Influence of Boiling Time on Chemical Composition and Properties of Tender and Mature Moringa Pods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Boiling the Moringa Pods
2.3. Analytical Determinations
2.4. Statistical Analysis
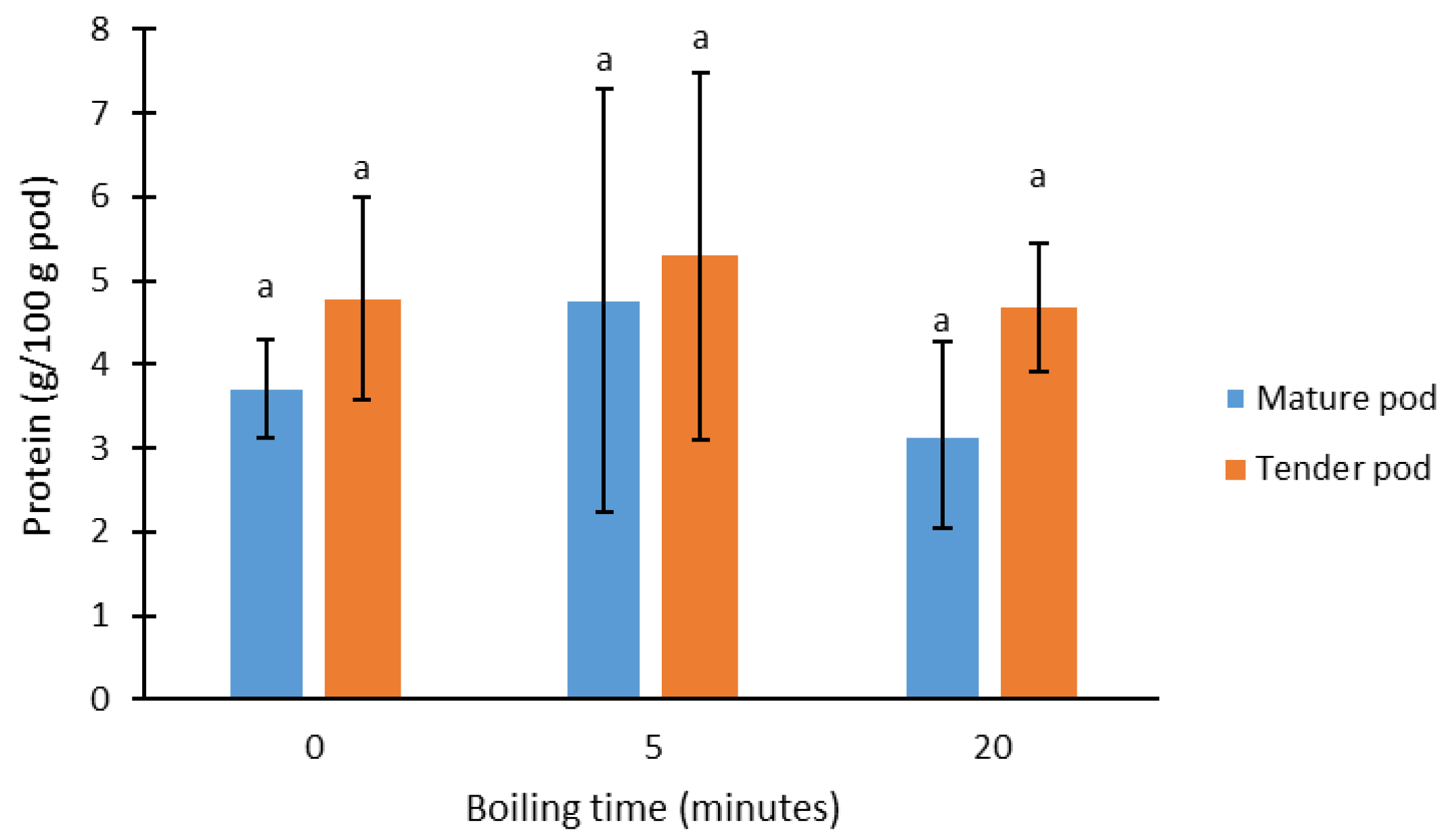
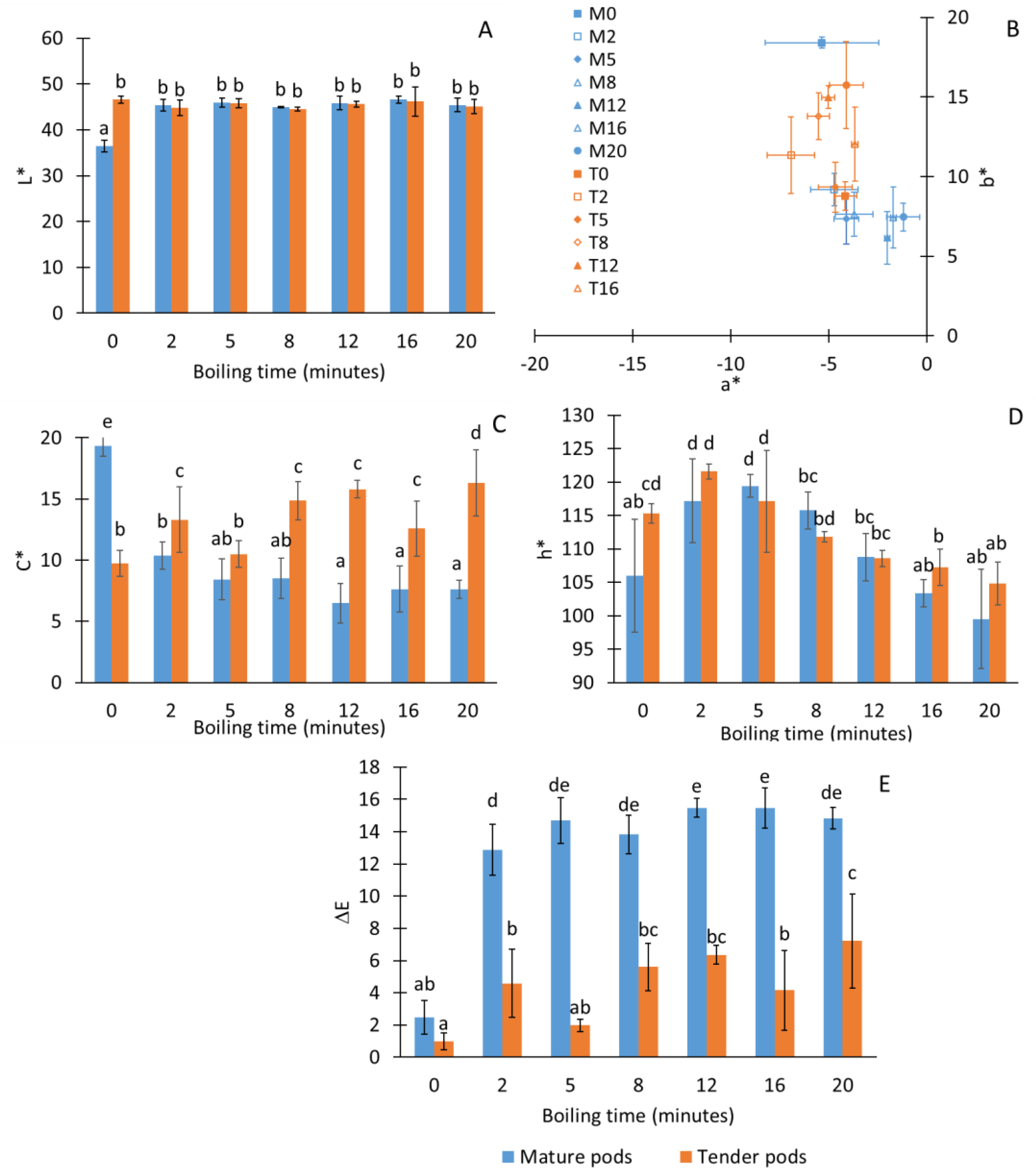
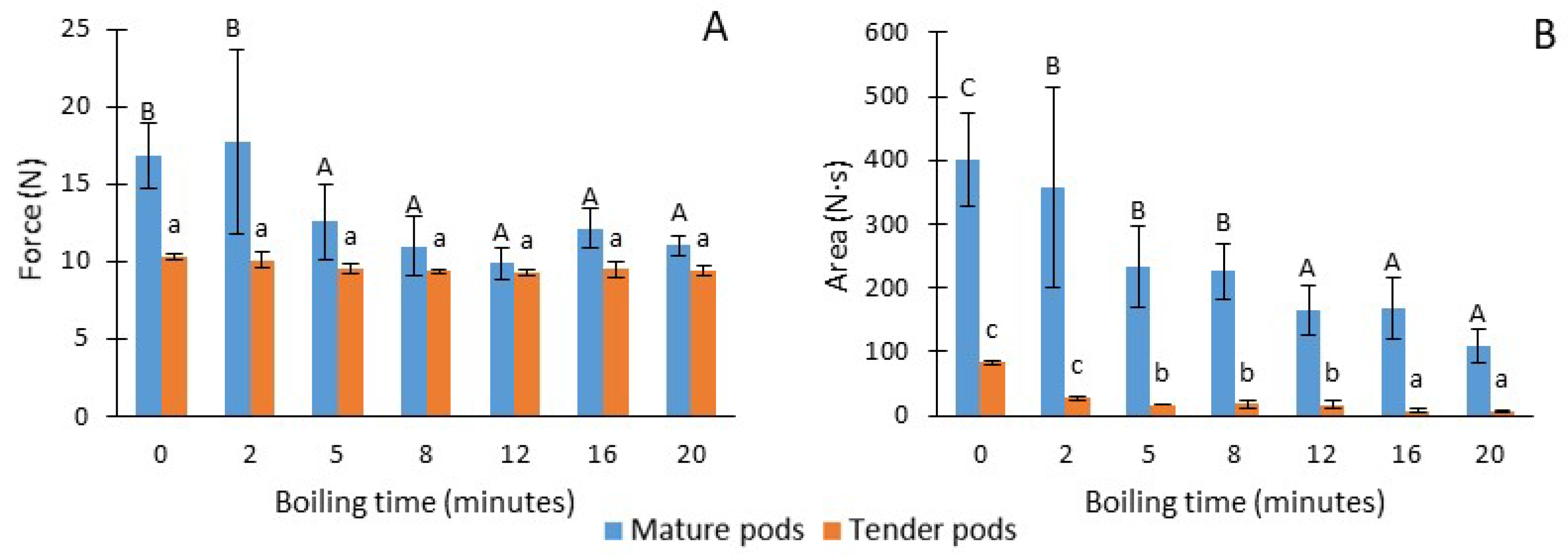
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godino, M. Moringa oleifera: Árbol Multiusos de Interés Forestal Para El Sur de La Península Ibérica; Fichas de Transferencia; Negocio Agroalimentario y Cooperativo; Cajamar Caja Rural: Almería, Spain, 2016; Volume 25, pp. 20–24. [Google Scholar]
- Joshi, P.; Jain, S. Nutrient Composition of Drumstick (Moringa oleifera) Pod Powder and Their Product Development. J. Dairy Foods Home Sci. 2011, 30, 285–289. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A Review on Nutritive Importance and Its Medicinal Application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; Núñez-Gastélum, J.A.; Reyes-Moreno, C.; Ramírez-Wong, B.; López-Cervantes, J. Nutritional Quality of Edible Parts of Moringa oleifera. Food Anal. Methods 2010, 3, 175–180. [Google Scholar] [CrossRef]
- Gidamis, A.B.; Panga, J.T.; Sarwatt, S.V.; Chove, B.E.; Shayo, N.B. Nutrient and Antinutrient Contents in Raw and Cooked Young Leaves and Immature Pods of Moringa oleifera, Lam. Ecol. Food Nutr. 2003, 42, 399–411. [Google Scholar] [CrossRef]
- Owon, M.; Osman, M.; Ibrahim, A.; Salama, M.A.; Matthäus, B. Characterisation of Different Parts from Moringa oleifera Regarding Protein, Lipid Composition and Extractable Phenolic Compounds. Oilseeds Fats Crops Lipids 2021, 28, 45. [Google Scholar] [CrossRef]
