Studies on the Properties and Stability Mechanism of Double Emulsion Gels Prepared by Heat-Induced Aggregates of Egg White Protein-Oligosaccharides Glycosylation Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Heat-Induced Aggregates of IMO-EWP Conjugates by Glycation
2.3. Characteristic Testing of HIA
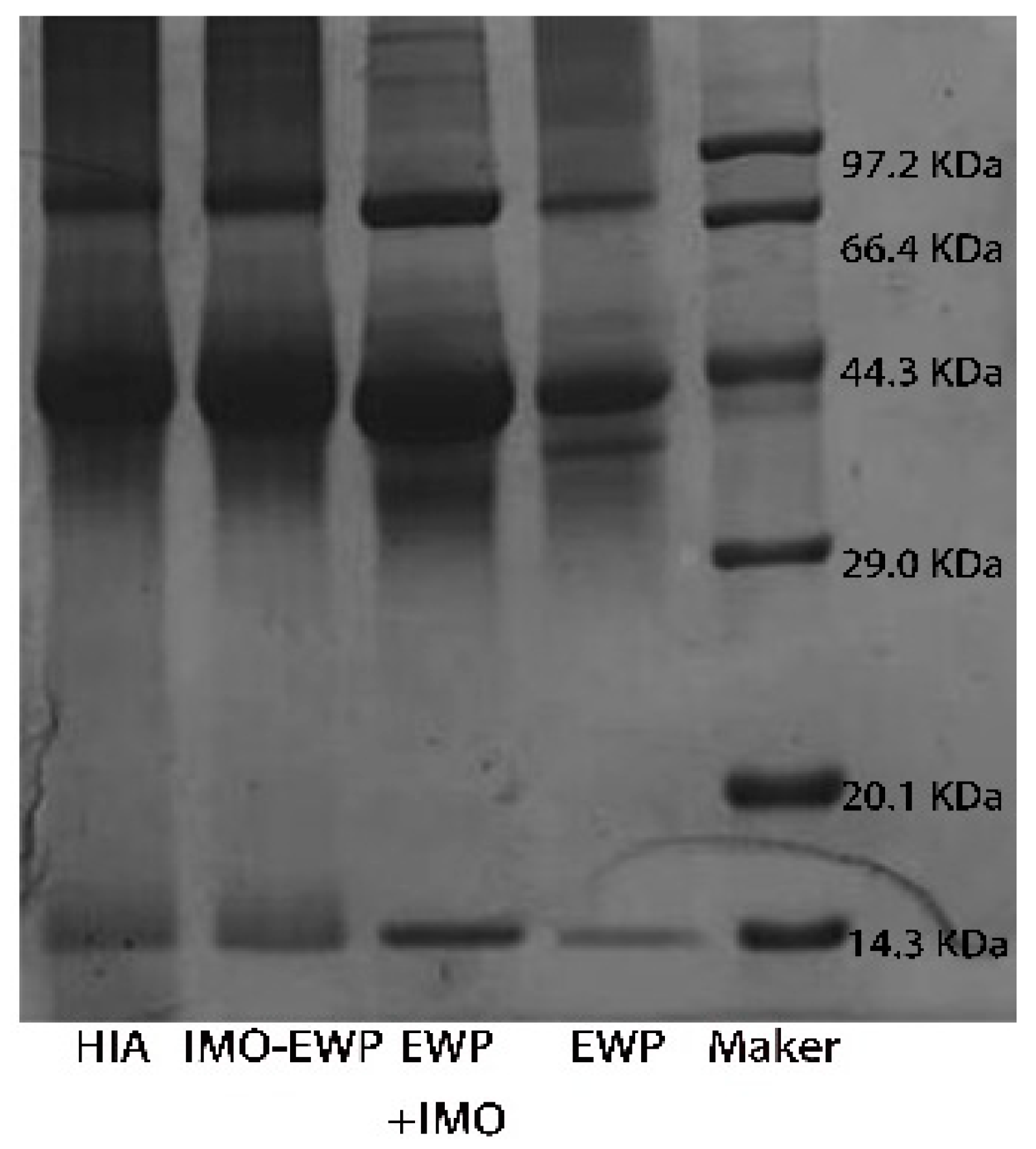
2.3.1. SDS-PAGE
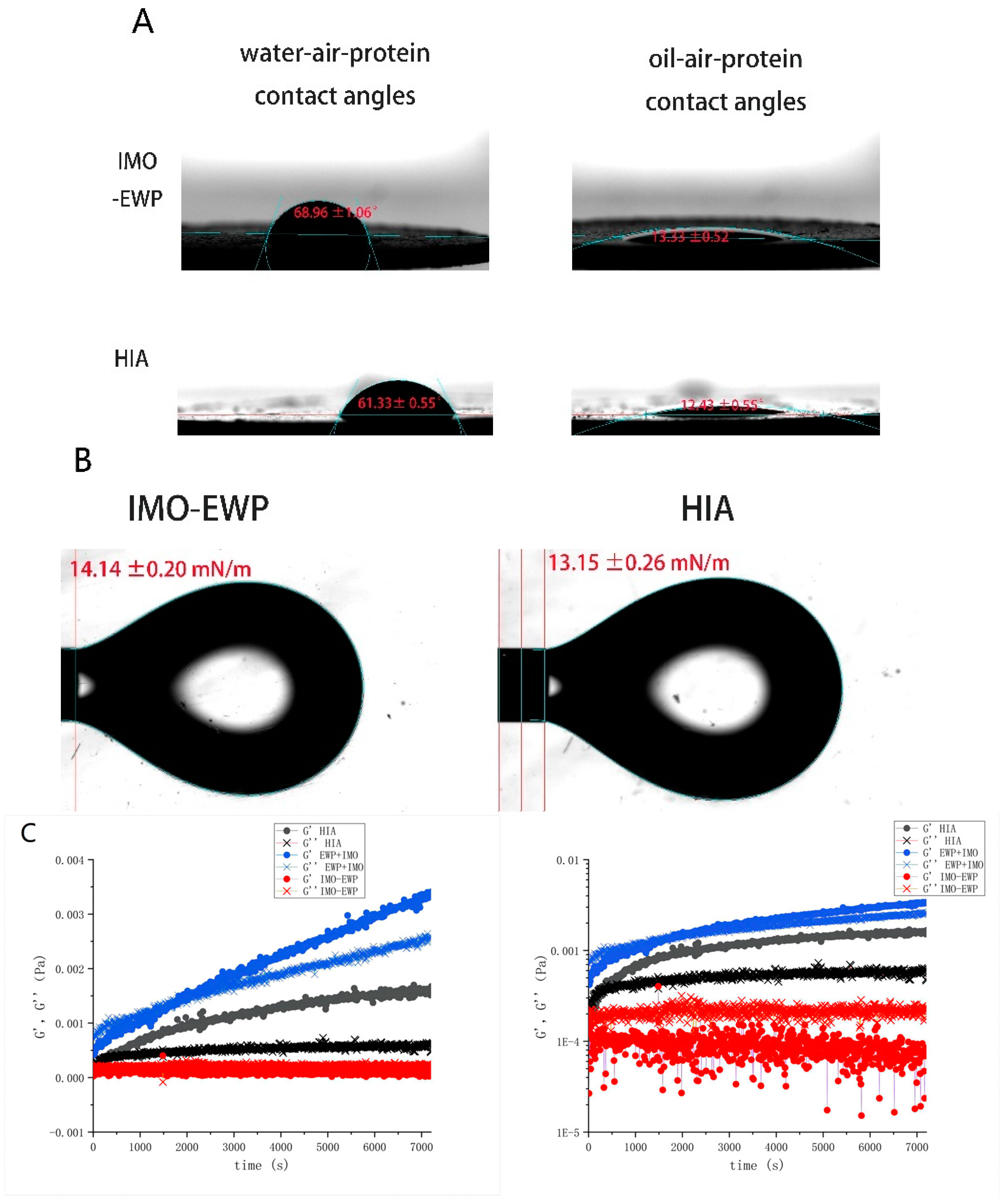
2.3.2. Contact Angle and Interfacial Tension of HIA
2.3.3. Interfacial Rheology of HIA
2.4. Preparation of W1/O/W2 Emulsions
2.5. Preparation of W1/O/W2 Emulsion-Gels System
2.6. Characteristic Testing of Emulsions
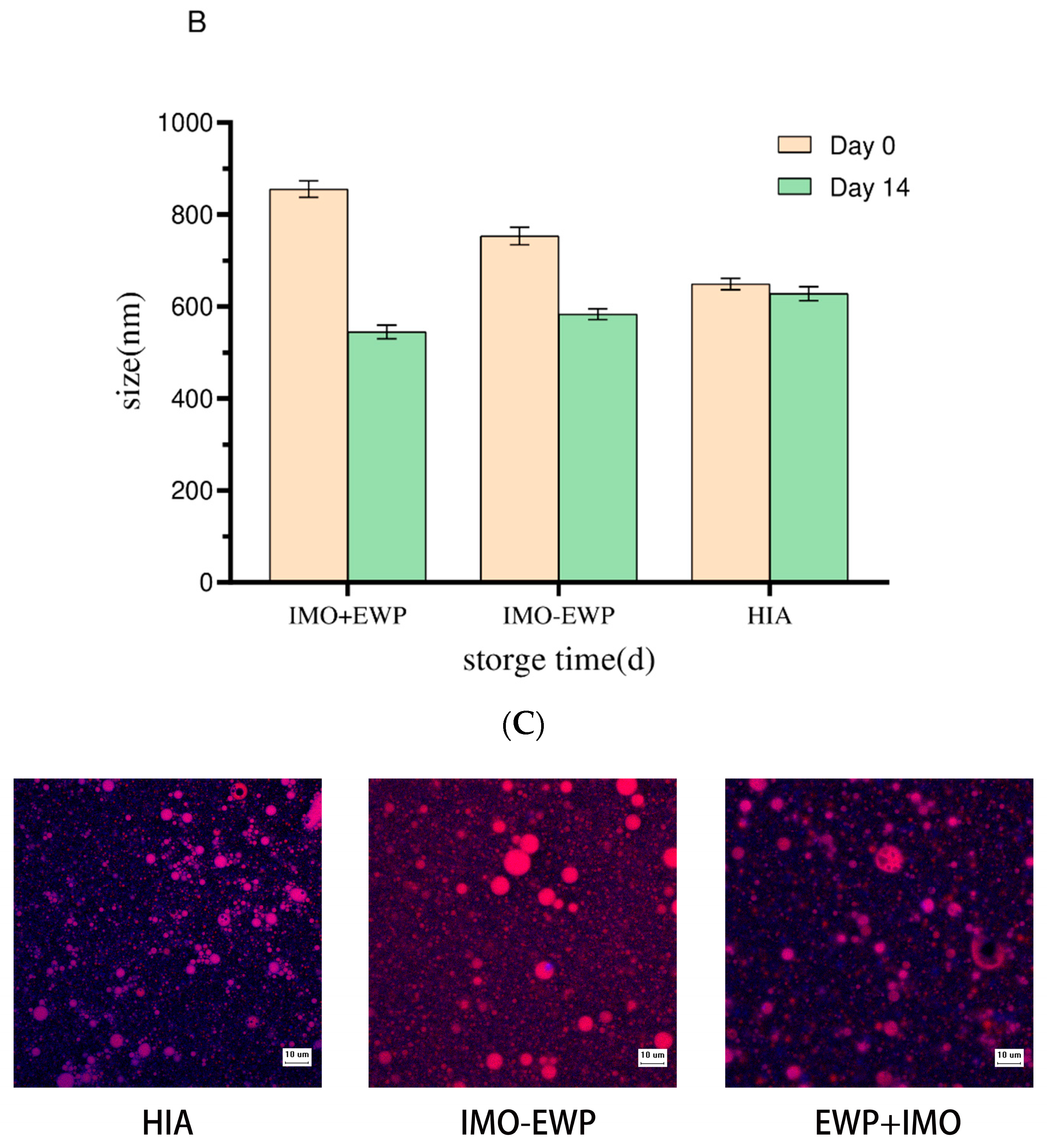
2.6.1. Morphology Observation
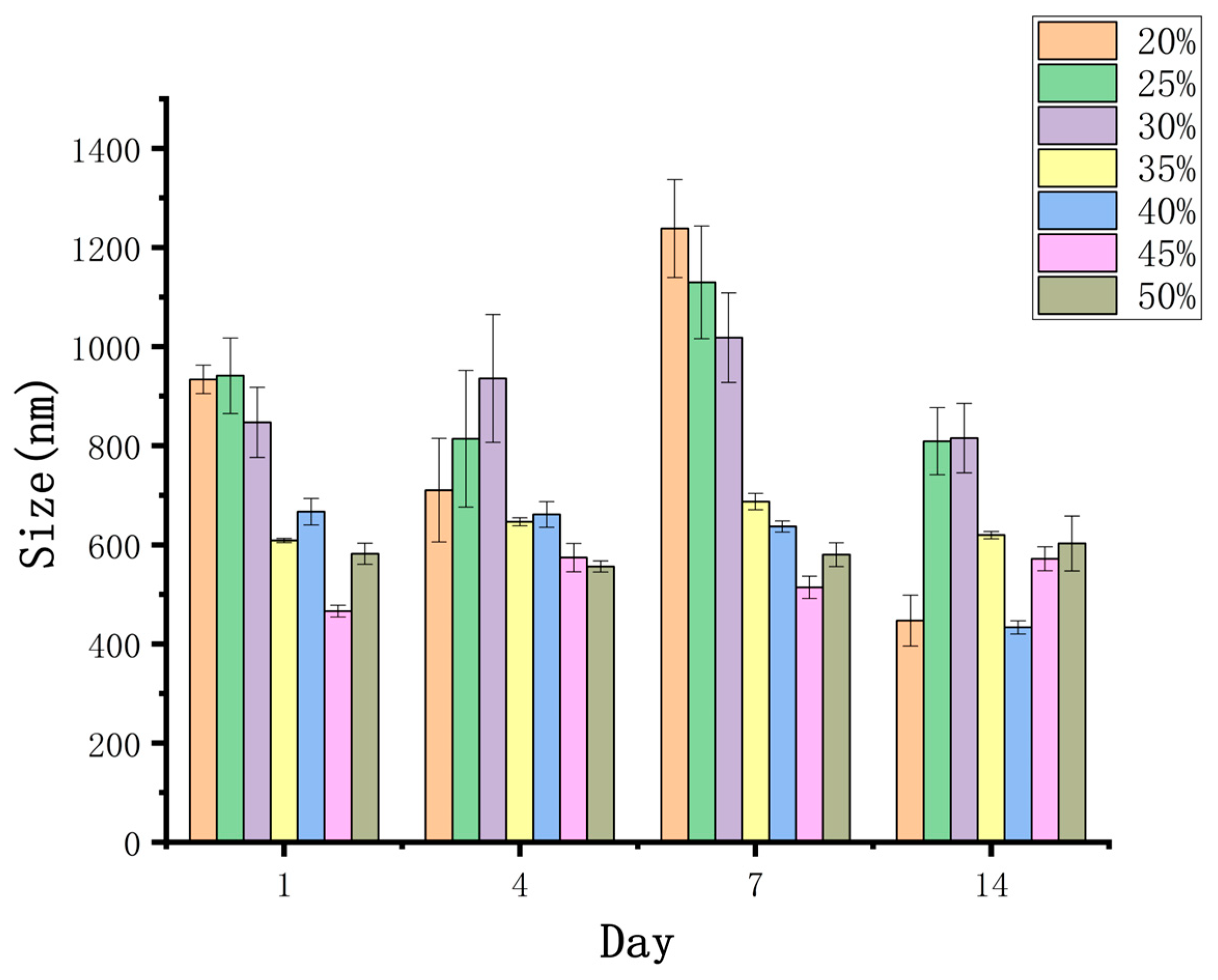
2.6.2. Particle Size and Zeta Potential Measurements
2.6.3. Rheological Analysis
2.7. Encapsulation Efficiency (EE)
2.8. Impact of Environmental Stresses on Stability of Emulsion-Gels System
2.8.1. Salt Stability
2.8.2. Heat Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of HIA
3.1.1. SDS-PAGE
3.1.2. Contact Angle and Interfacial Tension
3.1.3. Interface Rheology
3.2. Emulsion Behavior of HIA and IMO-EWP
3.3. Analysis of Emulsion-Gels System
3.3.1. Microstructure and Appearance of W1/O/W2 Emulsion Gels
3.3.2. Particle Size and Zeta Potential Analysis
3.3.3. Rheological Properties
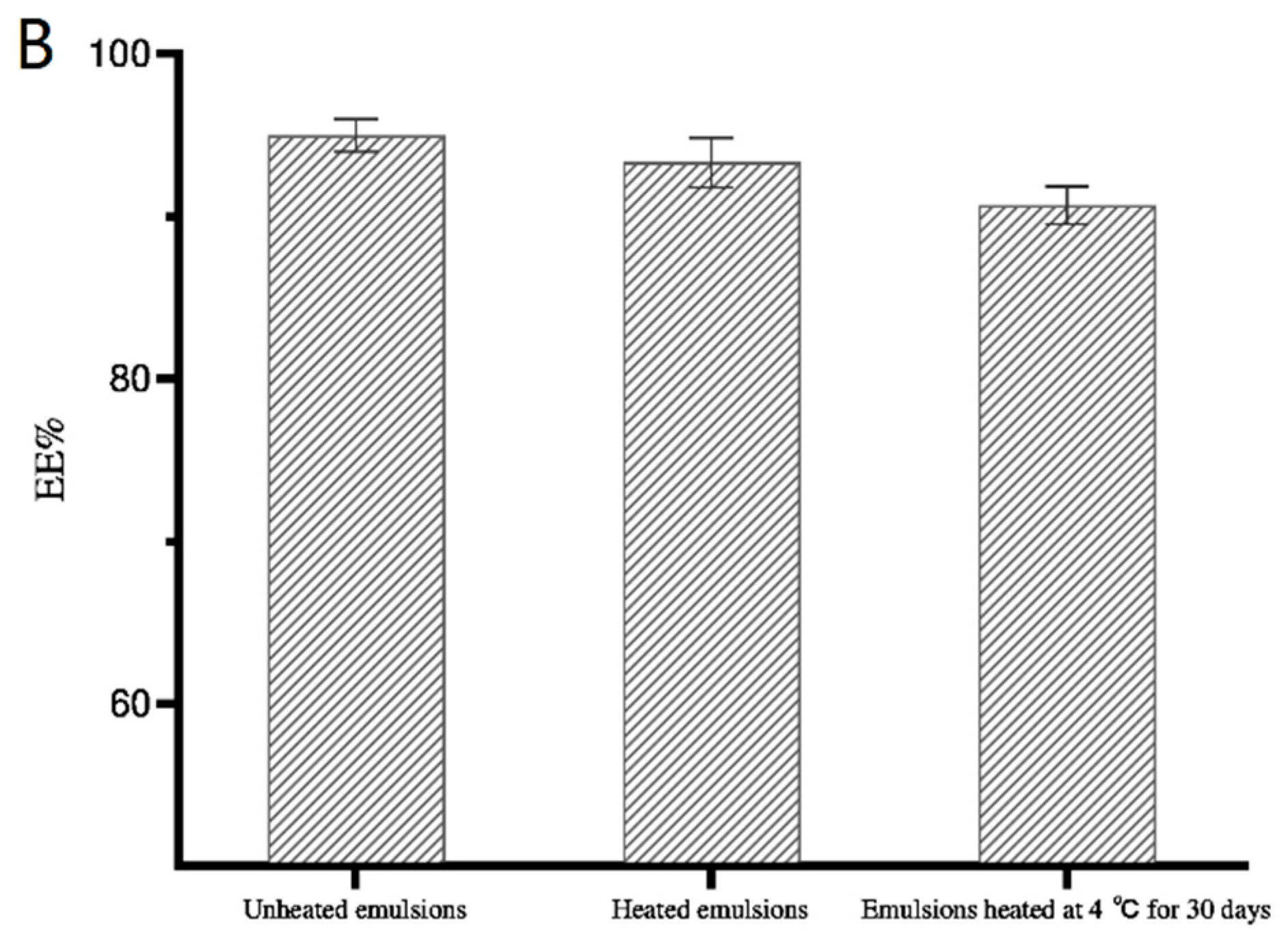
3.3.4. Encapsulation Efficiency
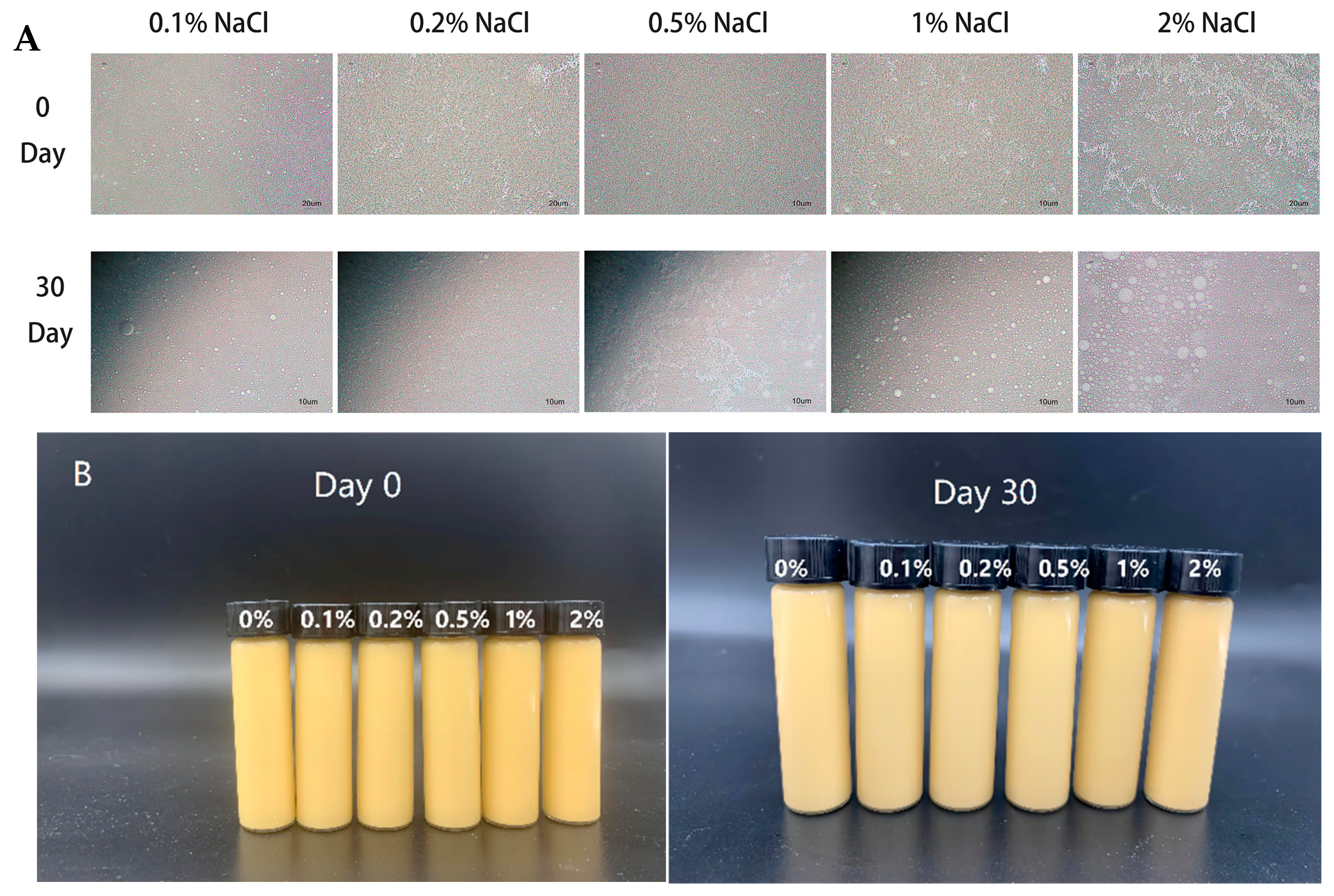
3.3.5. Salt Stability
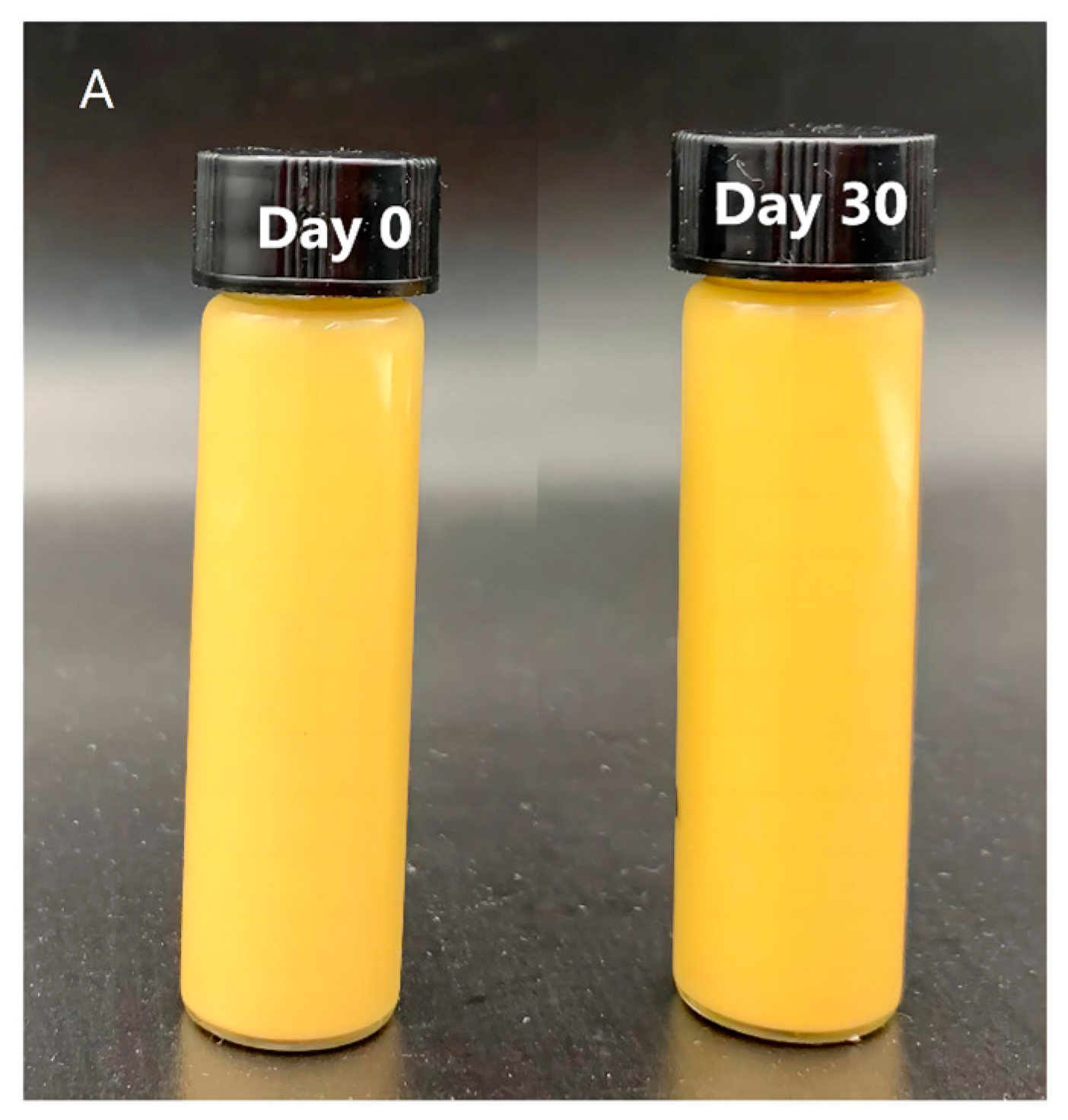
3.3.6. Heat Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oster, C.G.; Kissel, T. Comparative study of DNA encapsulation into PLGA microparticles using modified double emulsion methods and spray drying techniques. J. Microencapsul. 2005, 22, 235–244. [Google Scholar] [CrossRef]
