Lactic Acid Fermentation Ameliorates Intrinsic Toxicants in Brassica campestris L. Leaves Harvested at Different Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of B. campestris Leaf Samples
2.2. Morphological Attributes of B. campestris Plant at Different Growth Stages
2.3. Sample Preparation of B. campestris Leaves
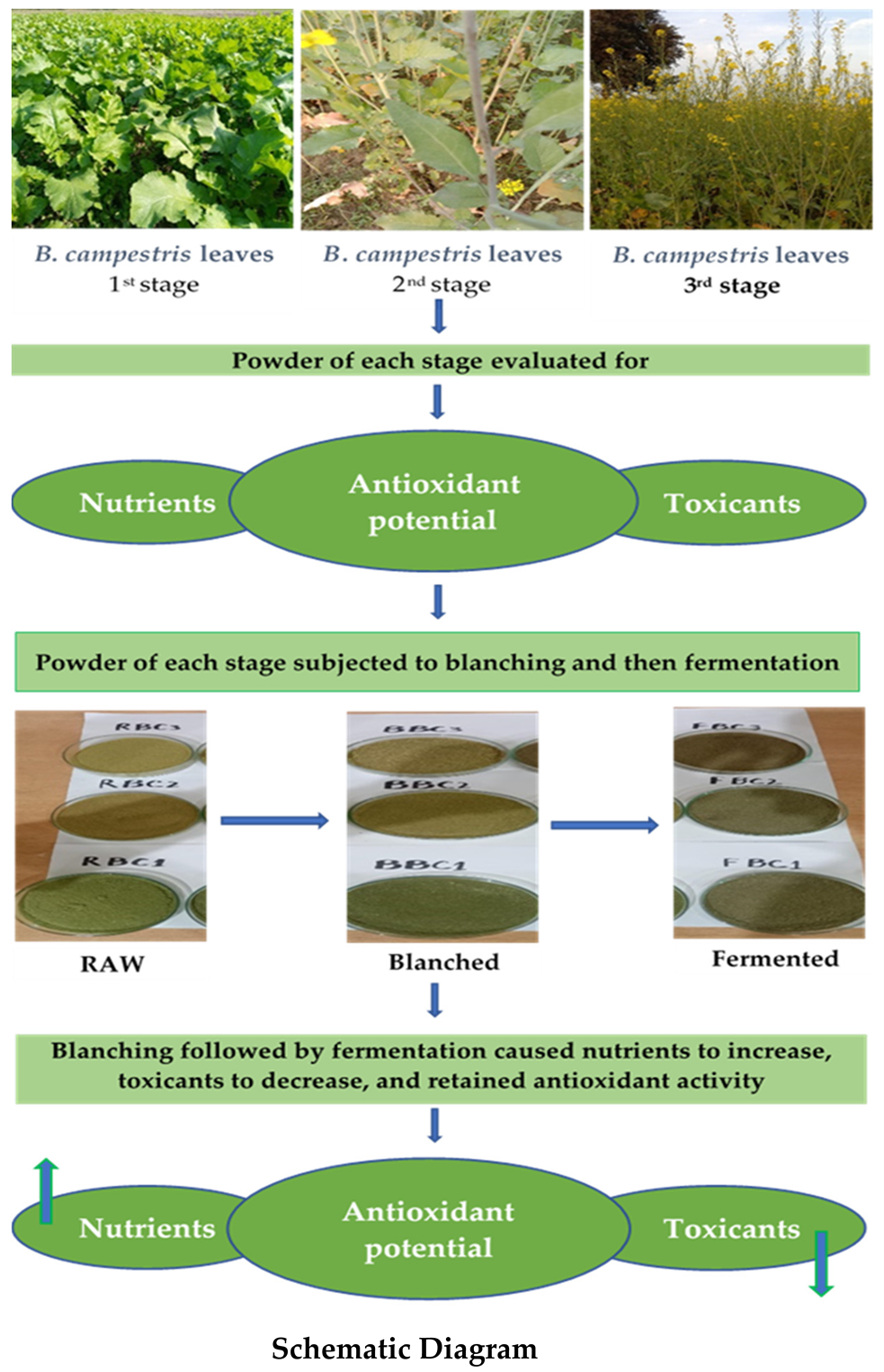
2.4. Fermentation of B. campestris Fresh Leaves Harvested at Different Growth Stages
2.5. Determination of pH and Acidity during the Fermentation Process
2.6. Dehydration of Raw, Blanched and Fermented B. campestris Leaves
2.7. Chlorophyll a and b, Total Chlorophyll and Anthocyanin Content Analysis
2.8. Proximate Analysis of the Samples Harvested at Different Growth Stages
2.9. Color Tonality of B. campestris Fresh Leaves and Powders
2.10. DPPH Radical Scavenging Activity
2.11. Total Phenolic Content (TPC)
2.12. Ferric Reducing Antioxidant Power (FRAP) Assay
2.13. Total Flavonoid Content (TFC)
2.14. Estimation of Phytates (Phytic Acid)
2.15. Determination of Tannin Contents
2.16. Total Saponin Content
2.17. Oxalate Contents
2.18. Determination of the Alkaloid Contents
2.19. Statistical Analysis
3. Results
3.1. Morphological Attribute of B. campestris
3.2. pH and Acidity Changes during the Fermentation of B. campestris Leaves
3.3. Chlorophyll a and b, Total Chlorophyll and Anthocyanin Contents in B. campestris Leaves
3.4. Proximate Composition of B. campestris
3.5. Color Tonality of B. campestris Leaves
3.6. Antioxidants Potential of the B. campestris Leaf Powder Extracts
3.7. Anti-Nutritional Factors Found in the B. campestris Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, M.; Hassani, D.; Bilal, M.; Liao, J.; Huang, D. Elevation of secondary metabolites synthesis in Brassica campestris ssp. chinensis L. via exogenous inoculation of Piriformospora indica with appropriate fertilizer. PLoS ONE 2017, 12, e0177185. [Google Scholar]
- Agrawal, M.K.; Rathore, D.; Goyal, S.; Varma, A.; Varma, A. Antibacterial efficacy of Brassica campestris root, stem and leaves extracts. Int. J. Adv. Res. 2013, 1, 131–135. [Google Scholar]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Stace, C. New Flora of the British Isles; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Wagle, D.S. Nutritional and antinutritional factors of green leafy vegetables. J. Agric. Food Chem. 1988, 36, 472–474. [Google Scholar] [CrossRef]
- Ugwu, F.M.; Oranye, N.A. Effects of some processing methods on the toxic components of African breadfruit (Treculia africana). AJB 2006, 5, 2329–2333. [Google Scholar]
- Popova, A.; Mihaylova, D. Antinutrients in plant-based foods: A review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An overview of nutritional and antinutritional factors in green leafy vegetables. Hortic. Int. J. 2017, 1, 58–65. [Google Scholar] [CrossRef]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Berghetti, Á.L.P.; Araujo, M.M.; Tabaldi, L.A.; Aimi, S.C.; Tonetto, T.D.S.; Turchetto, F.; Brunetto, G. Morphological and physiological parameters in young plants of cordia trichotoma submitted to the application of phosphorus in the soil. Rev. Arvore. 2020, 44, e4404. [Google Scholar] [CrossRef]
