Silicon as a Functional Meat Ingredient Improves Jejunal and Hepatic Cholesterol Homeostasis in a Late-Stage Type 2 Diabetes Mellitus Rat Model
Abstract
:1. Introduction
2. Materials and Methods
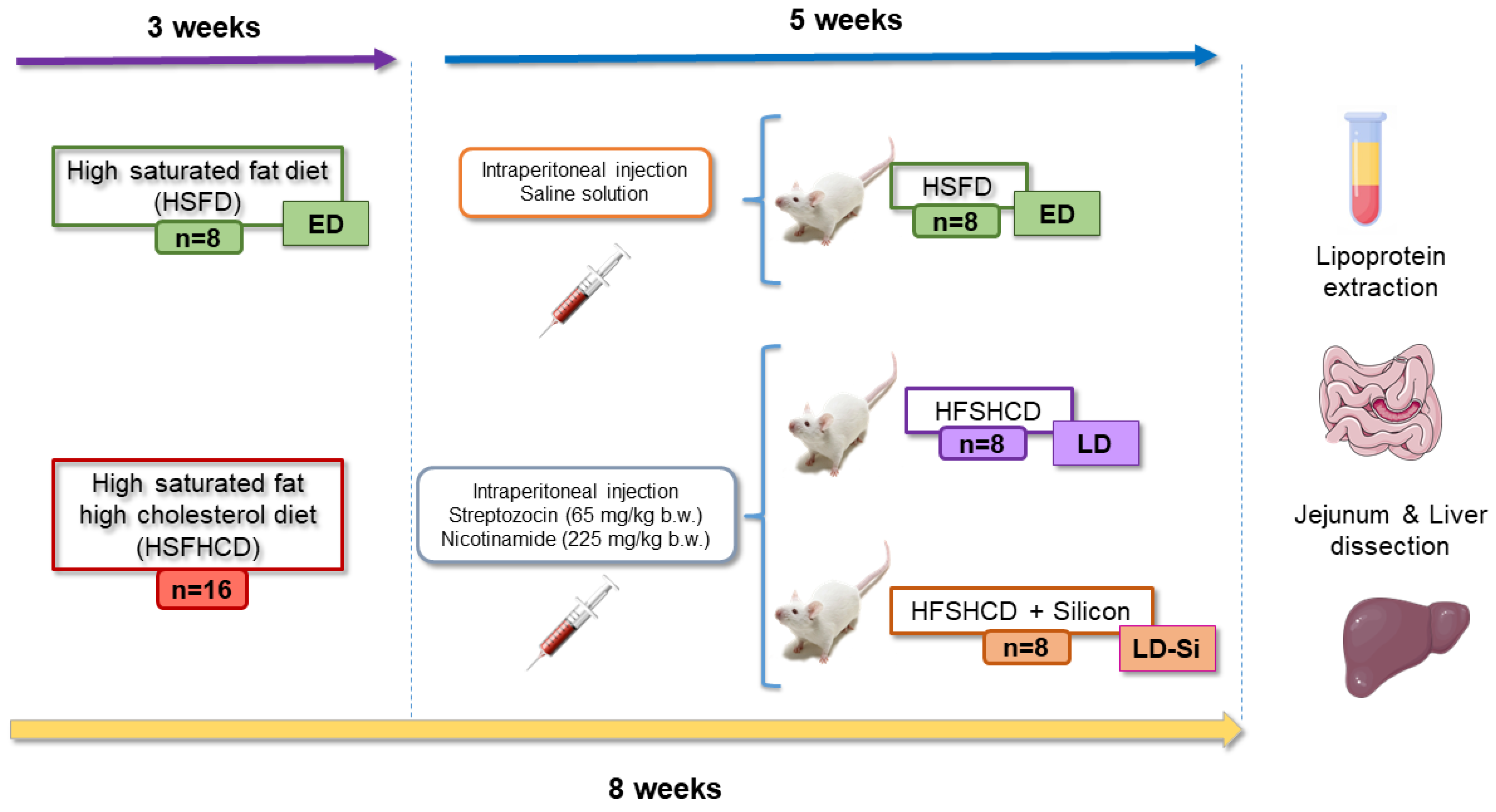
2.1. Diets, Animal Model, and Experimental Design
2.2. Plasma Glucose and Insulin Level Determinations
2.3. Plasma Lipoprotein Cholesterol Determinations
2.4. Fecal Fat Analyses
2.5. Histological Procedure
2.6. Immunohistochemical Staining