- Jin, H.J.; Chong, H.H.; Zhu, Y.M.; Zhang, M.Q.; Li, X.; Bazybek, N.; Wei, Y.; Gong, F.L.; He, Y.X.; Ma, G.H. Preparation and evaluation of amphipathic lipopeptide-loaded PLGA microspheres as sustained-release system for AIDS prevention. Eng. Life Sci. 2020, 20, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, R.; Xia, Q. An ascorbic acid delivery system based on (W1/O/W2) double emulsions encapsulated by Ca-alginate hydrogel beads. J. Drug Deliv. Sci. Technol. 2020, 60, 101929. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.T.; Shi, F.; Liu, Y.; Hu, W.J.; Yang, Z.J. Influence of β-cyclodextrin concentration on the physicochemical properties and skin permeation behavior of vitamin C-loaded Pickering water-in-oil-in-water (W1/O/W2) double emulsions. J. Drug Deliv. Sci. Technol. 2022, 72, 103368. [Google Scholar] [CrossRef]
- Klojdová, I.; Kumherová, M.; Veselá, K.; Horácková, S.; Stetina, J. Functional W1/O/W2 model food product with encapsulated colostrum and high protein content. Eur. Food Res. Technol. 2022, 248, 899–903. [Google Scholar] [CrossRef]
- El Kadri, H.; Lalou, S.; Mantzouridou, F.; Gkatzionis, K. Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Res. Int. 2018, 107, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Özer, Ö.; Muguet, V.; Roy, E.; Grossiord, J.L.; Seiller, M. Stability study of W/O/W viscosified multiple emulsions. Drug Dev. Ind. Pharm. 