- Kitum, V.C.; Kinyanjui, P.K.; Mathara, J.M.; Sila, D.N. Effect of Lb. plantarum BFE 5092 fermentation on antinutrient and oligosaccharide composition of whole red haricot bean (Phaseolus vulgaris L). Int. J. Food Sci. 2020, 2020, 8876394. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Applications of Biotechnology to Fermented Foods: Report of an Ad Hoc Panel of the Board on Science and Technology for International Development; National Research Council: Ottawa, ON, Canada, 1992.
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S. Effects of Salt Concentration on the Physicochemical Properties and Microbial Safety of Spontaneously Fermented Cabbage. Master’s Thesis, The University of Maine, Orono, ME, USA, 2019; pp. 1–119. [Google Scholar]
- Matela, K.S.; Pillai, M.K.; Thamae, T. Evaluation of pH, titratable acidity, syneresis and sensory profiles of some yoghurt samples from the kingdom of Lesotho. Food Res. 2019, 3, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Galla, N.R.; Pamidighantam, P.R.; Karakala, B.; Gurusiddaiah, M.R.; Akula, S. Nutritional, textural and sensory quality of biscuits supplemented with spinach (Spinacia oleracea L.). Int. J. Gastron Food Sci. 2017, 7, 20–26. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Olaniyan, S.A.; Adebayo, L.O.; Ademola, A.A. Malted sorghum-soy composite flour: Preparation, chemical and physico-chemical properties. J. Food Sci. Technol. 2015, 6, 467. [Google Scholar]
- Kamble, P.N.; Giri, S.P.; Mane, R.S.; Tiwana, A. Estimation of chlorophyll content in young and adult leaves of some selected plants. UJERT 2015, 5, 306–310. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis 2004; AOAC: Washington, DC, USA, 2004. [Google Scholar]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Mosha, T.C.; Gaga, H.E. Nutritive value and effect of blanching on the trypsin and chymotrypsin inhibitor activities of selected leafy vegetables. Plant Foods Hum. Nutr. 1999, 54, 271–283. [Google Scholar] [CrossRef]
- Hossain, M.; Ahmed, K.; Chowdhury, F.; Roksana, K.; Islam, S.; Barman, A. Experimental study on grain weight, moisture, ash, carbohydrates, protein, oil, total energy and minerals content of different varieties of rapeseed and mustard (Brassica spp.). Int. J. Sci. Res. Publ. 2015, 5, 394–400. [Google Scholar]