2.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.8. Western-Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Feed Intake, Ponderal Parameters, Total Feces, and Fat Excretion
3.2. Plasma Concentrations of Glucose, Insulin, HOMA-IR, Total Cholesterol, Triglycerides, VLDLtg, VLDLc, LDLc, IDLc, HDLc, and Atherogenic Index
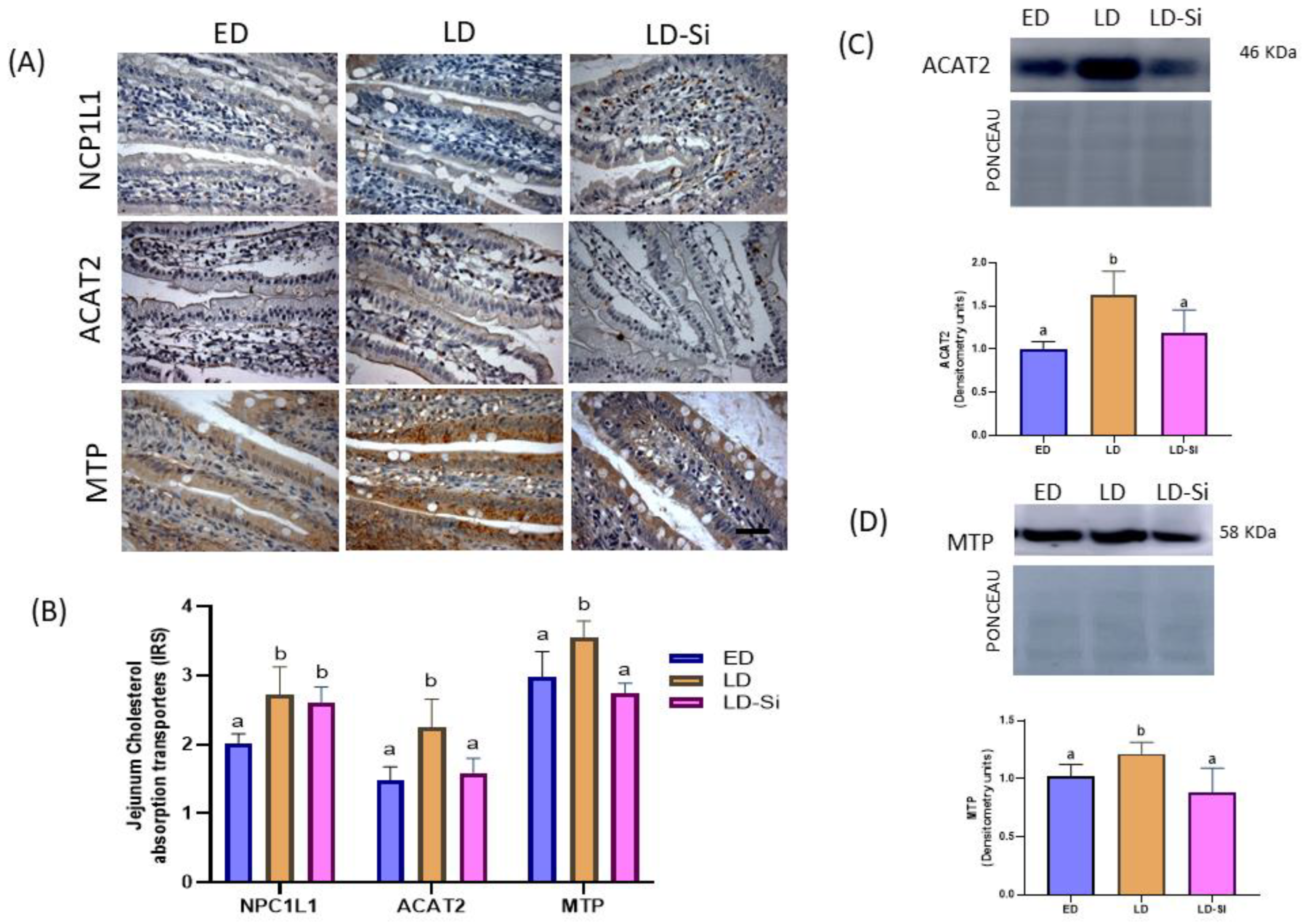
3.3. Molecular Markers of Jejunal Cholesterol Absorption
3.4. Cholesterol Efflux, Transintestinal Cholesterol Excretion (TICE), and Jejunal ABCA1 Levels
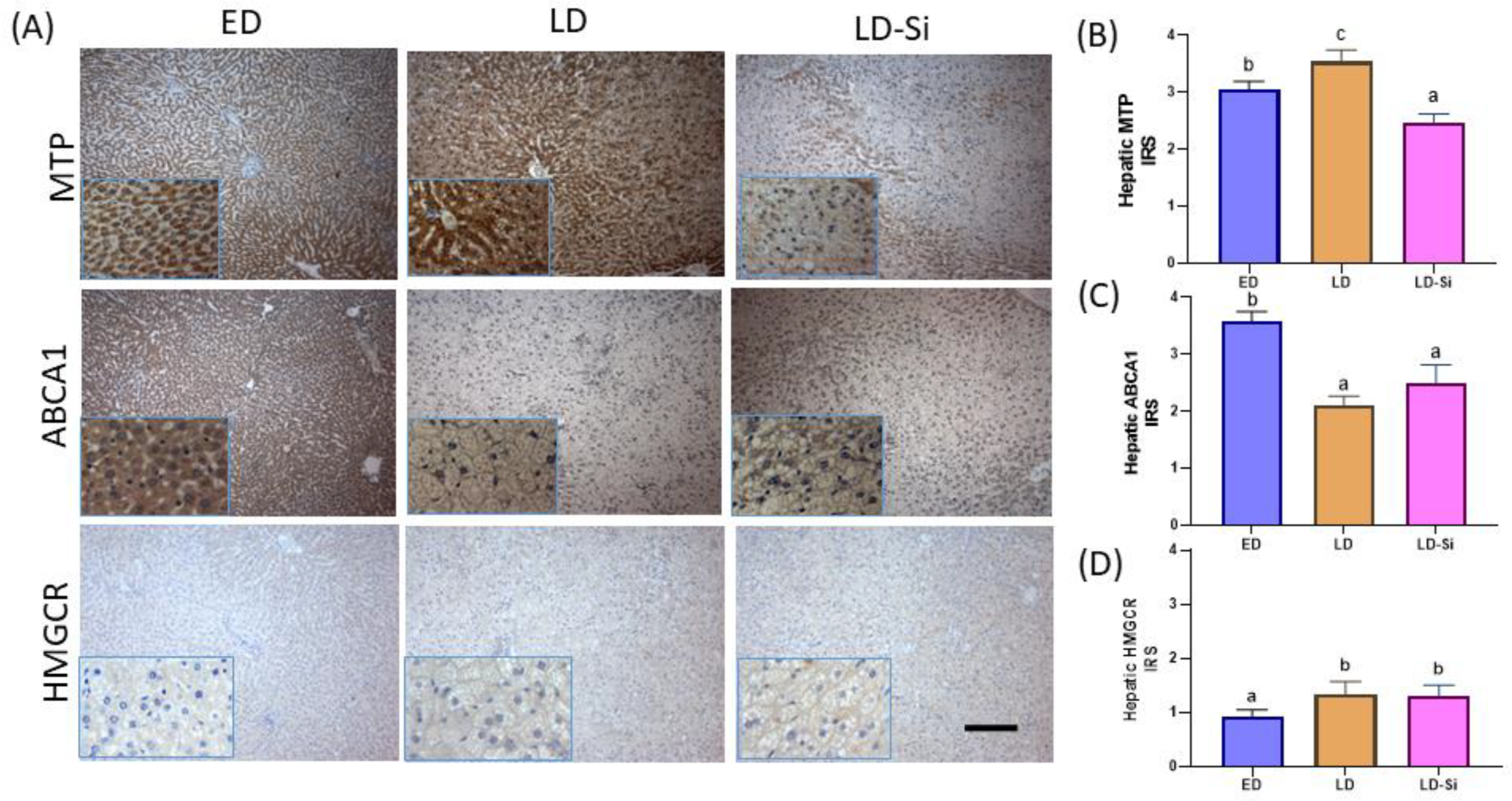