- El-Massry, F.H.M.; Mossa, M.E.M.; Youssef, S.M. Moringa oleifera Plant “Value and Utilization in Food Processing”. Egypt. J. Agric. Res. 2013, 91, 1597–1909. [Google Scholar] [CrossRef]
- Tobias, F.L. Moringa oleifera the Tree of Nutrition. Cienc. Salud Virtual 2010, 2, 130–138. [Google Scholar]
- Foidl, N.; Makkar, H.P.S.; Becker, K.; Foild, N.; Km, S. The Potential of Moringa oleifera for Agricultural and Industrial Uses. What Development Potential for Moringa Products? 2001, October–November, 1–20. Available online: https://d1wqtxts1xzle7.cloudfront.net/48719799/the_potential_of_moringa_oleifera_for_agricultural_and_industrial_uses-libre.pdf?1473491706=&response-content-disposition=inline%3B+filename%3Dthe_potential_of_moringa_oleifera_for_ag.pdf&Expires=1718027399&Signature=PtMR~pFy~XVz9gDU9RFjJK4S3qJHbCUKchbYFCOufVpKiZekwPLCBT3~Ke0CWQLz~UryMnOh8mBPHXSsorsdpx0d8NHvRx1KmH1488RJl3cM8lWj3QzN2Uh21RmfOYLC5PGgDRbbCqm~r8tNzBAmBvdWZ-SQeOJAHlbw58NoZ3Ewmiqf3ManNLtpTbffwzYhapKwlG9RgfA-5eMTcNjouoaSzhvmi2jrxitztyOtgx6l9XoseZ6AANrXG19uaeEQ4WwkxSoSv99BQFXTckliUxDPxZYe8zSWXm0FI~WmXVHm0tv1S-HdPo1-69rEDqYINTvnAhcsy0MGWqmEZJGKnw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 3 April 2024).
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 14. [Google Scholar] [CrossRef]
- Sandeep, G.; Anitha, T.; Vijayalatha, K.R.; Sadasakthi, A. Moringa for Nutritional Security (Moringa oleifera Lam.). Int. J. Bot. Stud. 2019, 4, 21–24. [Google Scholar]