- Ohizua, E.R.; Adeola, A.A.; Idowu, M.A.; Sobukola, O.P.; Afolabi, T.A.; Ishola, R.O.; Falomo, A. Nutrient composition, functional, and pasting properties of unripe cooking banana, pigeon pea, and sweet potato flour blends. Food Sci. Nutr. 2017, 5, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Neeharika, B.; Patel, K.; Suneetha, J.; Kumari, B.A. Blanched bathua leaves: Influence on phytochemical and antioxidant properties. Int. J. Conserv. Sci. 2019, 7, 793–799. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, J.K.; Kim, S.G.; Kim, S.H.; Chun, T.; Imm, J.Y. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int. J. Food Sci. Technol. 2019, 46, 2513–2519. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Zeb, A.; Mahmood, F.; Muhammad, S.; Shah, S.A.; Chaudry, M.A.; Qureshi, M.J. Modification of rapid method for phytic acid determination in wheat and its production. Biol. Sci.-PJSIR 2004, 47, 322–324. [Google Scholar]
- Sharma, A.; Aacharya, M.; Punetha, H.; Sharma, S.; Kumari, N.; Rai, P.K. Biochemical characterization and correlations in Brassica juncea genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2408–2417. [Google Scholar] [CrossRef]
- Saxena, V.A.S.U.N.D.H.A.R.A.; Mishra, G.; Saxena, A.; Vishwakarma, K.R. A comparative study on quantitative estimation of tannins in Terminalia chebula, Terminalia belerica, Terminalia arjuna and Saraca indica using spectrophotometer. Asian J. Pharm. Clin. Res. 2013, 6, 148–149. [Google Scholar]
- Dini, C.; Doporto, M.C.; García, M.A.; Viña, S.Z. Nutritional profile and anti-nutrient analyses of Pachyrhizus ahipa roots from different accessions. Food Res. Int. 2013, 54, 255–261. [Google Scholar] [CrossRef]
- Mishra, D.P.; Mishra, N.; Musale, H.B.; Samal, P.; Mishra, S.P.; Swain, D.P. Determination of seasonal and developmental variation in oxalate content of Anagallis arvensis plant by titration and spectrophotometric method. Pharma Innov. 2017, 6 Part B, 105. [Google Scholar]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life 2012, 31, 198. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.S.; Zasada, J.C.; Tappeiner, J.C., II; Stone, D.M. Plant morphological characteristics as a tool in monitoring response to silvicultural activities. Commun. Role Silvic. Manag. Natl. For. 1997, 37–41. [Google Scholar]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Chapter 5. Composition and activity of microbiota in sourdough and their effect on bread quality and safety. In Trends in Wheat and Bread Making; Charis, M., Galanakis, Eds.; Elsevier-Academic Press: Cambridge, MA, USA, 2021; pp. 129–172. ISBN 978-0-12-821048-2. [Google Scholar]
- Fraberger, V.; Özülkü, G.; Petrova, P.; Nada, K.; Petroc, K.; Domig, K.J.; Rocha, J.M. Chapter 10. Sourdough as a source of technological, antimicrobial and probiotic microorganisms. In Sourdough Innovations: Novel Uses of Metabolites, Enzymes and Microbiota from Sourdough Processing; Garcia-Vaquero, M., Rocha, J.M., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2023; p. 464. ISBN 978-0-367-67497-7. [Google Scholar]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic acid fermentation of vegetables and fruits. Microorg. Ferment. Tradit. Foods 2014, 108–140. [Google Scholar]