3.5. Hepatic MTP, ABCA1, and HMGCR Levels
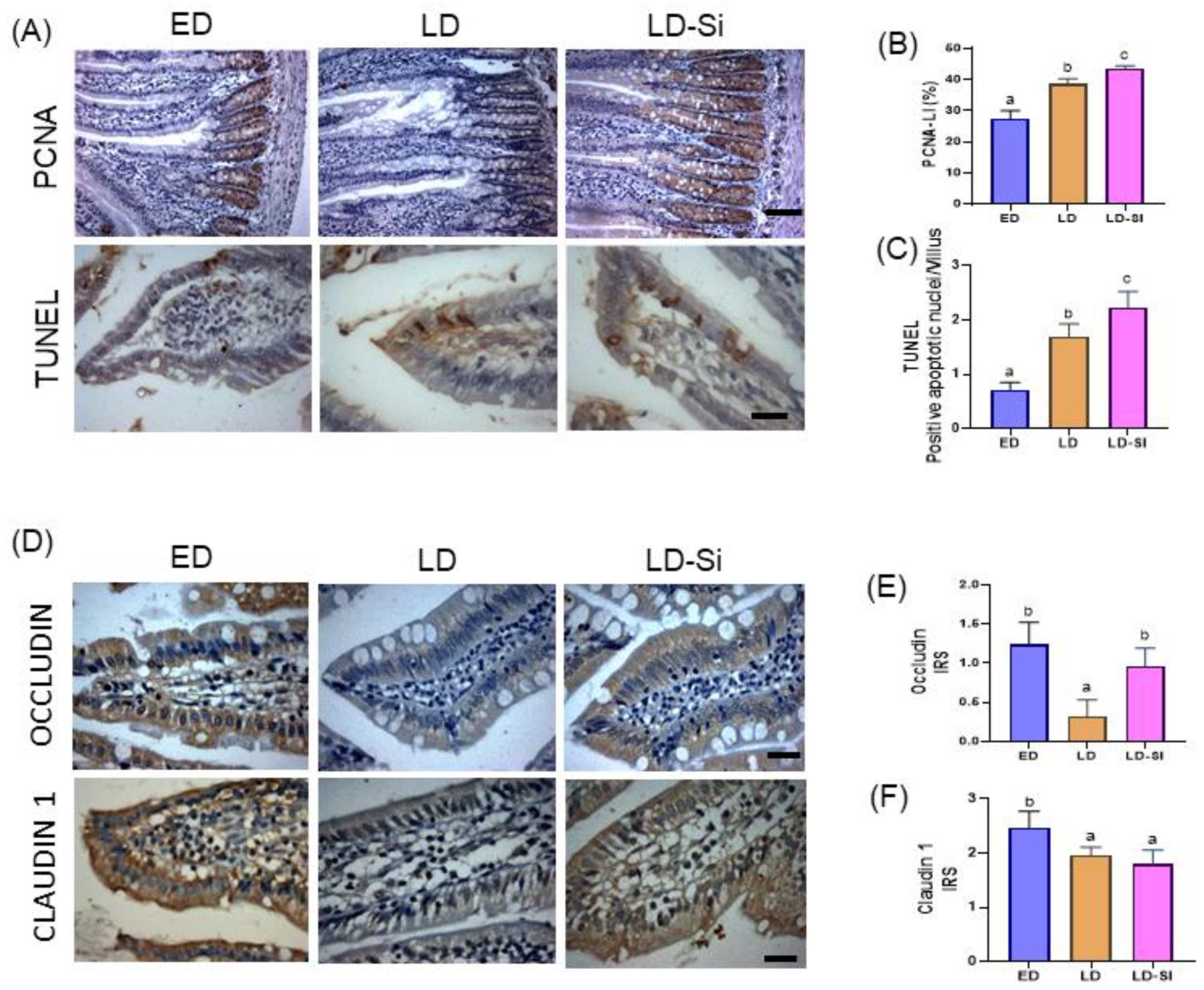
3.6. Morphometric Parameters of the Absorptive Area, Epithelium Turnover, Differentiation, and Barrier Integrity of the Jejunum
3.7. Heatmap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Mbue, N.D.; Mbue, J.E.; Anderson, J.A. Management of Lipids in Patients with Diabetes. Nurs. Clin. N. Am. 2017, 52, 605–619. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025, 9th ed. Available online: https://www.dietaryguidelines.gov/ (accessed on 12 February 2024).