- Golla, S.P.; Ramesh Kumar, R.; Veerapandian, C.; Rangarajan, J.; Mariya Anthony, T.A. Meta Analysis on Color, Total Flavonoids, Antioxidants, Frictional, Mechanical, and Cutting Force of Moringa Pods. J. Food Process. Eng. 2022, 45, 14136. [Google Scholar] [CrossRef]
- Kamble, D.B.; Bashir, K.; Singh, R.; Rani, S. Effect of Moringa Oleífera Pod Addition on the Digestibility, Cooking Quality, and Structural Attributes of Functional Pasta. J. Food Process. Preserv. 2022, 46, e16163. [Google Scholar] [CrossRef]
- Núñez-Gastélum, A.J.; Maciel-Ortiz, E.F.; Sáyago-Ayerdi, G.S.; del Martínez-Ruiz, R.N.; Alvarez-Parrilla, E.; de la Rosa, A.L.; Mercado-Mercado, G. Incorporation of Moringa oleifera Pods onto Breads Improves Nutrient Contents, Phytochemicals Bioaccessibility and Reduces the Predicted Glycemic Index. Emir. J. Food Agric. 2023, 35, 604–610. [Google Scholar] [CrossRef]
- Ansari, F.; Singh, A.; Patidar, S.; Rana, G.K. Production and Evaluation of Immunity Boosting Instant Soup Mix from Moringa oleifera Lam. Pod Powder. Bangladesh J. Bot. 2022, 51, 75–81. [Google Scholar] [CrossRef]
- Ahmad, S.; Khalique, A.; Pasha, T.N.; Mehmood, S.; Hussain, K.; Ahmad, S.; Rasheed, B.; Awais, M.M.; Bhatti, S.A. Influence of Feeding Moringa oleifera Pods as Phytogenic Feed Additive on Performance, Blood Metabolites, Chemical Composition and Bioactive Compounds of Breast Meat in Broiler. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 195–202. [Google Scholar] [CrossRef]