- Misci, C.; Taskin, E.; Dall’Asta, M.; Fontanella, M.C.; Bandini, F.; Imathiu, S.; Puglisi, E. Fermentation as a tool for increasing food security and nutritional quality of indigenous African leafy vegetables: The case of Cucurbita sp. Food Microbiol. 2021, 99, 103820. [Google Scholar] [CrossRef]
- Ebah, E.E.; Wusuum, B.; Akande, T.; Emmanuel, O.O.; Ikala, R.O.; Ode, T.A. Effect of lactic-acid fermentation on the shelf life of vegetables. Am. J. Innova. Res. App. Sci. 2019, 90, 328–334. [Google Scholar]
- Tanaka, A.; Tanaka, R. The biochemistry, physiology, and evolution of the chlorophyll cycle. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 183–212. [Google Scholar]
- Mulay, J.; Kokate, S. Estimation of chlorophyll content in young and adult leaves of some selected plants in polluted areas. IJARIIT 2019, 5, 1300–1307. [Google Scholar]
- Różyło, R.; Piekut, J.; Dziki, D.; Smolewska, M.; Gawłowski, S.; Wójtowicz, A.; Gawlik-Dziki, U. Effects of Wet and Dry Micronization on the GC-MS Identification of the Phenolic Compounds and Antioxidant Properties of Freeze-Dried Spinach Leaves and Stems. Molecules 2022, 27, 8174. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Smith, W.K.; Vogelmann, T.C. Ontogenetic differences in mesophyll structure and chlorophyll distribution in Eucalyptus globulus ssp. globulus (Myrtaceae). Am. J. Bot. 1999, 6, 198–207. [Google Scholar] [CrossRef]
- Abid, R.; Islam, M.; Saeed, H.; Ahmad, A.; Imtiaz, F.; Yasmeen, A.; Rathore, H.A. Antihypertensive potential of Brassica rapa leaves: An in vitro and in silico approach. Front. Pharmacol. 2022, 13, 996755. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zuo, J. Nutritional and Anti-Nutritional Composition of Rapeseed Meal and Its Utilization as a Feed Ingredient for Animal; International Consultative Group for Research on Rapeseed: Wuhan, China, 2007; pp. 265–270. [Google Scholar]
- Budniak, L.; Slobodianiuk, L.; Marchyshyn, S.; Basaraba, R.; Banadyga, A. The antibacterial and antifungal activities of the extract of Gentiana cruciata L. herb. Pharmacologyonline 2021, 2, 188–197. [Google Scholar]
- Arshad, N.; Akhtar, S.; Ismail, T.; Saeed, W.; Qamar, M.; Özogul, F.; Bartkiene, E.; Rocha, J.M. The Comparative Effect of Lactic Acid Fermentation and Germination on the Levels of Neurotoxin, Anti-Nutrients, and Nutritional Attributes of Sweet Blue Pea (Lathyrus sativus L.). Foods 2023, 12, 2851. [Google Scholar] [CrossRef] [PubMed]
- Naseem, A.; Akhtar, S.; Ismail, T.; Qamar, M.; Sattar, D.-e.-s.; Saeed, W.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Growth Stages and Lactic Acid Fermentation on Anti-Nutrients and Nutritional Attributes of Spinach (Spinacia oleracea). Microorganisms 2023, 11, 2343. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Egbewole, O.O.; Ifesan, B.T. Effect of fermentation on nutritional composition of selected commonly consumed green leafy vegetables in Nigeria. Int. J. Appl. Sci. Biotechnol. 2014, 2, 291–297. [Google Scholar] [CrossRef]
