Microbiota Effects in Additive- and Allergen-Free Cooked Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Nutritional Composition of Meat Products
2.3. In Vitro Gastrointestinal Digestion
2.4. In Vitro Gut Microbial Fermentation
2.5. Amino Acid Analysis
2.6. Determination of Tryptophan
2.7. In Vitro Digestibility, Digestible Indispensable Amino Acid Ratio (DIAAR), and Digestible Indispensable Amino Acid Score (DIAAS) Calculations
2.8. Determination of Short-Chain Fatty Acids
2.9. High-Throughput Amplicon Sequencing
2.10. Bioinformatic Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition of Meat Products
3.2. Amino Acid Composition of the Products
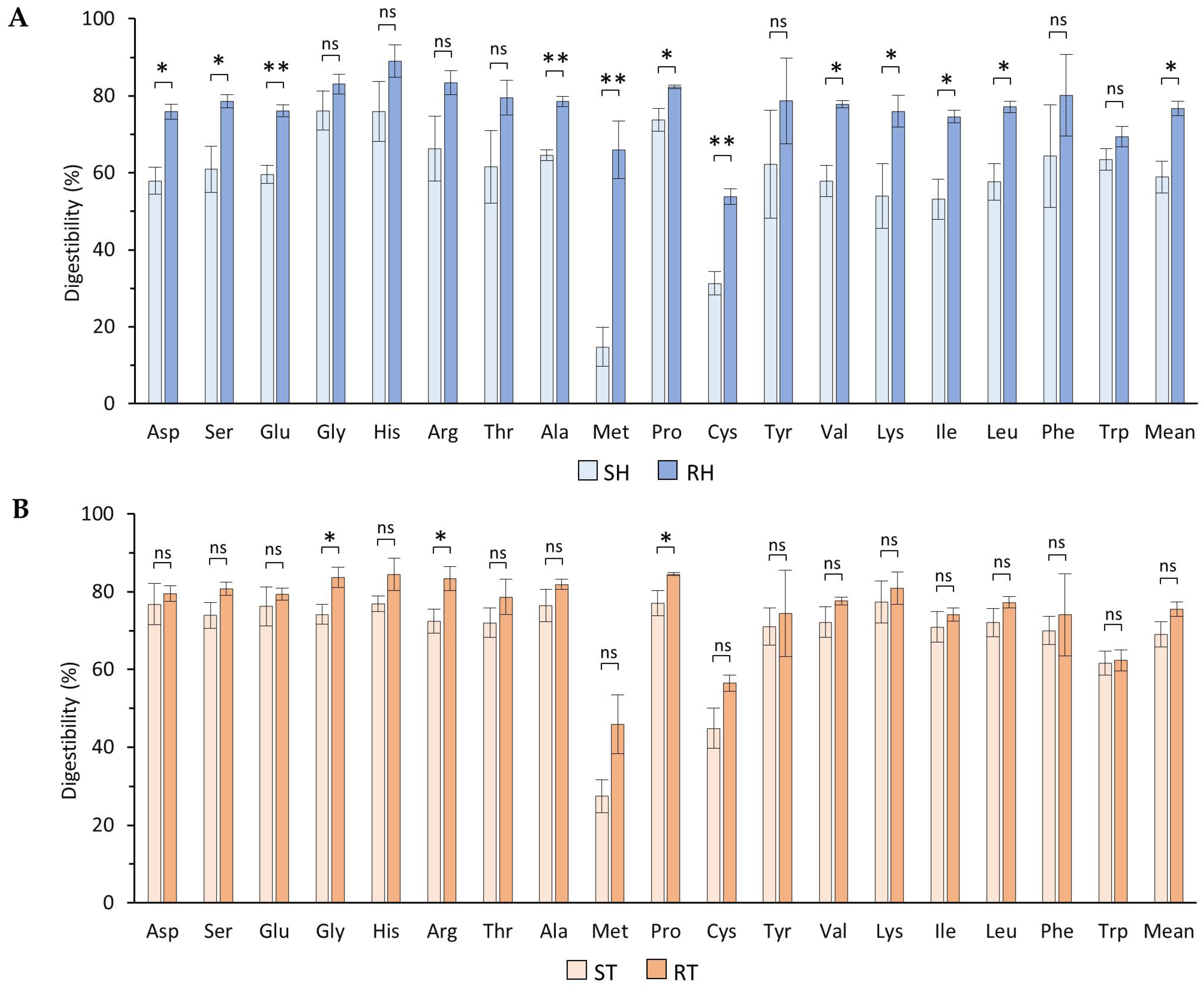
3.3. In Vitro Protein Digestibility
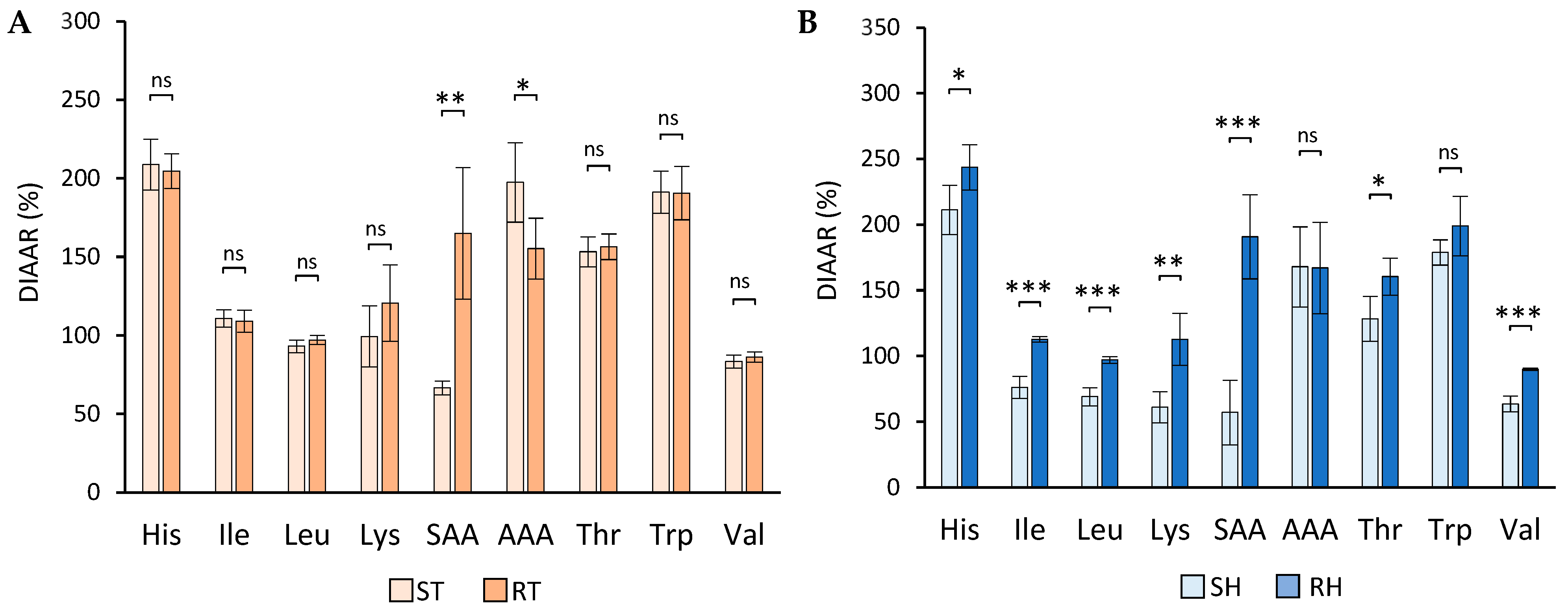
3.4. In Vitro DIAAR and DIAAS Values
3.5. Gut Microbiota Composition
3.5.1. Alpha and Beta Diversity
3.5.2. Relative Abundance
3.6. Production of Short-Chain Fatty Acids (SCFAs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report; American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Johnston, B.; De Smet, S.; Leroy, F.; Mente, A.; Stanton, A. Non-communicable disease risk associated with red and processed meat consumption—Magnitude, certainty, and contextuality of risk? Anim. Front. 2023, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wójcik, G.; et al. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations from the Nutritional Recommendations (NUTRIRECS) Consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef]
- Abraham, J.; Anxieties, A.I. Concerns and Facts about Meat Consumption and Health: A Short Review. J. Food Anim. Sci. 2020, 1, 66–80. [Google Scholar] [CrossRef]
- de Castro Cardoso Pereira, P.M.; dos Reis Baltazar Vicente, A.F. Meat Nutritional Composition and Nutritive Role in the Human Diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Benefits and Quality of Meat Products: A Review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.; Sharma, D.K.; Kishore, K. Toxicity of Food Additives. In Food Safety and Human Health; Academic Press: Cambridge, MA, USA, 2019; pp. 67–98. [Google Scholar] [CrossRef]
