Chicken Juice Enhances C. jejuni NCTC 11168 Biofilm Formation with Distinct Morphological Features and Altered Protein Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media, and Culture Conditions
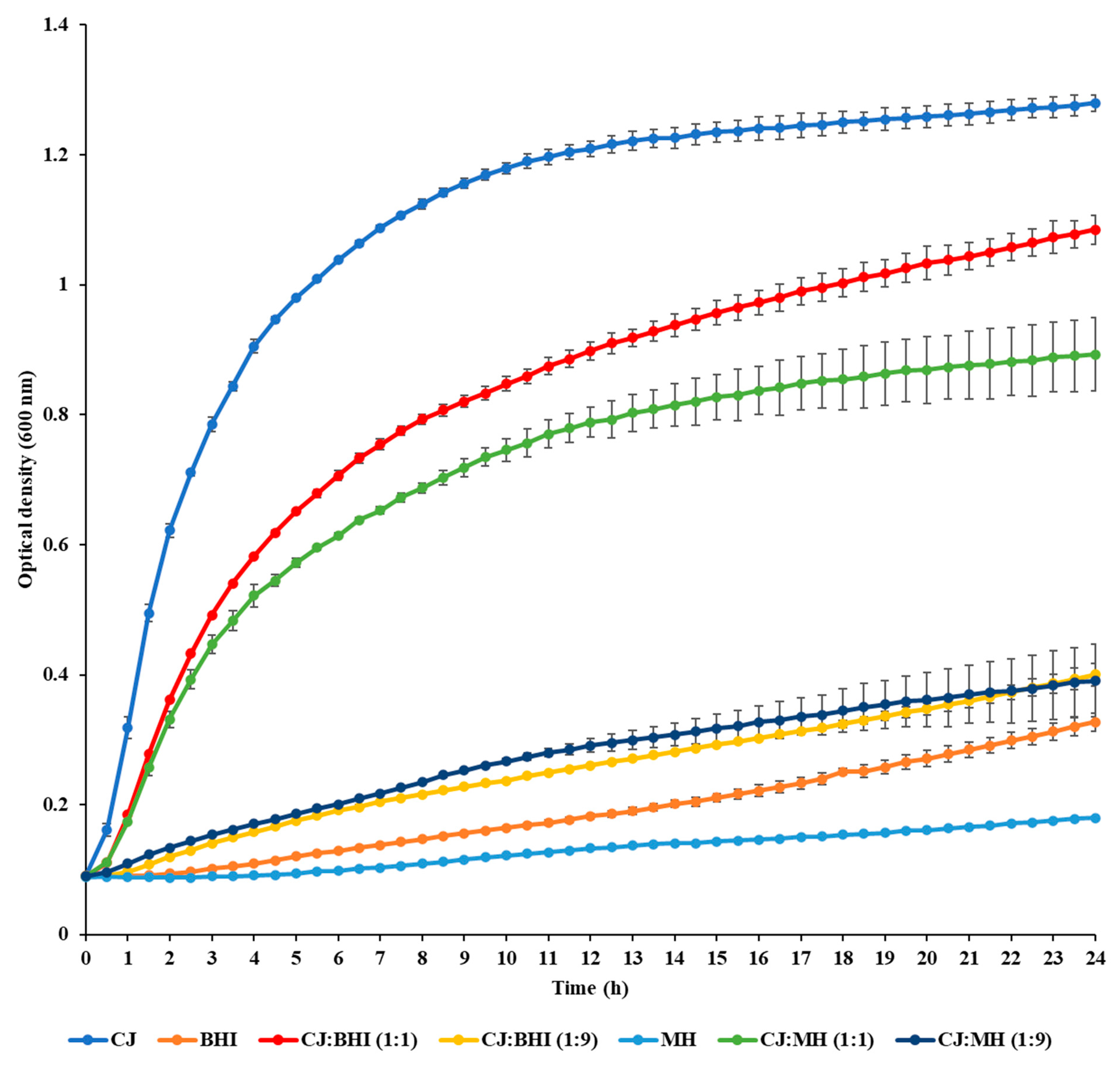
2.2. Measurement of Growth
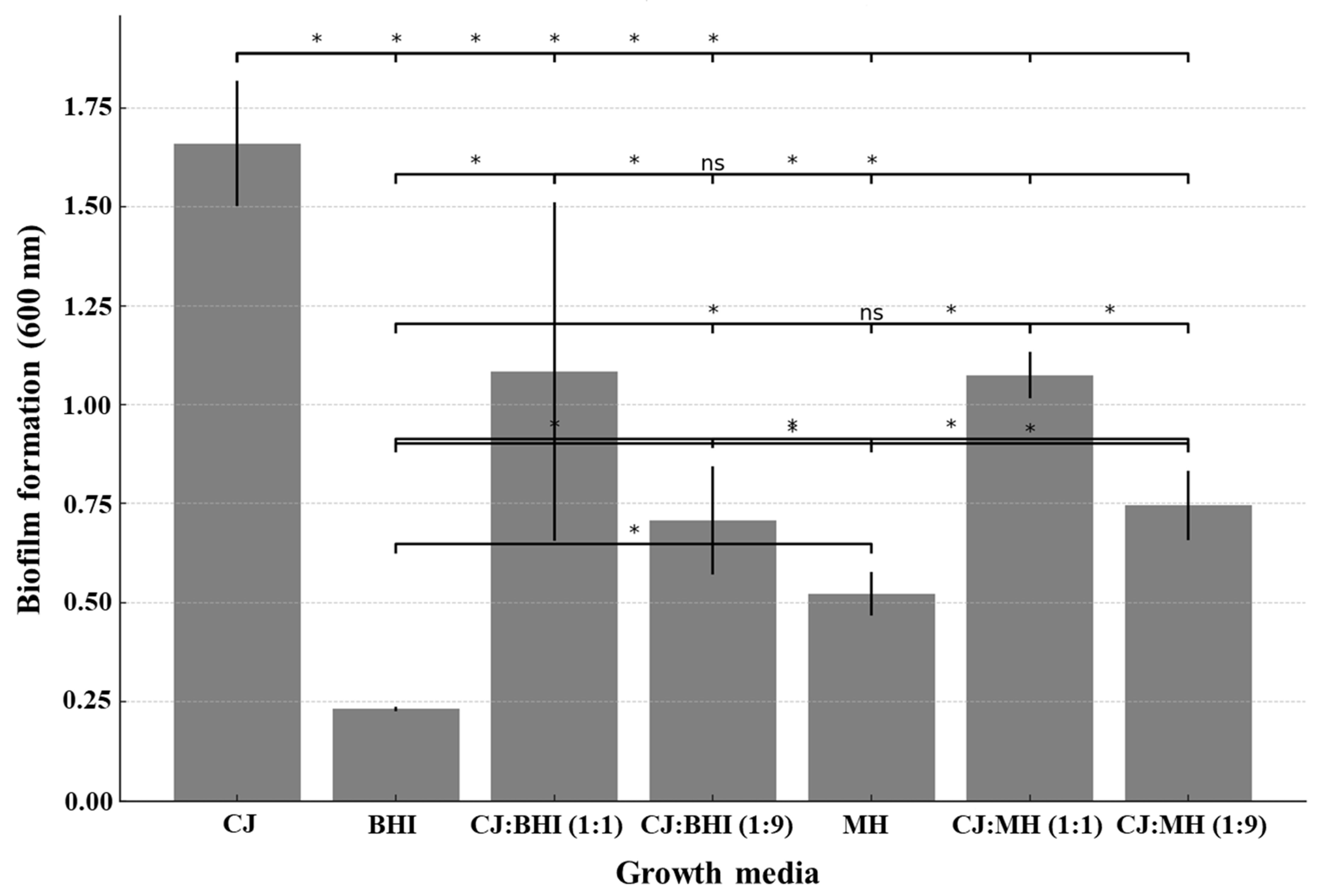
2.3. Measurement of Biofilm Formation
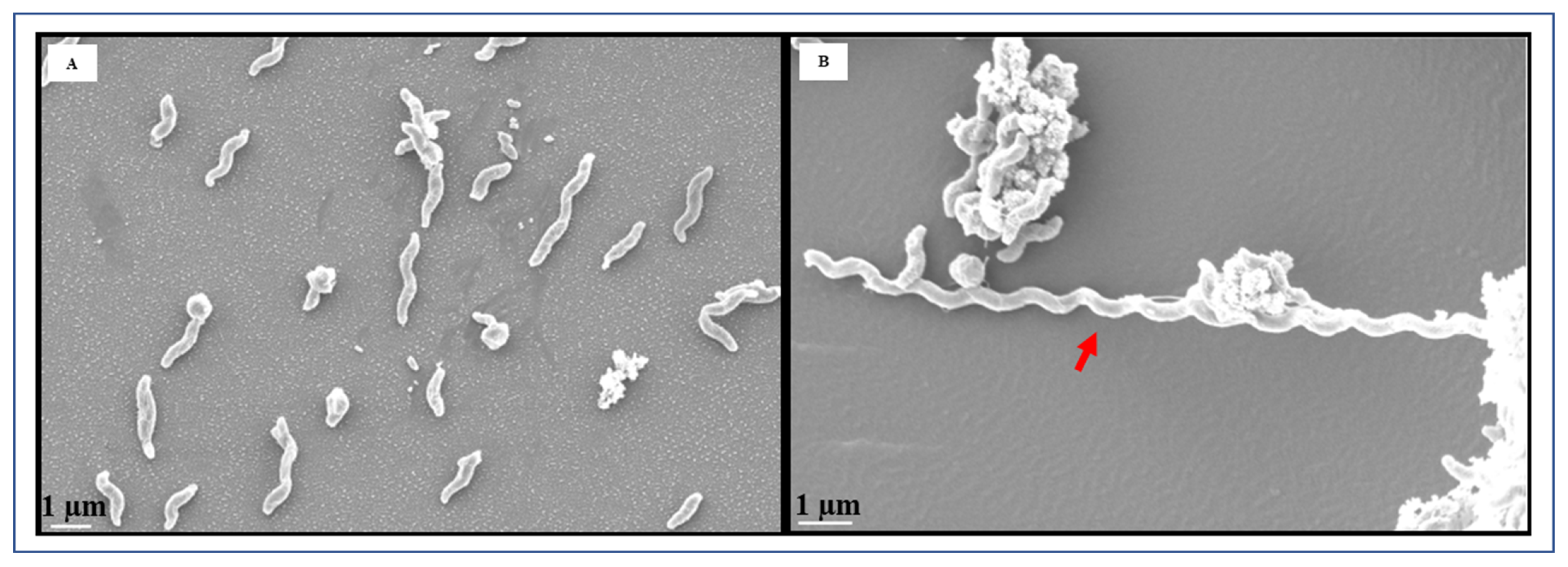
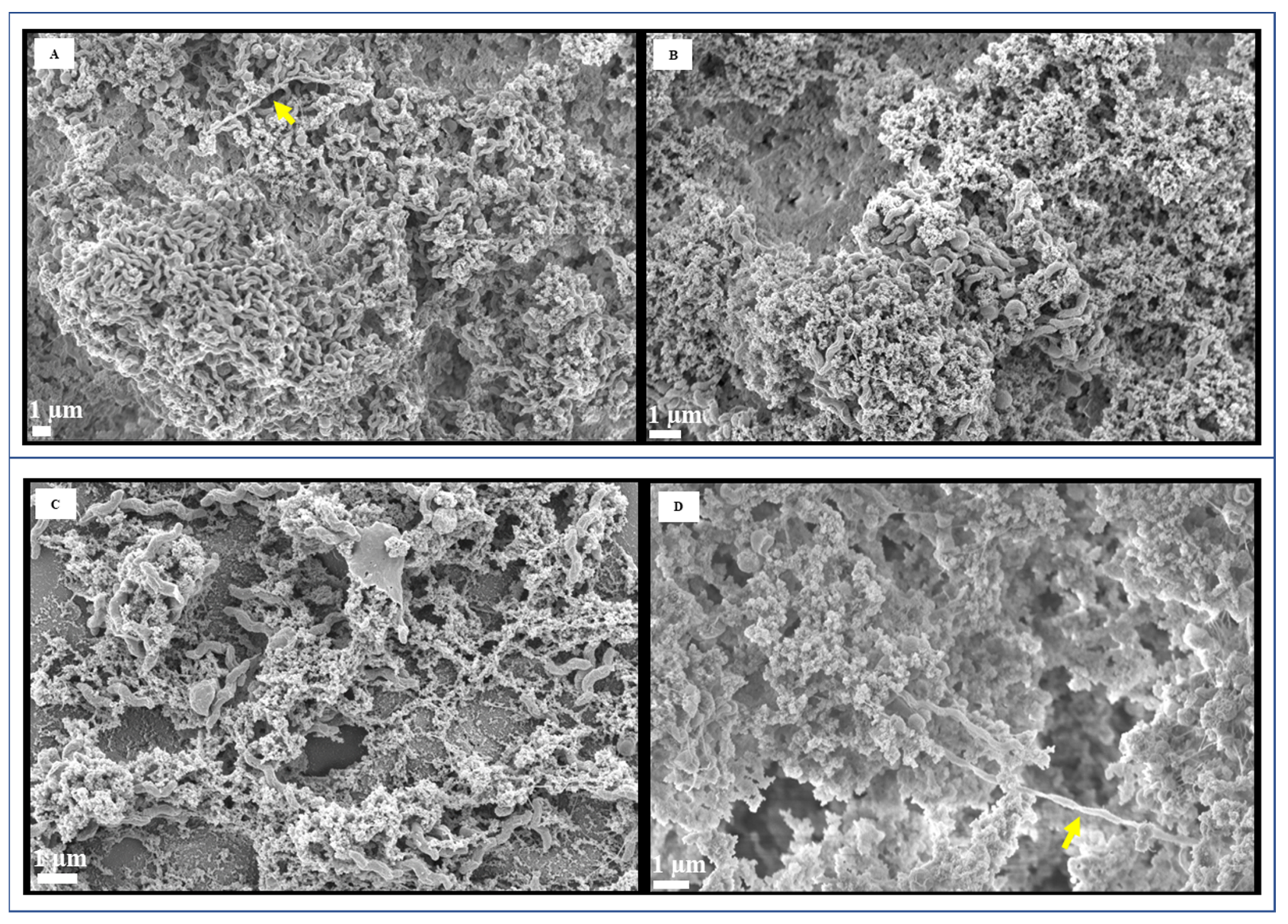
2.4. Electron Microscopy
2.5. Protein Extraction
2.6. Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC-MS/MS)
3. Results
3.1. Growth Rate and Biofilm Formation in CJ
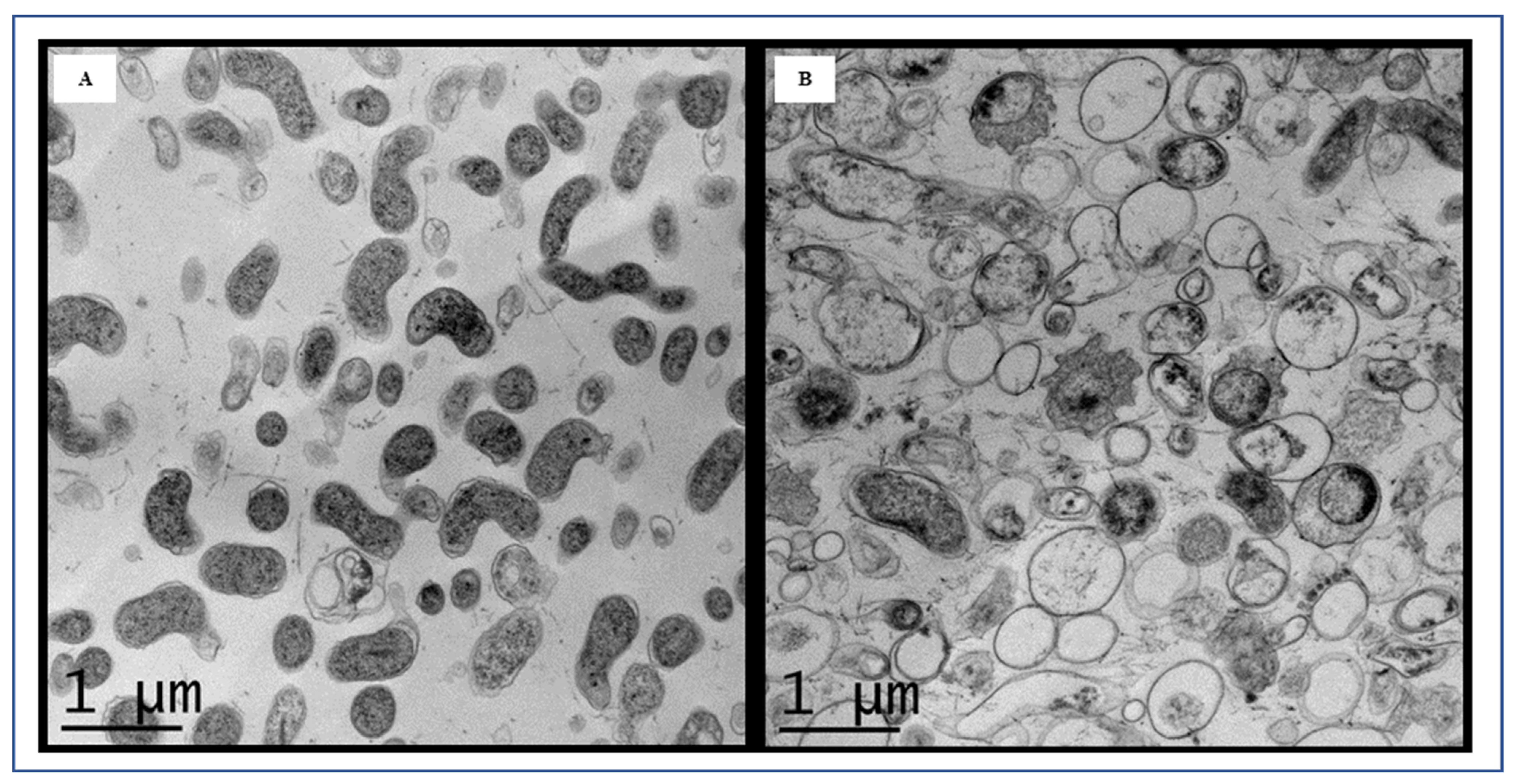
3.2. Microscopic Image Analysis by FESEM and TEM
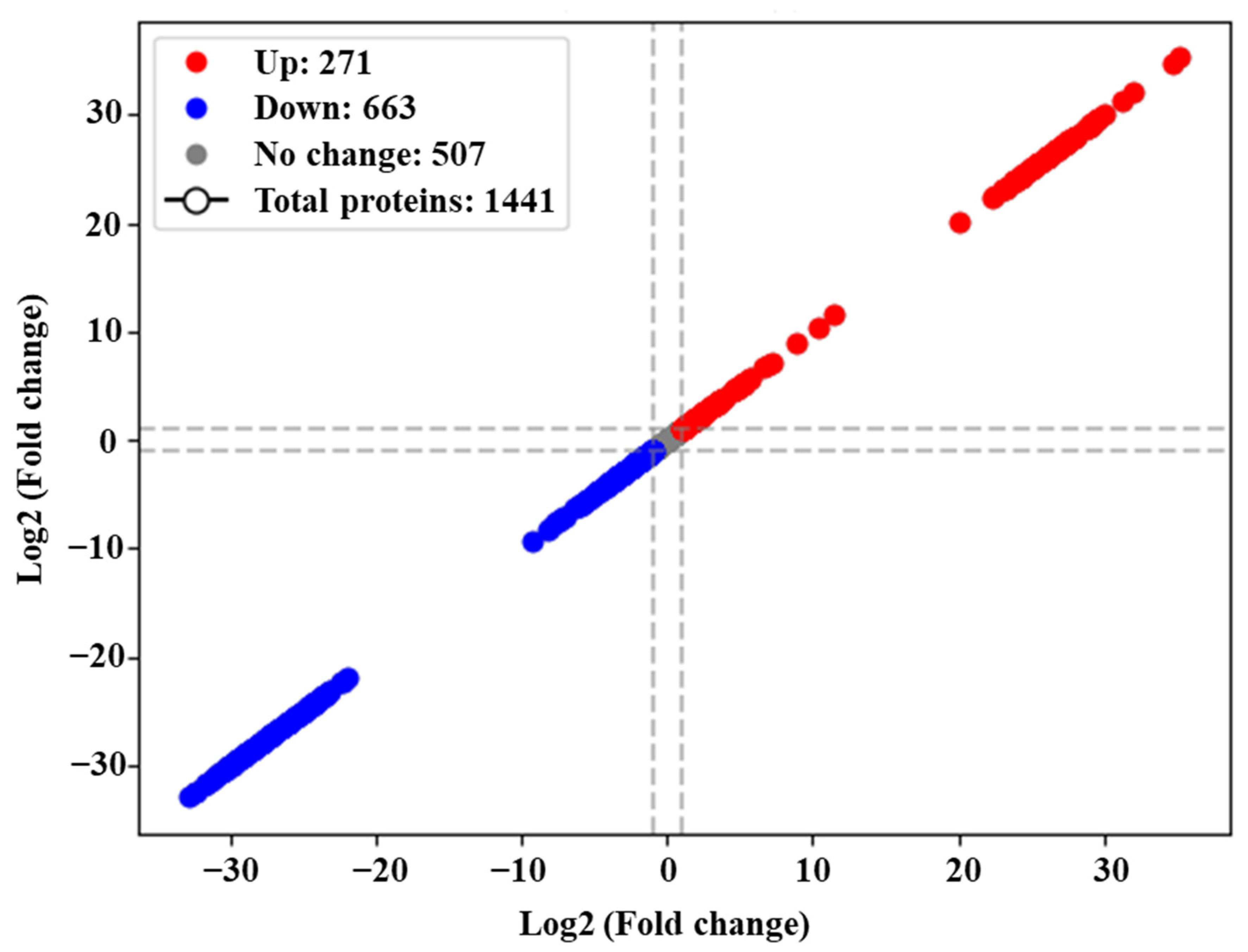
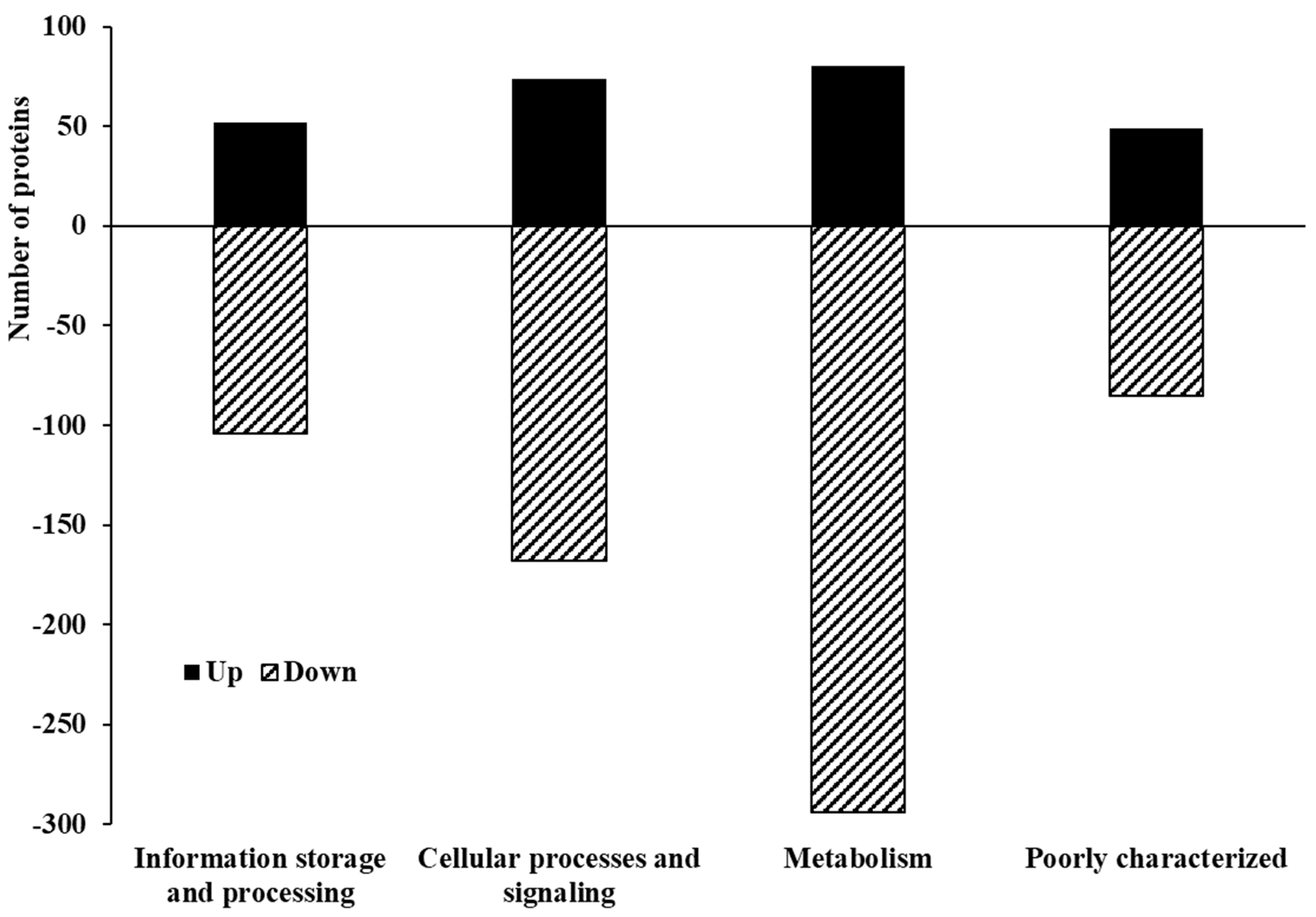
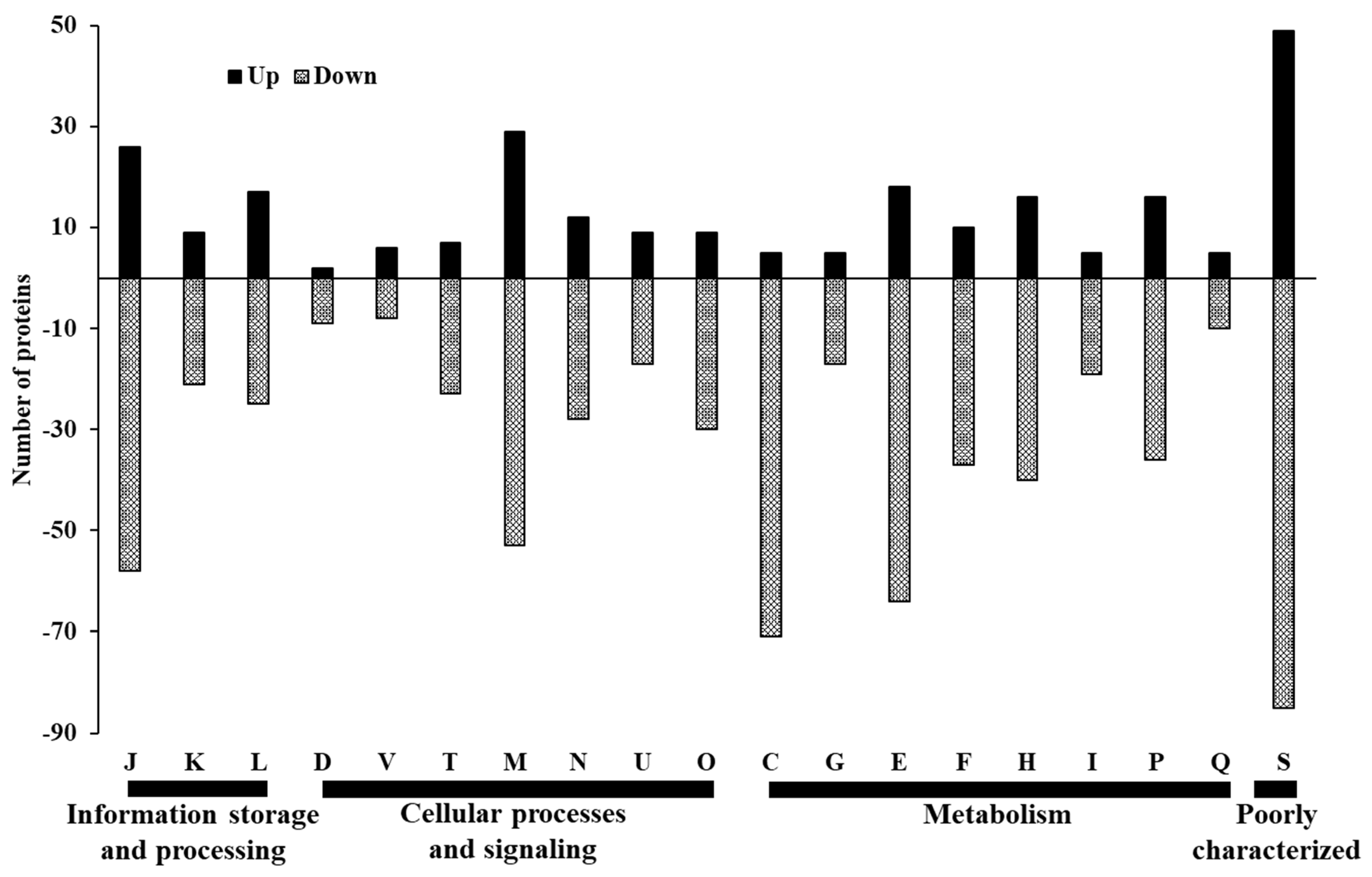
3.3. Proteome Profile of C. jejuni Biofilms in Chicken Juice after 72 h of Incubation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allos, B.M. Campylobacter jejuni Infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [PubMed]