- Ayo-Omogie, H.N.; Jolayemi, O.S.; Chinma, C.E. Fermentation and blanching as adaptable strategies to improve nutritional and functional properties of unripe Cardaba banana flour. J. Agric. Food Res. 2021, 6, 100214. [Google Scholar] [CrossRef]
- Akinola, S.; Badejo, A.; Osundahunsi, O.; Edema, M. Effect of preprocessing techniques on pearl millet flour and changes in technological properties. IJFST 2017, 52, 992–999. [Google Scholar] [CrossRef]
- Oyarekua, M. Sensory evaluation, nutritional quality and antinutritional factors of traditionally co-fermented cereals/cowpea mixtures as infant complementary food. Agric. Biol. J. N. Am. 2010, 1, 950–956. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Hussen, E.M.; Endalew, S.A. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement. Med. Ther. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Shyamala, B.N.; Gupta, S.; Lakshmi, A.J.; Prakash, J. Leafy vegetable extracts—Antioxidant activity and effect on storage stability of heated oils. IFSET 2005, 6, 239–245. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. AJCN 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Fuster, J.M.B.; Cortés, P.S.; Bestard, J.P.; Freixedas, F.G. Plant phosphates, phytate and pathological calcifications in chronic kidney disease. Nefrol. (Engl. Ed.) 2017, 37, 20–28. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Akhtar, S.; Qamar, M.; Saeed, W.; Ismail, T.; Esatbeyoglu, T. Effect of Thermal and Non-Thermal Processing on Nutritional, Functional, Safety Characteristics and Sensory Quality of White Cabbage Powder. Foods 2022, 11, 3802. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Akhtar, S.; Mehmood, T.; Qamar, M.; Saeed, W.; Younis, M.; Perveen, S.; Ismail, T.; Esatbeyoglu, T. Nutritional, safety and sensory quality evaluation of unleavened flatbread supplemented with thermal and non-thermal processed spinach powder. J. Agric. Food Res. 2024, 16, 101114. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.A.; Chanliaud, E.; Rémésy, C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.R.; Hsieh, S.C.; Huang, H.Y.; Chou, C.C. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J. Biosci. Bioeng. 2013, 115, 552–556. [Google Scholar] [CrossRef]
- Abifarin, T.O.; Otunola, G.A.; Afolayan, A.J. Nutritional composition and antinutrient content of Heteromorpha arborescens (Spreng.) Cham. & Schltdl. leaves: An underutilized wild vegetable. Food Sci. Nutr. 2021, 9, 172–179. [Google Scholar]

| Plant Age (Week) | Root Size (inch) | Stem Size (inch) | Leaf Size (inch) | Leaf Width (inch) | Plant Height (inch) | Leaf Count |
|---|---|---|---|---|---|---|
| 4th | 7.22 ± 0.81 c | 12.10 ± 3.12 c | 9.17 ± 2.60 b | 3.80 ± 0.76 b | 19.32 ± 3.43 c | 9.00 ± 1.58 c |
| 6th | 10.04 ± 1.36 b | 45.79 ± 8.03 b | 10.71 ± 2.69 b | 4.58 ± 3.48 ab | 55.83 ± 8.06 b | 45.00 ± 6.20 b |
| 8th | 11.09 ± 2.20 a | 74.14 ± 7.59 a | 15.64 ± 3.25 a | 5.95 ± 3.66 a | 85.23 ± 8.03 a | 62.00 ± 5.10 a |