2000, 26, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Shima, M.; Tanaka, M.; Kimura, Y.; Adachi, S.; Matsuno, R. Hydrolysis of the oil phase of a W/O/W emulsion by pancreatic lipase. J. Control. Release 2004, 94, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Benna-Zayani, M.; Kbir-Ariguib, N.; Trabelsi-Ayadi, M.; Grossiord, J.L. Stabilisation of W/O/W double emulsion by polysaccharides as weak gels. Colloids Surf. A-Physicochem. Eng. Asp. 2008, 316, 46–54. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. W/O/W double emulsions stabilized with WPI-polysaccharide complexes. Colloids Surf. A-Physicochem. Eng. Asp. 2007, 294, 20–32. [Google Scholar] [CrossRef]
- Choi, M.-J.; Choi, D.; Lee, J.; Jo, Y.-J. Encapsulation of a bioactive peptide in a formulation of W1/O/W2-type double emulsions: Formation and stability. Food Struct. 2020, 25, 100145. [Google Scholar] [CrossRef]
- Liang, X.P.; Wu, J.Q.; Yang, X.G.; Tu, Z.B.; Wang, Y. Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particle dynamics. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 546, 107–114. [Google Scholar] [CrossRef]
- Xing, Y.; Li, R.; Xue, L.; Chen, M.; Lu, X.; Duan, Z.; Zhou, W.; Li, J. Double emulsion (W/O/W) gel stabilised by polyglycerol polyricinoleate and calcium caseinate as mangiferin carrier: Insights on formulation and stability properties. Int. J. Food Sci. Technol. 2022, 57, 5268–5279. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.Q.; Deng, S.M.; Liu, W.; Yan, C.; Zhu, Y.Q.; Cheng, C.; Liu, C.M. Coencapsulation of (-)-Epigallocatechin-3-gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Xuan, Z.; Xing, R.; Duo, Z.; Yongkang, Y.; Bin, L. Fabrication of natural W1/O/W2 double emulsions stabilized with gliadin colloid particles and soybean lecithin. Food Hydrocoll. 2023, 144, 108978. [Google Scholar] [CrossRef]