- Arrieta, F.; Iglesias, P.; Pedro-Botet, J.; Becerra, A.; Ortega, E.; Obaya, J.C.; Nubiola, A.; Maldonado, G.F.; Campos, M.d.M.; Petrecca, R.; et al. Diabetes Mellitus and Cardiovascular Risk: Update of the Recommendations of the Diabetes and Cardiovascular Disease Working Group of the Spanish Diabetes Society. Clin. Investig. Arter. 2018, 30, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Pascual, V.; Díaz, J.L.; Millán Nuñez-Cortés, J.; Pérez-Martínez, P. Nutritional Recommendations in the Prevention and Treatment of Atherogenic Dyslipidemia. Clin. Investig. Arter. 2023, 35, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gant, C.M.; Binnenmars, S.H.; Harmelink, M.; Soedamah-Muthu, S.S.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Real-Life Achievement of Lipid-Lowering Treatment Targets in the DIAbetes and LifEstyle Cohort Twente: Systemic Assessment of Pharmacological and Nutritional Factors. Nutr. Diabetes 2018, 8, 24. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional Foods and Dietary Supplements for the Management of Dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional Foods for Dyslipidaemia and Cardiovascular Risk Prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef]
- Jain, A.; Rani, V. Food and Cardiac Health: Protective Effects of Food on Cardiovascular System. In Exploring the Nutrition and Health Benefits of Functional Foods; Shekhar, H.U., Howlader, Z.H., Kabir, Y., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 1–15. ISBN 9781522505921. [Google Scholar]
- Jesch, E.D.; Carr, T.P. Food Ingredients That Inhibit Cholesterol Absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of Cholesterol Homeostasis in Health and Diseases: From Mechanisms to Targeted Therapeutics. Signal Transduct. Target Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Timothy Garvey, W.; Karen Lau, K.H.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Wolk, A. Potential Health Hazards of Eating Red Meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Basiak-Rasała, A.; Różańska, D.; Zatońska, K. Food Groups in Dietary Prevention of Type 2 Diabetes. Rocz. Panstw. Zakl. Hig. 2019, 70, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Prasow, M.; Domaradzki, P.; Litwińczuk, A.; Kowalczyk, M. Bioactive Compounds in Meat and Their Importance in Human Nutrition. Medycyna Ogólna i Nauki o Zdrowiu 2019, 25, 170–180. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Carrascal, J.R.; Bügel, S.; Astrup, A. Meat and Human Health—Current Knowledge and Research Gaps. Foods 2021, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Park, Y. Healthier Meat Products as Functional Foods. Meat Sci. 2010, 86, 49–55. [Google Scholar] [CrossRef]
- Macho-González, A.; Bastida, S.; Garcimartín, A.; López-Oliva, M.E.; González, P.; Benedí, J.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Functional Meat Products as Oxidative Stress Modulators: A Review. Adv. Nutr. 2021, 12, 1514–1539. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Ahmad, S.; Azad, Z.R.A.A.; Adnan, M.; Ashraf, S.A. Incorporating Dietary Fiber from Fruit and Vegetable Waste in Meat Products: A Systematic Approach for Sustainable Meat Processing and Improving the Functional, Nutritional and Health Attributes. PeerJ 2023, 11, 14977. [Google Scholar] [CrossRef]
- Garcimartín, A.; López-Oliva, M.E.; Macho-González, A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Hypoglycaemic and Hypotriglyceridaemic Postprandial Properties of Organic Silicon. J. Funct. Foods 2017, 29, 290–294. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; González-Muñoz, M.J.; Macho-González, A.; Benedi, J.; Garcimartín, A.; López-Oliva, E.; Santos-López, J.A.; Bastida, S. When Silicon Transmutes in Gold. J. Negat. No Posit. Results 2020, 5, 202–211. [Google Scholar] [CrossRef]
- Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Effects of Silicon Inclusion in Restructured Meat-Enriched Diet on Lipoprotein Profile and Composition in Aged Wistar Rats. Atherosclerosis 2014, 235, e202–e203. [Google Scholar] [CrossRef]