| F-ratio | 52.7 ** | 708 ** | 44.9 ** | 4.36 * | 747 ** | 164 ** |
| Samples | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Anthocyanin (mg/g) |
|---|---|---|---|---|
| RBC1 | 31.98 ± 0.06 c | 51.82 ± 0.11 ab | 83.80 ± 0.09 bc | 134.33 ± 4.53 bc |
| BBC1 | 31.75 ± 0.05 c | 51.46 ± 0.07 ab | 83.21 ± 0.11 bc | 131.74 ± 5.98 bc |
| FBC1 | 32.81 ± 0.03 a | 52.04 ± 0.08 a | 84.85 ± 0.11 ab | 124.02 ± 4.33 c |
| RBC2 | 32.27 ± 0.48 b | 49.59 ± 3.92 bc | 81.85 ± 3.44 cd | 159.50 ± 8.87 a |
| BBC2 | 31.70 ± 0.04 c | 51.28 ± 0.03 ab | 82.98 ± 0.07 bc | 138.52 ± 7.63 b |
| FBC2 | 32.99 ± 0.02 a | 52.87 ± 0.04 a | 85.86 ± 0.04 a | 136.29 ± 8.30 b |
| RBC3 | 32.93 ± 0.02 a | 39.16 ± 0.02 e | 72.08 ± 0.04 f | 158.85 ± 4.04 a |
| BBC3 | 32.32 ± 0.04 b | 44.72 ± 0.01 d | 77.04 ± 0.03 e | 156.28 ± 5.03 a |
| FBC3 | 33.06 ± 0.07 a | 47.69 ± 0.03 c | 80.75 ± 0.08 d | 135.25 ± 5.91 b |
| F-ratio | 31.4 ** | 35.2 ** | 42.4 ** | 12.9 ** |
| Plant Part | Moisture (%) | Total Ash (%) | Crude Fat (%) | Crude Protein (%) | Crude Fiber (%) | NFE (%) | Energy (Kcal/100 g) |
|---|---|---|---|---|---|---|---|
| RBC1 | 0.91 ± 0.06 bc | 5.36 ± 0.16 b | 9.88 ± 1.05 cd | 9.02 ± 0.62 d | 9.58 ± 0.15 | 64.47 ± 0.79 a | 381.07 ± 6.66 abc |
| BBC1 | 0.91 ± 0.07 bc | 5.25 ± 0.22 b | 9.46 ± 0.05 bcd | 9.48 ± 1.21 d | 9.36 ± 0.64 | 64.48 ± 1.39 a | 385.91 ± 3.11 a |
| FBC1 | 0.82 ± 0.06 c | 5.98 ± 0.50 b | 9.16 ± 0.52 cd | 10.43 ± 0.47 cd | 10.71 ± 0.07 | 64.25 ± 0.37 a | 382.21 ± 1.18 ab |
| RBC2 | 1.05 ± 0.09 b | 5.58 ± 0.19 b | 9.36 ± 0.08 d | 11.03 ± 1.66 bcd | 9.23 ± 0.39 | 61.66 ± 2.40 ab | 370.62 ± 1.43 c |
| BBC2 | 0.91 ± 0.10 bc | 5.58 ± 0.02 b | 10.10 ± 0.06 bc | 11.93 ± 1.95 bc | 10.07 ± 2.47 | 61.41 ± 4.41 ab | 383.88 ± 10.28 a |
| FBC2 | 0.77 ± 0.03 c | 7.87 ± 0.81 a | 9.00 ± 0.21 b | 14.22 ± 1.17 a | 9.77 ± 2.08 | 59.12 ± 1.65 bc | 387.19 ± 8.80 a |
| RBC3 | 1.37 ± 0.16 a | 8.12 ± 0.68 a | 9.64 ± 0.14 cd | 8.85 ± 0.79 d | 9.32 ± 0.98 | 63.17 ± 1.35 a | 372.56 ± 6.92 bc |
| BBC3 | 1.01 ± 0.10 b | 5.57 ± 0.07 b | 12.01 ± 0.51 a | 11.85 ± 1.29 c | 9.19 ± 1.20 | 57.46 ± 1.79 c | 385.34 ± 4.69 a |
| FBC3 | 0.79 ± 0.03 c | 8.52 ± 0.43 a | 9.11 ± 0.37 cd | 12.81 ± 1.85 ab | 9.93 ± 0.89 | 60.99 ± 2.43 abc | 382.40 ± 5.79 ab |
| F-ratio | 12.3 ** | 46.2 ** | 12.3 ** | 4.62 ** | 0.47 ns | 3.10 * | 2.66 * |
| Treatment | L* | a* | b* |
|---|---|---|---|
| FrBC1 | 54.80 ± 1.02 e | –9.72 ± 1.29 g | 21.37 ± 3.08 f |
| RBC1 | 60.69 ± 1.33 bc | –9.29 ± 0.16 fg | 34.14 ± 0.76 b |
| BBC1 | 57.17 ± 0.31 d | –6.72 ± 0.39 e | 29.30 ± 0.70 c |
| FBC1 | 56.68 ± 0.49 d | –0.89 ± 0.29 b | 24.94 ± 0.98 de |
| FrBC2 | 53.52 ± 1.78 f | –8.35 ± 0.98 f | 16.36 ± 0.54 h |
| RBC2 | 59.26 ± 2.14 c | −1.23 ± 0.62 b | 36.06 ± 0.43 b |
| BBC2 | 59.14 ± 1.01 c | –4.60 ± 0.60 d | 34.36 ± 1.34 b |
| FBC2 | 55.39 ± 0.94 de | –0.92 ± 0.10 b | 23.53 ± 1.10 e |
| FrBC3 | 54.16 ± 1.32 ef | –9.04 ± 0.88 fg | 18.87 ± 1.81 g |
| RBC3 | 66.65 ± 0.89 a | –4.25 ± 0.57 d | 38.90 ± 1.05 a |