- Nair, M.S.; Nair, D.V.T.; Kollanoor Johny, A.; Venkitanarayanan, K. Use of Food Preservatives and Additives in Meat and Their Detection Techniques. In Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Academic Press: Cambridge, MA, USA, 2020; pp. 187–213. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Re-Evaluation of Phosphoric Acid–Phosphates—Di-, Tri- and Polyphosphates (E 338–341, E 343, E 450–452) as Food Additives and the Safety of Proposed Extension of Use. EFSA J. 2019, 17, e05674. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An Overview on Applications and Side Effects of Antioxidant Food Additives. EMIR J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary κ-Carrageenan Facilitates Gut Microbiota-Mediated Intestinal Inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan Gum and Adherent Invasive Escherichia coli in a Piglet Model of Inflammatory Bowel Disease: Impact on Intestinal Mucosa-Associated Microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments; EHP Publishing: Durham, NC, USA, 2001; Volume 109. [Google Scholar] [CrossRef]
- Guimaraes, D.A.; Batista, R.I.M.; Tanus-Santos, J.E. Nitrate and Nitrite-Based Therapy to Attenuate Cardiovascular Remodelling in Arterial Hypertension. Basic Clin. Pharmacol. Toxicol. 2021, 128, 9–17. [Google Scholar] [CrossRef]
- Deveci, G.; Tek, N.A. N-Nitrosamines: A Potential Hazard in Processed Meat Products. J. Sci. Food Agric. 2024, 104, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Sambu, S.; Hemaram, U.; Murugan, R.; Alsofi, A.A. Toxicological and Teratogenic Effect of Various Food Additives: An Updated Review. Biomed Res. Int. 2022, 2022, 68294092022. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The Importance of Food Naturalness for Consumers: Results of a Systematic Review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Serrano, A.; Ros, G.; Nieto, G. Regulation of Inflammatory Response and the Production of Reactive Oxygen Species by a Functional Cooked Ham Reformulated with Natural Antioxidants in a Macrophage Immunity Model. Antioxidants 2019, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; da Silva Barretto, A.C. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural Antioxidants Used in Meat Products: A Brief Review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Avellaneda, A.; Gironés-Vilaplana, A.; Nieto, G.; Ros-Berruezo, G. Anti-Inflammatory and Antioxidant Effects of Regular Consumption of Cooked Ham Enriched with Dietary Phenolics in Diet-Induced Obese Mice. Antioxidants 2020, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Martínez-Zamora, L.; Peñalver, R.; Marín-Iniesta, F.; Taboada-Rodríguez, A.; López-Gómez, A.; Martínez-Hernández, G.B. Applications of Plant Bioactive Compounds as Replacers of Synthetic Additives in the Food Industry. Foods 2023, 13, 47. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive Overview of the Quality of Plant- and Animal-Sourced Proteins Based on the Digestible Indispensable Amino Acid Score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- FAO, Food and Agricultural Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition Report of an FAO Expert Consultation; FAO: Rome, Italy, 2013. [Google Scholar]
- Bailey, H.M.; Mathai, J.K.; Berg, E.P.; Stein, H.H. Most Meat Products Have Digestible Indispensable Amino Acid Scores That Are Greater than 100, but Processing May Increase or Reduce Protein Quality. Br. J. Nutr. 2020, 124, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Fulgoni, V.L., 3rd. Association of Pork (All Pork, Fresh Pork and Processed Pork) Consumption with Nutrient Intakes and Adequacy in US Children (Age 2–18 Years) and Adults (Age 19+ Years): NHANES 2011–2018 Analysis. Nutrients 2023, 15, 2293. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Domaradzki, P.; Niedzielak, M.; Stadnik, J. Nutritional Value and Physicochemical Properties of Male and Female Broad-Breasted Bronze Turkey Muscle. Foods 2024, 13, 1369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zheng, B.; Zhang, Y.; Zeng, H. Food Allergy and Gut Microbiota. Trends Food Sci. Technol. 2023, 140, 104141. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, K.; Wu, H.; Zhang, Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules 2023, 28, 631. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, J.; Chen, F. Effect of Domestic Cooking on Rice Protein Digestibility. Food Sci. Nutr. 2019, 7, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef] [PubMed]
- Madrid, J.; Martínez, S.; López, C.; Orengo, J.; López, M.J.; Hernández, F. Effects of Low Protein Diets on Growth Performance, Carcass Traits and Ammonia Emission of Barrows and Gilts. Anim. Prod. Sci. 2013, 53, 146–153. [Google Scholar] [CrossRef]
- Morales de León, J.; Bourges, H.; Camacho, M.E. Amino Acid Composition of Some Mexican Foods. Arch. Latinoam. Nutr. 2005, 55, 172–186. [Google Scholar] [PubMed]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High Antioxidant Action and Prebiotic Activity of Hydrolyzed Spent Coffee Grounds (HSCG) in a Simulated Digestion–Fermentation Model: Toward the Development of a Novel Food Supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewski, M.; Tretyn, A. Generating Amplicon Reads for Microbial Community Assessment with Next-generation Sequencing. J. Appl. Microbiol. 2020, 128, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A Novel Approach for Accurate Taxonomic Classification of Microbiome Sequences. Microbiome 2018, 6, 140. [Google Scholar] [CrossRef]