- Garcimartín, A.; Santos-López, J.A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-Enriched Restructured Pork Affects the Lipoprotein Profile, VLDL Oxidation, and LDL Receptor Gene Expression in Aged Rats Fed an Atherogenic Diet. J. Nutr. 2015, 145, 2039–2045. [Google Scholar] [CrossRef]
- Santos-López, J.A.; Garcimartín, A.; Merino, P.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Effects of Silicon vs. Hydroxytyrosol-Enriched Restructured Pork on Liver Oxidation Status of Aged Rats Fed High-Saturated/High-Cholesterol Diets. PLoS ONE 2016, 11, 0147469. [Google Scholar] [CrossRef]
- Hernández-Martín, M.; Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bocanegra, A.; Redondo-Castillejo, R.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-Enriched Meat Positively Improves Plasma Lipidaemia and Lipoproteinaemia, LDLr, and Insulin Capability and the Signalling Pathway Induced by an Atherogenic Diet in Late-Stage Type 2 Diabetes Mellitus Rats. Food Funct. 2024, 15, 1513–1526. [Google Scholar] [CrossRef]
- Hernández-Martín, M.; Bocanegra, A.; Redondo-Castillejo, R.; Macho-González, A.; Sánchez-Muniz, F.J.; Benedí, J.; Bastida, S.; García-Fernández, R.A.; Garcimartín, A.; López-Oliva, M.E. Could Duodenal Molecular Mechanisms Be Involved in the Hypocholesterolemic Effect of Silicon Used as Functional Ingredient in Late-Stage Type 2 Diabetes Mellitus? Mol. Nutr. Food Res. 2022, 66, 2200104. [Google Scholar] [CrossRef] [PubMed]
- Schultz Moreira, A.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Benedi, J.; Sánchez-Muniz, F.J. Wakame and Nori in Restructured Meats Included in Cholesterol-Enriched Diets Affect the Antioxidant Enzyme Gene Expressions and Activities in Wistar Rats. Plant Foods Hum. Nutr. 2010, 65, 290–298. [Google Scholar] [CrossRef]
- Terpstra, A.H.M.; Woodward, C.J.H.; Sanchez-Muniz, F.J. Improved Techniques for the Separation of Serum Lipoproteins by Density Gradient Ultracentrifugation: Visualization by Prestaining and Rapid Separation of Serum Lipoproteins from Small Volumes of Serum. Anal. Biochem. 1981, 111, 149–157. [Google Scholar] [CrossRef]
- Olivero-David, R.; Schultz-Moreira, A.; Vázquez-Velasco, M.; González-Torres, L.; Bastida, S.; Benedé-, J.; Isabel Sanchez-Reus, M.; José González-Muñoz, M.; Sánchez-Muniz, F.J. Effects of Nori- and Wakame-Enriched Meats with or without Supplementary Cholesterol on Arylesterase Activity, Lipaemia and Lipoproteinaemia in Growing Wistar Rats. Br. J. Nutr. 2011, 106, 1476–1486. [Google Scholar] [CrossRef]
- Olivero David, R.; Sánchez-Muniz, F.J.; Bastida, S.; Benedi, J.; González-Muñoz, M.J. Gastric Emptying and Short-Term Digestibility of Thermally Oxidized Sunflower Oil Used for Frying in Fasted and Nonfasted Rats. J. Agric. Food Chem. 2010, 58, 9242–9248. [Google Scholar] [CrossRef]
- Macho-González, A.; López-Oliva, M.E.; Merino, J.J.; García-Fernández, R.A.; Garcimartín, A.; Redondo-Castillejo, R.; Bastida, S.; Sánchez-Muniz, F.J.; Benedí, J. Carob Fruit Extract-Enriched Meat Improves Pancreatic Beta-Cell Dysfunction, Hepatic Insulin Signaling and Lipogenesis in Late-Stage Type 2 Diabetes Mellitus Model. J. Nutr. Biochem. 2020, 84, 108461. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. Beta-Cell ABCA1 Influences Insulin Secretion, Glucose Homeostasis and Response to Thiazolidinedione Treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and Nutritional Overview of Diet-Induced Metabolic Syndrome Models in Rats: What Is the Best Choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Podrini, C.; Cambridge, E.L.; Lelliott, C.J.; Carragher, D.M.; Estabel, J.; Gerdin, A.K.; Karp, N.A.; Scudamore, C.L.; Ramirez-Solis, R.; White, J.K. High-Fat Feeding Rapidly Induces Obesity and Lipid Derangements in C57BL/6N Mice. Mamm. Genome 2013, 24, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Boutjdir, M.; Rudel, L.L.; Hussain, M.M. Intestine-Specific MTP and Global ACAT2 Deficiency Lowers Acute Cholesterol Absorption with Chylomicrons and HDLs. J. Lipid Res. 2014, 55, 2261–2275. [Google Scholar] [CrossRef]
- Tomkin, G.; Owens, D. Dyslipidaemia of Diabetes and the Intestine. World J. Diabetes 2015, 6, 970–977. [Google Scholar] [CrossRef]