- Liu, F.; Lee, S.A.; Xue, J.; Riordan, S.M.; Zhang, L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 979055. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Facciola, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Lagana, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar] [PubMed]
- Keithlin, J.; Sargeant, J.; Thomas, M.K.; Fazil, A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public. Health 2014, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.C. Campylobacter jejuni enteritis associated with consumption of raw milk. J. Environ. Health 2003, 65, 20–21, 24, 26. [Google Scholar] [PubMed]
- Zimmer, M.; Barnhart, H.; Idris, U.; Lee, M.D. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 2003, 47, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.A.; Bloomfield, S.F.; Humphrey, T.J. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett. Appl. Microbiol. 1999, 29, 354–358. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Zhang, Z.; Banaei-Esfahani, A.; Lalanne, J.B.; Okano, H.; Collins, B.C.; Schmidt, A.; Schubert, O.T.; Lee, D.S.; Li, G.W.; et al. From coarse to fine: The absolute Escherichia coli proteome under diverse growth conditions. Mol. Syst. Biol. 2021, 17, e9536. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Park, M.; Chon, J.; Kweon, O.; Khan, S.A.; Shen, A.; Paredes, A. Concentration-Dependent Global Quantitative Proteome Response of Staphylococcus epidermidis RP62A Biofilms to Subinhibitory Tigecycline. Cells 2022, 11, 3488. [Google Scholar] [CrossRef] [PubMed]
- Hanning, I.; Jarquin, R.; Slavik, M. Campylobacter jejuni as a secondary colonizer of poultry biofilms. J. Appl. Microbiol. 2008, 105, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Joshua, G.W.P.; Guthrie-Irons, C.; Karlyshev, A.V.; Wren, B.W. Biofilm formation in Campylobacter jejuni. Microbiology 2006, 152, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Nicodeme, M.; Tresse, O.; Houard, E.; Jugiau, F.; Courtillon, C.; El Manaa, K.; Laisney, M.J.; Chemaly, M. Characterization of Campylobacter spp. transferred from naturally contaminated chicken legs to cooked chicken slices via a cutting board. Int. J. Food Microbiol. 2013, 164, 7–14. [Google Scholar] [CrossRef]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef] [PubMed]