- Budda, S.; Butryee, C.; Tuntipopipat, S.; Rungsipipat, A.; Wangnaithum, S.; Lee, J.-S.; Kupradinun, P. Suppressive Effects of Moringa oleifera Lam Pod Against Mouse Colon Carcinogenesis Induced by Azoxymethane and Dextran Sodium Sulfate. Asian Pac. J. Cancer Prev. 2011, 12, 3221–3228. [Google Scholar] [PubMed]
- Praengam, K.; Muangnoi, C.; Dawilai, S.; Tuntipopipat, S. Digested Moringa oleifera Boiled Pod Exhibits Anti-Inflammatory Activity in Caco-2 Cells. J. Herbs Spices Med. Plants 2015, 21, 148–160. [Google Scholar] [CrossRef]
- Muangnoi, C.; Chingsuwanrote, P.; Praengamthanachoti, P.; Svasti, S.; Tuntipopipat, S. Moringa oleifera Pod Inhibits Inflammatory Mediator Production by Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage Cell Lines. Inflammation 2012, 35, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, R.; Pellegrini, N.; Visconti, A.; Graziani, G.; Chiavaro, E.; Miglio, C.; Fogliano, V. Effects of Different Cooking Methods on Antioxidant Profile, Antioxidant Capacity, and Physical Characteristics of Artichoke. J. Agric. Food Chem. 2008, 56, 8601–8608. [Google Scholar] [CrossRef]
- Rashmitha, R.; Preetham, G.S.; Chandrasekar, V.; Nambi, V.E. Influence of Ingredients and Pressure Cooking on the Textural Properties of Moringa Pods. Pharma Innov. J. 2022, 11, 207–210. [Google Scholar]
- AOAC. AOAC Method 930.15—Analysis of Water Content. In Official Methods of Analysis; AOAC International: Arlington, VA, USA, 2005. [Google Scholar]
- AOAC. AOAC Method 978.18—Water Activity of Canned Vegetables. In Official Methods International; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Chang, S.K.C.; Zhang, Y. Protein Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 315–330. [Google Scholar]