- Allard, G.; Ryan, F.J.; Jeffery, I.B.; Claesson, M.J. SPINGO: A Rapid Species-Classifier for Microbial Amplicon Sequences. BMC Bioinform. 2015, 16, 324. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Gardner, C.D.; Hartle, J.C.; Garrett, R.D.; Offringa, L.C.; Wasserman, A.S. Maximizing the Intersection of Human Health and the Health of the Environment with Regard to the Amount and Type of Protein Produced and Consumed in the United States. Nutr. Rev. 2019, 77, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.A.; Bechtel, P.J.; Hernot, D.C.; Parsons, C.M.; Swanson, K.S.; Smiley, S.; Fahey, G.C. Protein Digestibility Evaluations of Meat and Fish Substrates Using Laboratory, Avian, and Ileally Cannulated Dog Assays. J. Anim. Sci. 2010, 88, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of Oxidation on in Vitro Digestibility of Skeletal Muscle Myofibrillar Proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef] [PubMed]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Gao, S.; Fu, Z.; Zhang, L.; Li, B.; Tan, Y.; Hong, H.; Luo, Y. Oxidation and Side-Chain Modifications Decrease Gastrointestinal Digestibility and Transport of Proteins from Salted Bighead Carp Fillets after Frozen Storage. Food Chem. 2023, 428, 136747. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Zou, X.; He, J.; Xu, X.; Zhou, G.; Li, C. In Vitro Protein Digestibility of Pork Products Is Affected by the Method of Processing. Food Res. Int. 2017, 92, 88–94. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Liu, X.; Zhang, Y.; Zhao, K.; Zhang, K.; Wang, W. Effects of Different Cooking Methods on Physicochemical, Textural Properties of Yak Meat and Its Changes with Intramuscular Connective Tissue during in Vitro Digestion. Food Chem. 2023, 422, 136188. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Kumar, S.; Bhat, H.F.; Aadil, R.M.; Bekhit, A.E.D.A. Ultrasonication as an Emerging Technology for Processing of Animal Derived Foods: A Focus on in Vitro Protein Digestibility: Ultrasound and Animal Proteins. Trends Food Sci. Technol. 2022, 124, 309–322. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Yong, H.I.; Choi, Y.-S.; Jung, S. Comparison of the in Vitro Protein Digestibility of Protaetia Brevitarsis Larvae and Beef Loin before and after Defatting. Food Chem. 2021, 338, 128073. [Google Scholar] [CrossRef]