- Ligowska, M.; Cohn, M.T.; Stabler, R.A.; Wren, B.W.; Brondsted, L. Effect of chicken meat environment on gene expression of Campylobacter jejuni and its relevance to survival in food. Int. J. Food Microbiol. 2011, 145 (Suppl. 1), S111–S115. [Google Scholar] [CrossRef]
- Birk, T.; Ingmer, H.; Andersen, M.T.; Jorgensen, K.; Brondsted, L. Chicken juice, a food-based model system suitable to study survival of Campylobacter jejuni. Lett. Appl. Microbiol. 2004, 38, 66–71. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, 2437. [Google Scholar]
- Wilkinson, J.D.; Callicott, R.; Salminen, W.F.; Sandhu, S.K.; Greenhaw, J.; Paredes, A.; Davis, K.; Jones, Y.; Paule, M.G.; Slikker, W., Jr.; et al. A randomized controlled laboratory study on the long-term effects of methylphenidate on cardiovascular function and structure in rhesus monkeys. Pediatr. Res. 2019, 85, 398–404. [Google Scholar] [CrossRef]
- Sung, K.; Chon, J.; Kweon, O.; Nho, S.; Kim, S.; Park, M.; Paredes, A.; Lim, J.H.; Khan, S.A.; Phillips, K.S.; et al. Dynamic Adaptive Response of Pseudomonas aeruginosa to Clindamycin/Rifampicin-Impregnated Catheters. Antibiotics 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Moggridge, S.; Muller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Moggridge, S.; Sorensen, P.H.; Morin, G.B.; Hughes, C.S. Extending the Compatibility of the SP3 Paramagnetic Bead Processing Approach for Proteomics. J. Proteome Res. 2018, 17, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Jean-Toussaint, R.; Tian, Y.; Chaudhuri, A.D.; Haughey, N.J.; Sacan, A.; Ajit, S.K. Proteome characterization of small extracellular vesicles from spared nerve injury model of neuropathic pain. J. Proteom. 2020, 211, 103540. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.T.; Franz, M.; Kazi, F.; Donaldson, S.L.; Morris, Q.; Bader, G.D. Cytoscape Web: An interactive web-based network browser. Bioinformatics 2010, 26, 2347–2348. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron Through Oxidative Stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Birk, T.; Rosenquist, H.; Brondsted, L.; Ingmer, H.; Bysted, A.; Christensen, B.B. A comparative