- Talens, P.; Martınez-Navarrete, N.; Fito, P.; Chiralt, A. Changes in Optical and Mechanical Properties during Osmodehydrofreezing of Kiwi Fruit. Innov. Food Sci. Emerg. Technol. 2001, 3, 191–199. [Google Scholar] [CrossRef]
- Contreras, C.; Martín, M.E.; Martínez-Navarrete, N.; Chiralt, A. Effect of Vacuum Impregnation and Microwave Application on Structural Changes Which Occurred during Air-Drying of Apple. LWT Food Sci. Technol. 2005, 38, 471–477. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Landines, E.F.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of Drying Process and Particle Size of Persimmon Fibre on Its Physicochemical, Antioxidant, Hydration and Emulsifying Properties. J. Food Sci. Technol. 2017, 54, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant Characteristics of Honey from Mozambique Based on Specific Flavonoids and Phenolic Acid Compounds. J. Food Compos. Anal. 2020, 86, 103377. [Google Scholar] [CrossRef]
- Canett Romero, R.; Víctor Hugo Domínguez Corrales, V.H.; Torres Montaño, G. Important Aspects of Moringa oleifera: An Alternative to Treat Anemia Due to Iron Deficiency. Biotecnia 2016, 18, 3–9. [Google Scholar] [CrossRef]
- BEDCA. Base de Datos Española de Composición de Alimentos. Available online: https://www.bedca.net/ (accessed on 3 April 2024).
- U.S. Department of Agriculture. FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/ (accessed on 3 April 2024).