- Tian, H.; Xiang, D.; Li, C. Tea polyphenols encapsulated in W/O/W emulsions with xanthan gum-locust bean gum mixture: Evaluation of their stability and protection. Int. J. Biol. Macromol. 2021, 175, 40–48. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Belwal, T.; Aalim, H.; Li, L.; Lin, X.; Liu, S.; Ma, C.; Li, Q.; Zou, Y.; Luo, Z. Protein-polysaccharide complex coated W/O/W emulsion as secondary microcapsule for hydrophilic arbutin and hydrophobic coumaric acid. Food Chem. 2019, 300, 125171. [Google Scholar] [CrossRef] [PubMed]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Amid, B.T.; Kazemi, M.; Hedayatnia, S. Encapsulation properties, release behavior and physicochemical characteristics of water-in-oil-in-water (W/O/W) emulsion stabilized with pectin-pea protein isolate conjugate and Tween 80. Food Hydrocoll. 2016, 61, 599–608. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C. Bilayer electrostatic deposition: An effective strategy to enhance physical stability of double emulsion. Food Hydrocoll. 2023, 145, 109083. [Google Scholar] [CrossRef]
- Estévez, M.; Güell, C.; De Lamo-Castellví, S.; Ferrando, M. Encapsulation of grape seed phenolic-rich extract within W/O/W emulsions stabilized with complexed biopolymers: Evaluation of their stability and release. Food Chem. 2019, 272, 478–487. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, J.; Mulvihill, D.M. Sodium caseinate-maltodextrin conjugate stabilized double emulsions: Encapsulation and stability. Food Res. Int. 2010, 43, 224–231. [Google Scholar] [CrossRef]