- Xue, S.; Wang, C.; Kim, Y.H.B.; Bian, G.; Han, M.; Xu, X.; Zhou, G. Application of High-Pressure Treatment Improves the in Vitro Protein Digestibility of Gel-Based Meat Product. Food Chem. 2020, 306, 125602. [Google Scholar] [CrossRef]
- Bailey, H.M.; Stein, H.H. Can the Digestible Indispensable Amino Acid Score Methodology Decrease Protein Malnutrition. Anim. Front. 2019, 9, 18–23. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, C.; Zhao, D.; Zhou, C.; Li, C. Long-Term Intake of Pork Meat Proteins Altered the Composition of Gut Microbiota and Host-Derived Proteins in the Gut Contents of Mice. Mol. Nutr. Food Res. 2020, 64, e2000291. [Google Scholar] [CrossRef]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of Short-Term Dietary Intervention and Probiotic Mix Supplementation on the Gut Microbiota of Elderly Obese Women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef]
- Qin, P.; Zou, Y.; Dai, Y.; Luo, G.; Zhang, X.; Xiao, L. Characterization a Novel Butyric Acid-Producing Bacterium Collinsella Aerofaciens Subsp. Shenzhenensis Subsp. Nov. Microorganisms 2019, 7, 78. [Google Scholar] [CrossRef]
- Bag, S.; Ghosh, T.S.; Das, B. Complete Genome Sequence of Collinsella Aerofaciens Isolated from the Gut of a Healthy Indian Subject. Genome Announc. 2017, 5, e01361-17. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a Key Member of the Human Gut Microbiota in the Production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Derrien, M.; Turroni, F.; Ventura, M.; van Sinderen, D. Insights into Endogenous Bifidobacterium Species in the Human Gut Microbiota during Adulthood. Trends Microbiol. 2022, 30, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of Food Additives on the Composition and Function of Gut Microbiota: A Review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Gasbarrini, A.; Mele, M.C. Food Additives, Gut Microbiota, and Irritable Bowel Syndrome: A Hidden Track. Int. J. Environ. Res. Public Health 2020, 17, 8816. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The Impact of Food Additives, Artificial Sweeteners and Domestic Hygiene Products on the Human Gut Microbiome and Its Fibre Fermentation Capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-Induced Colitis Is Associated with Decreased Population of Anti-Inflammatory Bacterium, Akkermansia muciniphila, in the Gut Microbiota of C57BL/6J Mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- López-Moreno, A.; Suárez, A.; Avanzi, C.; Monteoliva-Sánchez, M.; Aguilera, M. Probiotic Strains and Intervention Total Doses for Modulating Obesity-Related Microbiota Dysbiosis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1921. [Google Scholar] [CrossRef]
- Qiu, K.; Zhang, X.; Jiao, N.; Xu, D.; Huang, C.; Wang, Y.; Yin, J. Dietary Protein Level Affects Nutrient Digestibility and Ileal Microbiota Structure in Growing Pigs. Anim. Sci. J. 2018, 89, 537–546. [Google Scholar] [CrossRef]


| ST | RT | SH | RH | |
|---|---|---|---|---|
| Energy value (KJ/Kcal/100 g) | 292/69 | 379/89 | 387/92 | 405/95.7 |
| Fats (%) | 1 | 0.7 | 1.5 | 1.5 |
| Saturated fats (%) | 0.3 | 0.2 | 0.5 | 0.5 |
| Carbohydrates (%) | 0.5 | 0.5 | 1.5 | 1.0 |
| Sugars (%) | 0.5 | 0.3 | 1.3 | 0.4 |
| Proteins (%) | 14.5 | 20.2 | 18.0 | 20.0 |
| Salt (%) | 1.8 | 1.6 | 1.9 | 1.7 |
| Dietary fibre (%) | 0 | 0.3 | 0 | 0.3 |
| Antioxidant capacity (FRAP (µM Trolox/100 g)) | 1199 | 1700 | 1019 | 1741 |
| Total polyphenols (mg gallic acid/100 g) | 172 | 403 | 223 | 342 |
| ST | RT | SH | RH | |
|---|---|---|---|---|
| Dispensable AA | ||||
| 0.83 ± 0.14 | 0.88 ± 0.21 | 0.79 ± 0.12 | 0.98 ± 0.18 |
| 0.49 ± 0.01 | 0.51 ± 0.00 | 0.51 ± 0.04 | 0.54 ± 0.01 |
| 1.33 ± 0.19 | 1.42 ± 0.29 | 1.32 ± 0.20 | 1.58 ± 0.23 |
| 0.53 ± 0.02 | 0.66 ± 0.02 | 0.92 ± 0.05 | 0.70 ± 0.07 |
| 0.84 ± 0.04 | 0.98 ± 0.02 | 0.97 ± 0.02 | 1.02 ± 0.05 |
| 0.50 ± 0.03 | 0.61 ± 0.03 | 0.60 ± 0.06 | 0.66 ± 0.10 |
| 0.42 ± 0.03 | 0.46 ± 0.02 | 0.62 ± 0.03 | 0.52 ± 0.07 |
| Total 1 | 4.93 ± 0.34 | 5.51 ± 0.52 | 5.73 ± 0.58 | 6.01 ± 0.24 |
| (49%) | (49%) | (49%) | (49%) | |
| Indispensable AA | ||||
| 0.44 ± 0.05 | 0.44 ± 0.09 | 0.52 ± 0.07 | 0.54 ± 0.07 |
| 0.54 ± 0.03 | 0.56 ± 0.21 | 0.61 ± 0.36 | 0.62 ± 0.14 |
| 0.63 ± 0.01 | 0.80 ± 0.01 | 0.63 ± 0.04 | 0.87 ± 0.04 |
| 0.65 ± 0.01 | 0.54 ± 0.06 | 0.71 ± 0.10 | 0.59 ± 0.04 |
| 0.51 ± 0.08 | 0.42 ± 0.02 | 0.57 ± 0.03 | 0.46 ± 0.14 |
| 0.35 ± 0.01 | 0.67 ± 0.02 | 0.64 ± 0.05 | 0.64 ± 0.00 |
| 0.13 ± 0.14 | 0.21 ± 0.19 | 0.20 ± 0.11 | 0.20 ± 0.19 |
| 0.21 ± 0.01 | 0.23 ± 0.04 | 0.22 ± 0.05 | 0.23 ± 0.01 |
| 0.48 ± 0.01 | 0.50 ± 0.03 | 0.50 ± 0.08 | 0.55 ± 0.03 |
| 0.80 ± 0.09 | 0.86 ± 0.03 | 0.86 ± 0.04 | 0.94 ± 0.13 |