- Patras, A.; Tiwari, B.K.; Brunton, N.P. Influence of Blanching and Low Temperature Preservation Strategies on Antioxidant Activity and Phytochemical Content of Carrots, Green Beans and Broccoli. LWT Food Sci. Technol. 2011, 44, 299–306. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.; Roy, K.R.; Sadia, U.; Zzaman, W. Effect of Thermal Processing on Physicochemical and Antioxidant Properties of Raw and Cooked Moringa oleifera Lam. Pods. Int. J. Food Sci. 2022, 2022, 1502857. [Google Scholar] [CrossRef] [PubMed]
- Preti, R.; Rapa, M.; Vinci, G. Effect of Steaming and Boiling on the Antioxidant Properties and Biogenic Amines Content in Green Bean (Phaseolus vulgaris) Varieties of Different Colours. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]




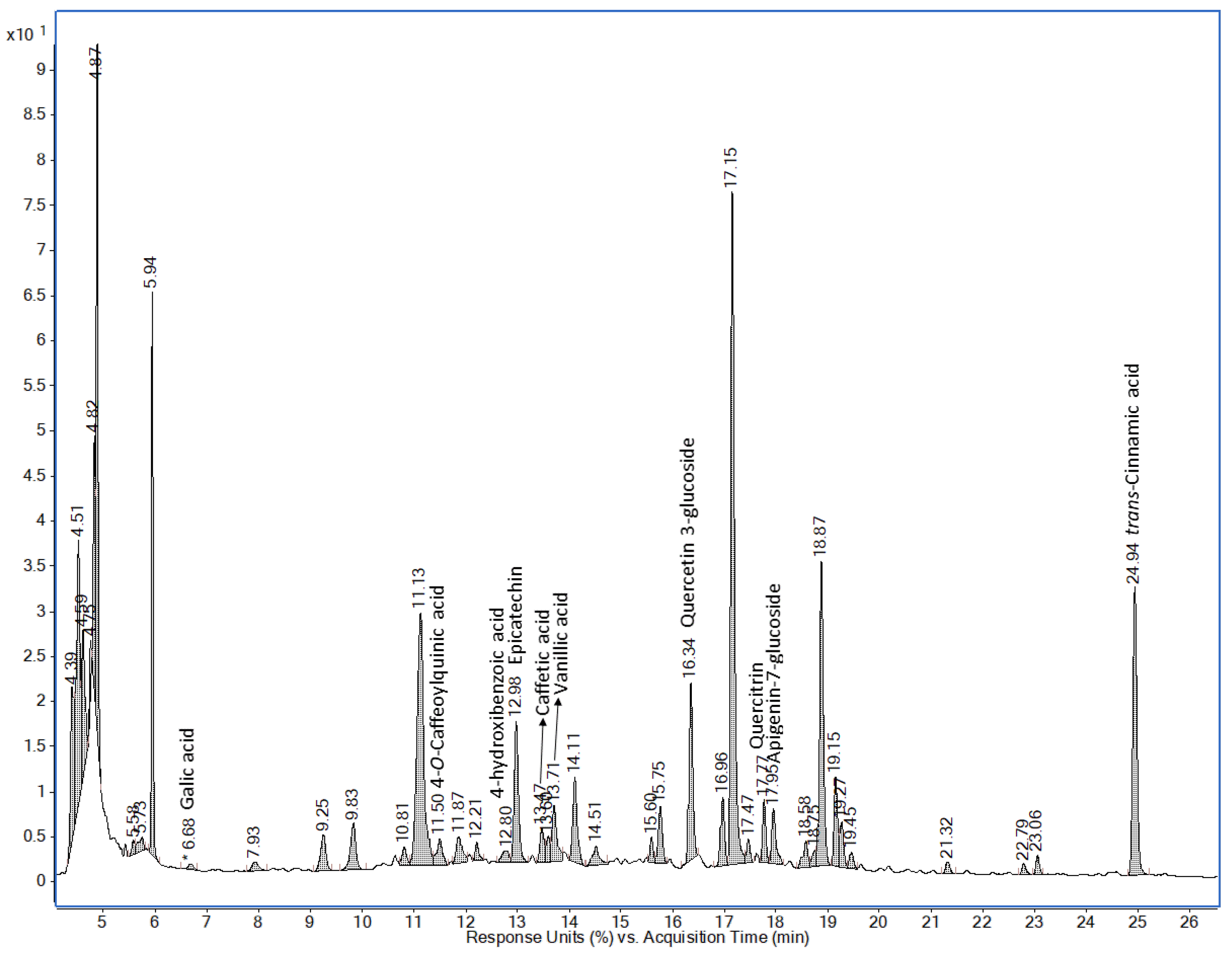

| Time (Minutes) | % A |
|---|---|
| 0 | 90 |
| 3 | 85 |
| 18 | 60 |
| 24 | 60 |
| 27 | 34 |
| 27 | 50 |
| 33 | 30 |
| 40 | 10 |
| 43 | 90 |
| 45 | 90 |
| Cooking Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | 20 min | |||||
| Mature Pods | Tender Pods | Mature Pods | Tender Pods | Mature Pods | Tender Pods | Mature Pods | Tender Pods | |
| phenolic acids | ||||||||
| Ellagic acid | 1.715 (0.56) | 8.047 (0.39) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-Coumaric acid | 0.956 (0.95) | 5.396 (0.25) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid | 0.429 (0.43) | 1.338 (0.28) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Trans-cinnamic acid | 0.111 (0.02) | 0.480 (0.08) | 0.566 (0.0009) | 2.279 (0.03) | 0.299 (0.013) | 0.249 (0.0005) | n.d. | n.d. |
| Gallic acid | n.d. | n.d. | n.d. | 0.527 (0.02) | n.d. | n.d. | n.d. | n.d. |
| 4-O-caffeoylquinic acid | n.d. | n.d. | 0.475 (0.004) | 1.375 (0.02) | 0.535 (0.0106) | 1.328 (0.014) | 0.611 (0.01) | 1.710 (0.015) |