- Pouria, G.; Jafari, S.M.; Hamed, H.; Aziz, H.; Habibollah, M. Pectin-whey protein complexes vs. small molecule surfactants for stabilization of double nano-emulsions as novel bioactive delivery systems. J. Food Eng. 2019, 245, 139–148. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Yu, J.; Karangwa, E.; Xia, S.; Zhang, X.; Jia, C. Mechanism of formation and stabilization of nanoparticles produced by heating electrostatic complexes of WPI-dextran conjugate and chondroitin sulfate. J. Agric. Food Chem. 2016, 64, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, C.; Liu, X.; Liao, W.; Zhang, L.; Chen, S.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Effects of microfluidization and thermal treatment on the characterization and digestion of curcumin loaded protein-polysaccharide-tea saponin complex nanoparticles. Food Funct. 2021, 12, 1192–1206. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wang, J.; Jiang, C.; Nie, X.; Hu, Z.; Ye, Q.; Meng, X.; Xiong, H. Development of composite nanoparticles from gum Arabic and carboxymethylcellulose-modified Stauntonia brachyanthera seed albumin for lutein delivery. Food Chem. 2022, 372, 131269. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Li, X.; Chang, C.; Zhang, M.; Gu, L.; Su, Y.; Yang, Y. Emulsifying properties of glycation or glycation-heat modified egg white protein. Food Res. Int. 2019, 119, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; McClements, D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Watt, S.; Wilde, P. Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocoll. 2014, 42, 215–222. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, M.; Xia, Q. Improved stability of (W1/O/W2) double emulsions based on dual gelation: Oleogels and hydrogels. J. Food Process Eng. 2019, 42, e13186. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Z.; Zhang, G.; Wang, X.; Zhao, Y.; Fan, Z.; Liu, M.; Han, J.; Wang, Z. Fabrication and spray-drying microencapsulation of vitamin C-loaded W1/O/W2 emulsions: Influence of gel polymers in the internal water phase on encapsulation efficiency, reconstituted stability, and controlled release properties. LWT-Food Sci. Technol. 2022, 170, 114113. [Google Scholar] [CrossRef]
- Wang, W.J.; Sun, R.; Xia, Q. Influence of gelation of internal aqueous phase on in vitro controlled release of W1/O/W2 double emulsions-filled alginate hydrogel beads. J. Food Eng. 2023, 337, 111246. [Google Scholar] [CrossRef]
- Su, Y.; Lu, C.; Chang, C.; Li, J.; Sun, Y.; Zhang, W.; Gong, L.; Gu, L.; Yang, Y. Preparation and characterization of W1/O/W2 emulsions stabilized by glycated and heat-modified egg white proteins. J. Sci. Food Agric. 2022, 102, 5795–5807. [Google Scholar] [CrossRef] [PubMed]
- Amid, B.T.; Mirhosseini, H. Stabilization of water in oil in water (W/O/W) emulsion using whey protein isolate-conjugated durian seed gum: Enhancement of interfacial activity through conjugation process. Colloids Surf. B-Biointerfaces 2014, 113, 107–114. [Google Scholar] [CrossRef] [PubMed]
- de Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef]
- Li, W.W.; Wang, Y.S.; Zhao, H.B.; He, Z.Y.; Zeng, M.M.; Qin, F.; Chen, J. Improvement of emulsifying properties of soy protein through selective hydrolysis: Interfacial shear rheology of adsorption layer. Food Hydrocoll. 2016, 60, 453–460. [Google Scholar] [CrossRef]