| 0.47 ± 0.01 | 0.50 ± 0.02 | 0.51 ± 0.01 | 0.57 ± 0.02 |
| Total IAA 2 | 5.20 ± 0.11 | 5.73 ± 0.09 | 5.97 ± 0.84 | 6.21 ± 0.07 |
| (51%) | (51%) | (51%) | (51%) | |
| Total BCAA 3 | 1.74 ± 0.01 | 1.86 ± 0.09 | 1.87 ± 0.17 | 2.06 ± 0.03 |
| (17%) | (17%) | (16%) | (17%) | |
| Total AA 4 | 10.14 ± 0.23 | 11.24 ± 0.43 | 11.70 ± 1.43 | 12.22 ± 0.17 |
| Meat Product | DIAAS (%) | Limiting IAA 1 | p-Value 2 |
|---|---|---|---|
| ST | 66.68 ± 4.39 | SAA (Met + Cys) | 0.001 |
| RT | 86.24 ± 3.24 | Val | |
| SH | 57.14 ± 24.59 | SAA (Met + Cys) | 0.037 |
| RH | 90.05 ± 0.91 | Val |
| Blank | Cooked Turkey Breast | Cooked Ham | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Reformulated | p-Value 1 | Control | Reformulated | p-Value 1 | |||
| Normal-Weight Group | ||||||||
| Genus | ||||||||
| Collinsella | 1.62 ± 0.24 | 1.6 ± 0.08 | 2.56 ± 0.23 | 0.019 | 1.77 ± 0.09 | 2.88 ± 0.23 | 0.011 | |
| OTU | ||||||||
| Otu_73 | Dorea longicatena | 0.08 ± 0.07 | 0.10 ± 0.08 | 0.30 ± 0.03 | 0.08 ± 0.03 | 0.44 ± 0.03 | 0.006 | |
| Otu_123 | Collinsella aerofaciens | 0.33 ± 0.02 | 0.03 ± 0.00 | 0.62 ± 0.03 | 0.001 | 0.04 ± 0.00 | 1.07 ± 0.07 | 0.002 |
| Otu_173 | Blautia obeum | 0.83 ± 0.06 | 0.66 ± 0.04 | 0.49 ± 0.01 | 0.022 | 0.62 ± 0.27 | 0.70 ± 0.15 | |
| Otu_228 | Butyricimonas paravirosa | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.19 ± 0.02 | 0.031 | 0.18 ± 0.03 | 0.13 ± 0.03 | |
| Obese group | ||||||||
| Phylo | ||||||||
| Firmicutes | 52.55 ± 5.25 | 37.93 ± 2.76 | 49.20 ± 0.47 | 0.030 | 39.94 ± 5.69 | 51.67 ± 1.04 | ||
| Actinobacteria | 12.33 ± 0.33 | 19.60 ± 0.44 | 11.24 ± 0.04 | 0.001 | 18.04 ± 0.20 | 10.81 ± 1.58 | 0.023 | |
| Genus | ||||||||
| Adlercreutzia | 1.00 ± 0.14 | 0.61 ± 0.04 | 1.30 ± 0.12 | 0.014 | 0.82 ± 0.08 | 0.97 ± 0.19 | ||
| Bifidobacterium | 10.24 ± 0.02 | 18.02 ± 0.28 | 8.66 ± 0.20 | <0.001 | 15.98 ±0.35 | 8.08 ± 0.53 | <0.001 | |
| Parabacteroides | 3.52 ± 0.20 | 2.38 ± 0.22 | 1.87 ± 0.11 | 2.50 ± 0.35 | 1.43 ± 0.09 | 0.021 | ||
| OTU | ||||||||
| Otu_38 | Bifidobacterium adolescentis | 4.02 ± 6.33 | 12.40 ± 0.26 | 3.20 ± 0.11 | <0.001 | 9.76 ± 0.09 | 2.80 ± 0.23 | 0.001 |
| Otu_74 | Adlercreutzia equolifaciens | 1.00 ± 0.33 | 0.61 ± 0.04 | 1.30 ± 0.12 | 0.017 | 0.82 ± 0.08 | 0.97 ± 0.19 | |
| Otu_83 | Bifidobacterium bifidum | 0.14 ± 0.04 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.00 | 0.09 ± 0.00 | 0.029 | |
| Otu_140 | Bacteroides eggerthii | 0.38 ± 0.06 | 0.41 ± 0.07 | 0.30 ± 0.05 | 0.49 ± 0.05 | 0.29 ± 0.03 | 0.040 | |