| BBC3 | 62.17 ± 0.44 b | –2.53 ± 0.05 c | 33.91 ± 1.22 b |
| FBC3 | 51.88 ± 1.22 g | 2.63 ± 0.21 a | 26.55 ± 1.96 d |
| F-ratio | 48.0 ** | 131 ** | 109 ** |
| Solvent | Sample | Acetone | Ethanol | n-Hexane | Methanol |
|---|---|---|---|---|---|
| DPPH (%) | RBC1 | 83.22 ± 3.11 a | 37.71 ± 0.82 b | 62.11 ± 0.67 c | 61.86 ± 0.03 g |
| BBC1 | 37.27 ± 2.28 de | 34.29 ± 0.87 cd | 42.03 ± 0.34 f | 85.66 ± 0.02 e | |
| FBC1 | 39.32 ± 3.16 d | 37.46 ± 0.21 b | 43.46 ± 0.25 ef | 91.88 ± 0.08 c | |
| RBC2 | 84.82 ± 3.73 a | 35.63 ± 0.47 bc | 77.06 ± 2.46 a | 59.15 ± 0.04 h | |
| BBC2 | 37.70 ± 4.27 de | 32.63 ± 0.24 de | 39.21 ± 0.47 g | 62.05 ± 0.01 g | |
| FBC2 | 51.09 ± 1.89 c | 49.77 ± 2.64 a | 50.48 ± 0.61 d | 94.32 ± 0.02 b | |
| RBC3 | 86.87 ± 1.30 a | 30.83 ± 0.29 ef | 75.20 ± 0.55 b | 98.80 ± 0.80 a | |
| BBC3 | 34.45 ± 0.05 e | 29.56 ± 0.56 f | 35.57 ± 0.55 h | 67.01 ± 0.08 f | |
| FBC3 | 71.24 ± 1.06 b | 34.45 ± 0.36 cd | 43.81 ± 0.37 e | 88.61 ± 0.06 d | |
| F-ratio | 222 ** | 56.2 ** | 820 ** | 10,604 ** | |
| TPC (mg GAE/g) | RBC1 | 140.30 ± 10.02 de | 357.99 ± 9.33 c | 341.36 ± 7.52 b | 306.17 ± 0.16 d |
| BBC1 | 186.13 ± 9.83 b | 363.93 ± 1.01 b | 93.88 ± 4.531 | 361.53 ± 10.81 b | |
| FBC1 | 245.59 ± 6.04 a | 388.28 ± 2.14 a | 203.39 ± 7.55 g | 377.91 ± 12.17 a | |
| RBC2 | 124.40 ± 5.50 e | 338.82 ± 1.02 d | 178.85 ± 9.78 h | 279.66 ± 12.03 e | |
| BBC2 | 155.39 ± 7.35 d | 370.37 ± 1.33 a | 302.96 ± 3.91 d | 287.22 ± 2.41 e | |
| FBC2 | 246.78 ± 5.31 a | 384.78 ± 1.14 b | 367.71 ± 9.42 a | 387.35 ± 7.96 a | |
| RBC3 | 153.57 ± 9.30 f | 375.24 ± 3.24 b | 316.82 ± 10.09 c | 345.16 ± 9.38 c | |
| BBC3 | 77.40 ± 4.98 g | 339.54 ± 0.87 c | 270.29 ± 3.98 f | 352.45 ± 5.97 b | |
| FBC3 | 171.22 ± 9.87 c | 356.54 ± 5.68 d | 283.43 ± 6.53 e | 360.84 ± 5.14 b | |
| F-ratio | 145 ** | 71.8 ** | 419 ** | 67.5 ** | |
| FRAP (µg/mL) | RBCI | 125.51 ± 0.18 a | 101.48 ± 1.95 f | 77.87 ± 1.19 bc | 98.07 ± 1.89 b |
| BBC1 | 87.65 ± 1.32 c | 119.67 ± 0.13 c | 75.09 ± 0.44 d | 66.53 ± 0.44 f | |
| FBC1 | 108.23 ± 0.13 b | 134.81 ± 0.36 b | 78.59 ± 0.42 b | 89.20 ± 1.42 d | |
| RBC2 | 88.29 ± 5.39 c | 110.21 ± 1.06 d | 73.75 ± 0.38 e | 57.69 ± 0.15 g | |
| BBC2 | 85.79 ± 2.01 c | 96.12 ± 1.02 g | 77.15 ± 0.38 c | 56.58 ± 0.36 g | |
| FBC2 | 86.47 ± 1.17 c | 105.12 ± 0.26 e | 74.23 ± 0.14 de | 75.47 ± 0.24 e | |
| RBC3 | 86.60 ± 1.68 c | 101.61 ± 1.14 f | 74.42 ± 0.44 de | 90.82 ± 0.42 c | |
| BBC3 | 73.42 ± 0.53 e | 101.15 ± 1.97 f | 77.10 ± 0.51 c | 88.40 ± 0.17 d | |
| FBC3 | 78.22 ± 0.94 d | 150.09 ± 0.15 a | 80.43 ± 1.64 a | 107.72 ± 0.13 a | |
| F-ratio | 172 ** | 784 ** | 26.8 ** | 1412 ** | |
| Flavonoids (mg CE/g) | RBC1 | 33.75 ± 2.81 g | 395.32 ± 7.87 b | 167.42 ± 7.58 d | 386.01 ± 8.33 d |
| BBC1 | 93.04 ± 4.74 cd | 422.42 ± 7.91 a | 214.77 ± 6.56 c | 414.02 ± 6.31 c | |
| FBC1 | 148.61 ± 3.12 b | 432.24 ± 12.71 a | 338.73 ± 2.46 a | 430.73 ± 8.20 b | |