- Lema, I.; Araújo, J.R.; Rolhion, N.; Demignot, S. Jejunum: The Understudied Meeting Place of Dietary Lipids and the Microbiota. Biochimie 2020, 178, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Clara, R.; Schumacher, M.; Ramachandran, D.; Fedele, S.; Krieger, J.P.; Langhans, W.; Mansouri, A. Metabolic Adaptation of the Small Intestine to Short- and Medium-Term High-Fat Diet Exposure. J. Cell Physiol. 2017, 232, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ravid, Z.; Bendayan, M.; Delvin, E.; Sane, A.T.; Elchebly, M.; Lafond, J.; Lambert, M.; Mailhot, G.; Levy, E. Modulation of Intestinal Cholesterol Absorption by High Glucose Levels: Impact on Cholesterol Transporters, Regulatory Enzymes, and Transcription Factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 873–885. [Google Scholar] [CrossRef]
- Mao, J.; Hu, X.; Xiao, Y.; Yang, C.; Ding, Y.; Hou, N.; Wang, J.; Cheng, H.; Zhang, X. Overnutrition Stimulates Intestinal Epithelium Proliferation through β-Catenin Signaling in Obese Mice. Diabetes 2013, 62, 3736–3746. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, F.; Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Yu, J.; Ding, G. Impact of a High-fat Diet on Intestinal Stem Cells and Epithelial Barrier Function in Middle-aged Female Mice. Mol. Med. Rep. 2020, 21, 1133–1144. [Google Scholar] [CrossRef]
- Dailey, M.J. Nutrient-Induced Intestinal Adaption and Its Effect in Obesity. Physiol. Behav. 2014, 136, 74–78. [Google Scholar] [CrossRef]
- Aliluev, A.; Tritschler, S.; Sterr, M.; Oppenländer, L.; Hinterdobler, J.; Greisle, T.; Irmler, M.; Beckers, J.; Sun, N.; Walch, A.; et al. Diet-Induced Alteration of Intestinal Stem Cell Function Underlies Obesity and Prediabetes in Mice. Nat. Metab. 2021, 3, 1202–1216. [Google Scholar] [CrossRef]
- Lamb, Y.N. Rosuvastatin/Ezetimibe: A Review in Hypercholesterolemia. Am. J. Cardiovasc. Drugs 2020, 20, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ding, X.; Feng, H.; Bi, Y.; Li, Y.; Shan, J.; Bian, H. Excessive Exogenous Cholesterol Activating Intestinal LXRα-ABCA1/G5/G8 Signaling Pathway Can Not Reverse Atherosclerosis in ApoE−/− Mice. Lipids Health Dis. 2023, 22, 51. [Google Scholar] [CrossRef]
- Vergès, B. Intestinal Lipid Absorption and Transport in Type 2 Diabetes. Diabetologia 2022, 65, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.; Xiao, C.; Nahmias, A.; Lewis, G.F. Role of the Gut in Diabetic Dyslipidemia. Front. Endocrinol. 2020, 11, 512985. [Google Scholar] [CrossRef]
- Petit, V.; Arnould, L.; Martin, P.; Monnot, M.C.; Pineau, T.; Besnard, P.; Niot, I. Chronic High-Fat Diet Affects Intestinal Fat Absorption and Postprandial Triglyceride Levels in the Mouse. J. Lipid Res. 2007, 48, 278–287. [Google Scholar] [CrossRef]
- Xu, H.; Xin, Y.; Wang, J.; Liu, Z.; Cao, Y.; Li, W.; Zhou, Y.; Wang, Y.; Liu, P. The TICE Pathway: Mechanisms and Potential Clinical Applications. Curr. Atheroscler. Rep. 2023, 25, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Groen, A.K.; Kuipers, F. Reverse Cholesterol Transport Revisited: Contribution of Biliary versus Intestinal Cholesterol Excretion. Arter. Thromb. Vasc. Biol. 2011, 31, 1726–1733. [Google Scholar] [CrossRef]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef]
- Iqbal, J.; Qarni, A.A.; Hawwari, A. Regulation of Intestinal Cholesterol Absorption: A Disease Perspective. Adv. Biol. Chem. 2017, 7, 60–75. [Google Scholar] [CrossRef]
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velde, A.E.; Vrins, C.L.J.; Van Den Oever, K.; Seemann, I.; Oude Elferink, R.P.J.; Van Eck, M.; Kuipers, F.; Groen, A.K. Regulation of Direct Transintestinal Cholesterol Excretion in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 203–208. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-Medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 331734. [Google Scholar] [CrossRef]