| 4-Hydroxybenzoic acid | n.d. | n.d. | 0.236 (0.0005) | 0.439 (0.010) | 0.226 (0.0005) | 0.469 (0.003) | 0.244 (0.03) | 0.432 (0.0003) |
| Caffeic acid | n.d. | n.d. | 0.454 (0.002) | 1.048 (0.06) | 0.456 (0.0002) | 0.934 (0.003) | 0.461 (0.001) | 0.931 (0.0012) |
| Vanillic acid | n.d. | n.d. | 0.624 (0.04) | 2.316 (0.04) | 0.569 (0.02) | 2.625 (0.04) | 0.576 (0.03) | 1.664 (0.17) |
| flavonoids | ||||||||
| Quercetin 3-glucoside | n.d. | n.d. | 2.348 (0.17) | 11.033 (0.09) | 2.616 (0.006) | 9.725 (0.03) | 2.68 (0.15) | 7.868 (0.08) |
| Quercitrin | n.d. | n.d. | 0.610 (0.013) | 2.978 (0.04) | 0.586 (0.0012) | 2.688 (0.02) | 0.673 (0.03) | 2.922 (0.03) |
| Apigenin-7-glucoside | n.d. | n.d. | 0.968 (0.020) | 2.526 (0.019) | 0.913 (0.005) | 1.967 (0.011) | 0.896 (0.07) | 1.595 (0.006) |
| Epicatechin | n.d. | n.d. | 11.797 (0.012) | 54.135 (0.28) | 5.897 (0.006) | 54.075 (0.0003) | 5.334 (0.02) | 27.346 (0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelló, M.L.; Sesé, T.; García-Mares, F.J.; Juan-Borrás, M.d.S.; Ortolá, M.D. Influence of Boiling Time on Chemical Composition and Properties of Tender and Mature Moringa Pods. Foods 2024, 13, 1823. https://doi.org/10.3390/foods13121823
Castelló ML, Sesé T, García-Mares FJ, Juan-Borrás MdS, Ortolá MD. Influence of Boiling Time on Chemical Composition and Properties of Tender and Mature Moringa Pods. Foods. 2024; 13(12):1823. https://doi.org/10.3390/foods13121823
Chicago/Turabian StyleCastelló, María Luisa, Tomás Sesé, Francisco José García-Mares, María del Sol Juan-Borrás, and María Dolores Ortolá. 2024. "Influence of Boiling Time on Chemical Composition and Properties of Tender and Mature Moringa Pods" Foods 13, no. 12: 1823. https://doi.org/10.3390/foods13121823