study of two food model systems to test the survival of Campylobacter jejuni at °18 degrees C. J. Food Prot. 2006, 69, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Karlyshev, A.V. Putative mechanisms and biological role of coccoid form formation in Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2012, 2, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.N.; Davis, B.; Tirado, S.M.; Duggal, M.; van Frankenhuyzen, J.K.; Deaville, D.; Wijesinghe, M.A.; Tessaro, M.; Trevors, J.T. Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Leeuwenhoek 2009, 96, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Attaran, B.; Falsafi, T. Identification of Factors Associated with Biofilm Formation Ability in the Clinical Isolates of Helicobacter pylori. Iran. J. Biotechnol. 2017, 15, 58–66. [Google Scholar] [CrossRef]
- Cellini, L.; Grande, R.; Di Campli, E.; Traini, T.; Di Giulio, M.; Lannutti, S.N.; Lattanzio, R. Dynamic colonization of Helicobacter pylori in human gastric mucosa. Scand. J. Gastroenterol. 2008, 43, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, E.; Bakhshi, B.; Kalani, B.S.; Talebi, M.; Irajian, G. Transcriptome analysis of biofilm formation under aerobic and microaerobic conditions in clinical isolates of Campylobacter spp. Res. Vet. Sci. 2021, 142, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tram, G.; Klare, W.P.; Cain, J.A.; Mourad, B.; Cordwell, S.J.; Korolik, V.; Day, C.J. RNA Sequencing Data Sets Identifying Differentially Expressed Transcripts during Campylobacter jejuni Biofilm Formation. Microbiol. Resour. Announc. 2020, 9, e00982-19. [Google Scholar] [CrossRef]
- Lopez-Fernandez, M.; Westmeijer, G.; Turner, S.; Broman, E.; Ståhle, M.; Bertilsson, S.; Dopson, M. Thiobacillus as a key player for biofilm formation in oligotrophic groundwaters of the Fennoscandian Shield. NPJ Biofilms Microbiomes 2023, 9, 41. [Google Scholar] [CrossRef]
- Benamara, H.; Rihouey, C.; Jouenne, T.; Alexandre, S. Impact of the biofilm mode of growth on the inner membrane phospholipid composition and lipid domains in Pseudomonas aeruginosa. Biochim. Biophys. Acta 2011, 1808, 98–105. [Google Scholar] [CrossRef]