- Kiss, R.S.; McManus, D.C.; Franklin, V.; Tan, W.L.; McKenzie, A.; Chimini, G.; Marcel, Y.L. The Lipidation by Hepatocytes of Human Apolipoprotein A–I Occurs by Both ABCA1-Dependent and -Independent Pathways. J. Biol. Chem. 2003, 278, 10119–10127. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Iqbal, J.; Fievet, C.; Timmins, J.M.; Pape, T.D.; Coburn, B.A.; Bissada, N.; Staels, B.; Groen, A.K.; et al. Intestinal ABCA1 Directly Contributes to HDL Biogenesis in Vivo. J. Clin. Investig. 2006, 116, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, M.H.; Song, Y.; Yee, J.; Song, G.; Gwak, H.S. ABCA1 69C>T Polymorphism and the Risk of Type 2 Diabetes Mellitus: A Systematic Review and Updated Meta-Analysis. Front. Endocrinol. 2021, 12, 639524. [Google Scholar] [CrossRef]
- Jacobo-Albavera, L.; Domínguez-Pérez, M.; Medina-Leyte, D.J.; González-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Fernández-Hernando, C. Regulation of Cholesterol Homeostasis. Cell. Mol. Life Sci. 2012, 69, 915–930. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.D.; Scott, R.A.; et al. HMG-Coenzyme A Reductase Inhibition, Type 2 Diabetes, and Bodyweight: Evidence from Genetic Analysis and Randomised Trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- Lotta, L.A.; Sharp, S.J.; Burgess, S.; Perry, J.R.B.; Stewart, I.D.; Willems, S.M.; Luan, J.; Ardanaz, E.; Arriola, L.; Balkau, B.; et al. Association between Low-Density Lipoprotein Cholesterol-Lowering Genetic Variants and Risk of Type 2 Diabetes: A Meta-Analysis. JAMA 2016, 316, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Borén, J. Overproduction of Very Low-Density Lipoproteins Is the Hallmark of the Dyslipidemia in the Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2008, 28, 1225–1236. [Google Scholar] [CrossRef]
- Hussain, M.M.; Nijstad, N.; Franceschini, L. Regulation of Microsomal Triglyceride Transfer Protein. Clin. Lipidol. 2011, 6, 293–303. [Google Scholar] [CrossRef]
- Choi, S.H.; Ginsberg, H.N. Increased Very Low Density Lipoprotein (VLDL) Secretion, Hepatic Steatosis, and Insulin Resistance. Trends Endocrinol. Metab. 2011, 22, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Hirowatari, Y.; Yoshida, H. Diabetic Dyslipidemia: Evaluation and Mechanism. Glob. Health Med. 2019, 1, 30–35. [Google Scholar] [CrossRef]
- Hori, M.; Satoh, M.; Furukawa, K.; Sakamoto, Y.I.; Hakamata, H.; Komohara, Y.; Takeya, M.; Sasaki, Y.; Miyazaki, A.; Horiuchi, S. Acyl-Coenzyme A:Cholesterol Acyltransferase-2 (ACAT-2) Is Responsible for Elevated Intestinal ACAT Activity in Diabetic Rats. Arter. Thromb. Vasc. Biol. 2004, 24, 1689–1695. [Google Scholar] [CrossRef]
- Iqbal, J.; Jahangir, Z.; Al-Qarni, A.A. Microsomal Triglyceride Transfer Protein: From Lipid Metabolism to Metabolic Diseases. Adv. Exp. Med. Biol. 2020, 1276, 37–52. [Google Scholar] [CrossRef]
- Zeka, K.; Ruparelia, K.; Arroo, R.R.J.; Budriesi, R.; Micucci, M. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases 2017, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Bernardo, M.A.; Singh, J.; de Mesquita, M.F. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.E.; Attie, A.D.; Biddinger, S.B. The Regulation of ApoB Metabolism by Insulin. Trends Endocrinol. Metab. 2013, 24, 391–397. [Google Scholar] [CrossRef]
- Sparks, J.D.; Sparks, C.E.; Adeli, K. Selective Hepatic Insulin Resistance, VLDL Overproduction, and Hypertriglyceridemia. Arter. Thromb. Vasc. Biol. 2012, 32, 2104–2112. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Parks, J.S.; Kuipers, F.; Hayden, M.R. Tissue-Specific Induction of Intestinal ABCA1 Expression with a Liver X Receptor Agonist Raises Plasma HDL Cholesterol Levels. Circ. Res. 2006, 99, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kuusisto, J. Diabetes Secondary to Treatment with Statins. Curr. Diab. Rep. 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Biscetti, F.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Nicolazzi, M.A.; Covino, M.; Gasbarrini, A.; Massetti, M.; Flex, A. Statins in High Cardiovascular Risk Patients: Do Comorbidities and Characteristics Matter? Int. J. Mol. Sci. 2022, 23, 9326. [Google Scholar] [CrossRef] [PubMed]