| RBC2 | 41.52 ± 1.46 f | 399.88 ± 7.36 b | 71.31 ± 3.07 e | 236.35 ± 2.60 e | |
| BBC2 | 89.00 ± 3.81 d | 403.06 ± 3.44 b | 284.99 ± 3.59 b | 380.95 ± 4.56 d | |
| FBC2 | 142.70 ± 5.82 b | 404.47 ± 2.84 b | 327.01 ±2.77 a | 409.72 ± 5.63 c | |
| RBC3 | 78.49 ± 1.51 e | 402.10 ± 3.17 b | 81.27 ± 1.56 e | 406.68 ± 2.27 c | |
| BBC3 | 97.50 ± 7.21 c | 395.27 ± 0.47 b | 175.31 ± 8.13 d | 411.23 ± 3.54 c | |
| FBC3 | 180.49 ± 3.90 a | 393.75 ± 9.19 b | 221.47 ± 2.32 c | 448.17 ± 4.43 a | |
| F-ratio | 1826 ** | 10.4 ** | 460 ** | 376 ** |
| Sample | Phytates (mg/100 g) | Tannins (mg/100 g) | Saponins (mg/100 g) | Oxalates (mg/100 g) | Alkaloids (mg/100 g) |
|---|---|---|---|---|---|
| RBC1 | 134.70 ± 0.20 a | 163.82 ± 4.75 ab | 30.85 ± 0.38 a | 1440.36 ± 47.91 c | 0.43 ± 0.07 cde |
| BBC1 | 75.57 ± 0.29 c | 162.57 ± 2.86 abc | 20.26 ± 3.12 c | 1234.86 ± 15.85 de | 0.39 ± 0.02 def |
| FBC1 | 44.01 ± 6.35 e | 147.70 ± 5.00 d | 11.98 ± 0.10 ef | 1155.14 ± 50.39 e | 0.37 ± 0.03 ef |
| RBC2 | 28.67 ± 0.12 g | 160.32 ± 7.23 bc | 27.88 ± 0.37 ef | 1819.37 ± 55.84 a | 0.46 ± 0.05 c |
| BBC2 | 26.46 ± 0.04 f | 154.24 ± 2.35 a | 12.68 ± 0.15 e | 1500.45 ± 46.05 c | 0.37 ± 0.05 def |
| FBC2 | 19.21 ± 0.07 h | 57.36 ± 3.28 e | 13.39 ± 0.25 de | 1186.04 ± 71.54 e | 0.35 ± 0.03 f |
| RBC3 | 87.04 ± 0.06 b | 168.92 ± 0.80 a | 14.98 ± 0.95 d | 1705.95 ± 51.93 b | 0.66 ± 0.03 a |
| BBC3 | 66.48 ± 0.39 d | 158.02 ± 3.12 bc | 9.91 ± 1.80 f | 1278.38 ± 40.25 d | 0.56 ± 0.01 b |
| FBC3 | 43.54 ± 0.05 e | 156.53 ± 4.94 c | 11.37 ± 0.96 ef | 1283.51 ± 51.61 d | 0.44 ± 0.04 cd |
| F-ratio | 845 ** | 209 ** | 103 ** | 66.6 ** | 19.8 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, M.; Akhtar, S.; Ismail, T.; Qamar, M.; Sattar, D.-e.-s.; Saeed, W.; Mubarak, M.S.; Bartkiene, E.; Rocha, J.M. Lactic Acid Fermentation Ameliorates Intrinsic Toxicants in Brassica campestris L. Leaves Harvested at Different Growth Stages. Foods 2024, 13, 1826. https://doi.org/10.3390/foods13121826
Younis M, Akhtar S, Ismail T, Qamar M, Sattar D-e-s, Saeed W, Mubarak MS, Bartkiene E, Rocha JM. Lactic Acid Fermentation Ameliorates Intrinsic Toxicants in Brassica campestris L. Leaves Harvested at Different Growth Stages. Foods. 2024; 13(12):1826. https://doi.org/10.3390/foods13121826
Chicago/Turabian StyleYounis, Muhammad, Saeed Akhtar, Tariq Ismail, Muhammad Qamar, Dur-e-shahwar Sattar, Wisha Saeed, Mohammad S. Mubarak, Elena Bartkiene, and João Miguel Rocha. 2024. "Lactic Acid Fermentation Ameliorates Intrinsic Toxicants in Brassica campestris L. Leaves Harvested at Different Growth Stages" Foods 13, no. 12: 1826. https://doi.org/10.3390/foods13121826
APA StyleYounis, M., Akhtar, S., Ismail, T., Qamar, M., Sattar, D.-e.-s., Saeed, W., Mubarak, M. S., Bartkiene, E., & Rocha, J. M. (2024). Lactic Acid Fermentation Ameliorates Intrinsic Toxicants in Brassica campestris L. Leaves Harvested at Different Growth Stages. Foods, 13(12), 1826. https://doi.org/10.3390/foods13121826