- Huang, Z.H.; Guo, B.Z.; Deng, C.; Tang, C.; Liu, C.M.; Hu, X.T. Fabrication and characterization of the W/O/W multiple emulsion through oleogelation of oil. Food Chem. 2021, 358, 129856. [Google Scholar] [CrossRef]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Mundo, J.L.M.; Tan, Y.; Pham, H.; McClements, D.J. Fabrication and characterization of W/O/W emulsions with crystalline lipid phase. J. Food Eng. 2020, 273, 109826. [Google Scholar] [CrossRef]
- Ying, X.; Gao, J.; Lu, J.; Ma, C.; Lv, J.; Adhikari, B.; Wang, B. Preparation and drying of water-in-oil-in-water (W/O/W) double emulsion to encapsulate soy peptides. Food Res. Int. 2021, 141, 110148. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, H.J.; Park, K.H.; Moon, T.W. Molecular characteristics of ovalbumin-dextran conjugates formed through the Maillard reaction. Food Chem. 2005, 92, 93–99. [Google Scholar] [CrossRef]
- Al-Hakkak, J.; Al-Hakkak, F. Functional egg white-pectin conjugates prepared by controlled Maillard reaction. J. Food Eng. 2010, 100, 152–159. [Google Scholar] [CrossRef]
- Baldursdottir, S.G.; Fullerton, M.S.; Nielsen, S.H.; Jorgensen, L. Adsorption of proteins at the oil/water interface-Observation of protein adsorption by interfacial shear stress measurements. Colloids Surf. B-Biointerfaces 2010, 79, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Tian, Z.G.; Chen, L.Y.; Emam-Djomeh, Z. Effect of CaCl2 on the stability and rheological properties of foams and high-sugar aerated systems produced by preheated egg white protein. Food Hydrocoll. 2020, 106, 105887. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Feng, L.P.; Saito, M.; Yin, L.J. Preparation and characterization of W/O/W double emulsions containing MgCl2. J. Dispers. Sci. Technol. 2018, 39, 349–355. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, S.; Zhang, H.; Cheng, Y.; Yin, L. Physical stability, microstructure and micro-rheological properties of water-in-oil-in-water (W/O/W) emulsions stabilized by porcine gelatin. Food Chem. 2018, 253, 63–70. [Google Scholar] [CrossRef]



| Gelation% | Average Particle Size (nm) | PDI | Zeta Potential (mv) |
|---|---|---|---|
| 0% | 653 ± 7 d | 0.42 ± 0.08 a | −37.83 ± 1.03 c |
| 1% | 3347 ± 320 a | 0.29 ± 0.03 b | −20.67 ± 0.12 b |
| 3% | 1157 ± 18 b | 0.44 ± 0.02 a | −19.10 ± 0.79 ab |
| 5% | 805 ± 29 c | 0.31 ± 0.04 b | −17.83 ± 0.84 a |
| 8% | 960 ± 8 c | 0.32 ± 0.02 b | −15.77 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Lu, C.; Chang, C.; Gu, L.; Li, J.; Guo, L.; Hu, S.; Huang, Z.; Yang, Y.; Su, Y. Studies on the Properties and Stability Mechanism of Double Emulsion Gels Prepared by Heat-Induced Aggregates of Egg White Protein-Oligosaccharides Glycosylation Products. Foods 2024, 13, 1822. https://doi.org/10.3390/foods13121822
Zhao Q, Lu C, Chang C, Gu L, Li J, Guo L, Hu S, Huang Z, Yang Y, Su Y. Studies on the Properties and Stability Mechanism of Double Emulsion Gels Prepared by Heat-Induced Aggregates of Egg White Protein-Oligosaccharides Glycosylation Products. Foods. 2024; 13(12):1822. https://doi.org/10.3390/foods13121822
Chicago/Turabian StyleZhao, Qianwen, Cheng Lu, Cuihua Chang, Luping Gu, Junhua Li, Lulu Guo, Shende Hu, Zijian Huang, Yanjun Yang, and Yujie Su. 2024. "Studies on the Properties and Stability Mechanism of Double Emulsion Gels Prepared by Heat-Induced Aggregates of Egg White Protein-Oligosaccharides Glycosylation Products" Foods 13, no. 12: 1822. https://doi.org/10.3390/foods13121822