- Hoogwerf, B.J. Statins May Increase Diabetes, but Benefit Still Outweighs Risk. Cleve Clin. J. Med. 2023, 90, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bardolia, C.; Amin, N.S.; Turgeon, J. Emerging Non-Statin Treatment Options for Lowering Low-Density Lipoprotein Cholesterol. Front. Cardiovasc. Med. 2021, 8, 789931. [Google Scholar] [CrossRef] [PubMed]


| ED Group | LD Group | LD-Si Group | p | |
|---|---|---|---|---|
| Daily total intake (g/day) | 17.8 ± 1.20 | 16.5 ± 0.55 | 17.1 ± 0.55 | NS |
| Daily cholesterol intake (mg/day) | 3.6 ± 0.36 a | 154.0 ± 12.1 b | 167.1 ± 8.5 b | <0.0001 |
| Body weight increase (g) | 134.4 ± 25 | 117.3 ± 32.3 | 127.6 ± 34.6 | NS |
| Small intestine weight (g) | 1.86 ± 0.16 | 1.90 ± 0.27 | 1.86 ± 0.21 | NS |
| Liver weight (g) | 10.34 ± 0.82 a | 18.43 ± 2.38 b | 17.43 ± 2.42 b | <0.001 |
| Fecal excretion (g/day dry matter) | 1.14 ± 0.08 a | 1.60 ± 0.06 b | 1.96 ± 0.24 c | <0.0001 |
| Fecal fat excretion (mg/g dry matter) | 80.0 ± 6.7 a | 170.6 ± 10.1 b | 254.0 ± 14.3 c | <0.0001 |
| ED Group | LD Group | LD-Si Group | p | |
|---|---|---|---|---|
| Glucose (mmol/L) | 13.92 ± 0. 91 a | 18.11 ± 1.65 b | 15.26 ± 2.07 a | <0.001 |
| Insulin (μUI/mL) | 15.84 ± 0.73 c | 5.41 ± 1.23 a | 8.11 ± 1.79 b | <0.0001 |
| HOMA-IR | 9.79 ± 0.64 b | 4.57 ± 1.55 a | 5.87 ± 1.5 a | <0.001 |
| Cholesterol (mmol/L) | 2.05 ± 0.07 a | 2.79 ± 0.05 c | 2.24 ± 0.04 b | <0.0001 |
| Triglycerides (mmol/L) | 1.79 ± 0.25 c | 0.88 ± 0.11 b | 0.60 ± 0.16 a | <0.001 |
| VLDLc (mmol/L) | 0.42 ± 0.05 a | 0.74 ± 0.09 b | 0.39 ± 0.07 a | <0.001 |
| VLDLtg (mmol/L) | 1.57 ± 0.05 b | 0.31 ± 0.07 a | 0.21 ± 0.02 a | <0.0001 |
| LDLc (mmol/L) | 0.03 ± 0.003 a | 0.33 ± 0.05 b | 0.23 ± 0.03 b | <0.001 |
| IDLc (mmol/L) | 0.02 ± 0.003 a | 0.52 ± 0.03 b | 0.60 ± 0.11 b | <0.001 |
| HDLc (mmol/L) | 1.45 ± 0.14 b | 1.07 ± 0.18 a | 1.10 ± 0.07 a | <0.0001 |
| AI (Non-HDLc/HDLc) | 0.43 ± 0.22 a | 1.48 ± 0.45 c | 1.04 ± 0.15 b | <0.001 |
| ED Group | LD Group | LD-Si Group | p | |
|---|---|---|---|---|
| Villi height (µm) | 959.5 ± 113.9 | 1003.5 ± 101.1 | 920.0 ± 107.2 | NS |
| Villi width (µm) | 251.2 ± 16.8 a | 292.5 ± 24.4 b | 244.6 ± 25.4 a | <0.01 |
| Villi area (mm2) | 0.79 ± 0.05 a | 0.85 ± 0.13 b | 0.71 ± 0.04 a | <0.001 |
| Crypt depth (µm) | 284.4 ± 23.6 a | 315.7 ± 58.7 ab | 354.5 ± 24.5 b | <0.01 |
| Villi height/width | 3.29 ± 0.30 | 3.16 ± 0.47 | 2.79 ± 0.24 | NS |
| Villi height/crypt depth | 3.69 ± 0.50 | 3.54 ± 0.23 | 3.38 ± 0.26 | NS |
| Villi PAS goblet cells (no. positive cells) | 27.18 ± 4.34 | 25.77 ± 6.57 | 25.75 ± 6.54 | NS |
| Villi AB goblet cells (no. positive cells) | 32.95 ± 5.35 | 32.19 ± 3.32 | 27.70 ± 3.47 | NS |
| Crypt PAS goblet cells (no. positive cells) | 13.95 ± 1.64 | 15.35 ± 1.58 | 15.70 ± 1.65 | NS |
| Crypt AB goblet cells (no. positive cells) | 14.33 ± 2.87 | 15.28 ± 2.19 | 14.45 ± 2.02 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martín, M.; Garcimartín, A.; Bocanegra, A.; Redondo-Castillejo, R.; Quevedo-Torremocha, C.; Macho-González, A.; García Fernández, R.A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J.; et al. Silicon as a Functional Meat Ingredient Improves Jejunal and Hepatic Cholesterol Homeostasis in a Late-Stage Type 2 Diabetes Mellitus Rat Model. Foods 2024, 13, 1794. https://doi.org/10.3390/foods13121794
Hernández-Martín M, Garcimartín A, Bocanegra A, Redondo-Castillejo R, Quevedo-Torremocha C, Macho-González A, García Fernández RA, Bastida S, Benedí J, Sánchez-Muniz FJ, et al. Silicon as a Functional Meat Ingredient Improves Jejunal and Hepatic Cholesterol Homeostasis in a Late-Stage Type 2 Diabetes Mellitus Rat Model. Foods. 2024; 13(12):1794. https://doi.org/10.3390/foods13121794
Chicago/Turabian StyleHernández-Martín, Marina, Alba Garcimartín, Aránzazu Bocanegra, Rocío Redondo-Castillejo, Claudia Quevedo-Torremocha, Adrián Macho-González, Rosa Ana García Fernández, Sara Bastida, Juana Benedí, Francisco José Sánchez-Muniz, and et al. 2024. "Silicon as a Functional Meat Ingredient Improves Jejunal and Hepatic Cholesterol Homeostasis in a Late-Stage Type 2 Diabetes Mellitus Rat Model" Foods 13, no. 12: 1794. https://doi.org/10.3390/foods13121794