| Otu_154 | Eggerthella lenta | 0.04 ± 0.10 | 0.18 ± 0.00 | 0.27 ± 0.02 | 0.031 | 0.25 ± 0.02 | 0.25 ± 0.01 | |
| Otu_183 | Bacteroides nordii | 0.17 ± 0.06 | 0.12 ± 0.01 | 0.22 ± 0.00 | 0.003 | 0.15 ± 0.02 | 0.15 ± 0.02 | |
| Otu_228 | Butyricimonas paravirosa | 0.49 ± 0.01 | 0.44 ± 0.13 | 0.50 ± 0.01 | 0.68 ± 0.04 | 0.52 ± 0.00 | 0.037 | |
| Acetic Acid | Propionic Acid | Butyric Acid | Total SCFA | |
|---|---|---|---|---|
| Normal-weight group | ||||
| Control | 20.60 ± 0.78 b | 10.38 ± 0.85 b | 4.10 ± 1.64 a | 35.08 ± 1.71 b |
| ST | 24.65 ± 0.82 b | 12.07 ± 0.76 ab | 3.92 ± 0.22 a | 40.63 ± 1.81 b |
| RT | 34.13 ± 5.62 a | 14.80 ± 0.28 a | 4.10 ± 0.46 a | 53.03 ± 6.36 a |
| SH | 25.71 ± 4.53 ab | 5.72 ± 2.00 c | 3.60 ± 0.40 a | 35.03 ± 6.94 b |
| RH | 26.46 ± 1.03 ab | 12.32 ± 1.54 ab | 4.13 ± 0.46 a | 42.90 ± 3.03 ab |
| Obese group | ||||
| Control | 18.66 ± 1.49 c | 7.38 ± 5.78 a | 2.79 ± 1.02 a | 28.83 ± 8.29 a |
| ST | 21.30 ± 0.43 c | 3.46 ± 4.89 a | 4.71 ± 2.07 a | 29.47 ± 3.25 a |
| RT | 31.65 ± 2.16 a | 1.31 ± 1.85 a | 3.17 ± 0.60 a | 36.13 ± 4.61 a |
| SH | 25.41 ± 1.36 b | 1.67 ± 1.14 a | 2.30 ± 0.76 a | 29.39 ± 1.75 a |
| RH | 27.84 ± 0.57 b | 2.49 ± 0.36 a | 3.78 ± 2.54 a | 34.11 ± 1.61 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuso, P.; Quizhpe, J.; Yepes, F.; Miranzo, D.; Avellaneda, A.; Nieto, G.; Ros, G. Improving the Nutritional Quality of Protein and Microbiota Effects in Additive- and Allergen-Free Cooked Meat Products. Foods 2024, 13, 1792. https://doi.org/10.3390/foods13121792
Ayuso P, Quizhpe J, Yepes F, Miranzo D, Avellaneda A, Nieto G, Ros G. Improving the Nutritional Quality of Protein and Microbiota Effects in Additive- and Allergen-Free Cooked Meat Products. Foods. 2024; 13(12):1792. https://doi.org/10.3390/foods13121792
Chicago/Turabian StyleAyuso, Pablo, Jhazmin Quizhpe, Fani Yepes, Domingo Miranzo, Antonio Avellaneda, Gema Nieto, and Gaspar Ros. 2024. "Improving the Nutritional Quality of Protein and Microbiota Effects in Additive- and Allergen-Free Cooked Meat Products" Foods 13, no. 12: 1792. https://doi.org/10.3390/foods13121792
APA StyleAyuso, P., Quizhpe, J., Yepes, F., Miranzo, D., Avellaneda, A., Nieto, G., & Ros, G. (2024). Improving the Nutritional Quality of Protein and Microbiota Effects in Additive- and Allergen-Free Cooked Meat Products. Foods, 13(12), 1792. https://doi.org/10.3390/foods13121792