- Lees-Miller, R.G.; Iwashkiw, J.A.; Scott, N.E.; Seper, A.; Vinogradov, E.; Schild, S.; Feldman, M.F. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol. Microbiol. 2013, 89, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Khan, M.M.; Chattagul, S.; Tran, B.Q.; Freiberg, J.A.; Nita-Lazar, A.; Shirtliff, M.E.; Sermswan, R.W.; Ernst, R.K.; Goodlett, D.R. Temporal proteomic profiling reveals changes that support Burkholderia biofilms. Pathog. Dis. 2019, 77, ftz005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Ramic, D.; Jug, B.; Simunovic, K.; Tusek Znidaric, M.; Kunej, U.; Toplak, N.; Kovac, M.; Fournier, M.; Jamnik, P.; Smole Mozina, S.; et al. The Role of luxS in Campylobacter jejuni Beyond Intercellular Signaling. Microbiol. Spectr. 2023, 11, e0257222. [Google Scholar] [CrossRef] [PubMed]
- Rampadarath, S.; Bandhoa, K.; Puchooa, D.; Jeewon, R.; Bal, S. Early bacterial biofilm colonizers in the coastal waters of Mauritius. Electron. J. Biotechnol. 2017, 29, 13–21. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amirkhani, A.; Chowdhury, D.; Mempin, M.; Molloy, M.P.; Deva, A.K.; Vickery, K.; Hu, H.H. Proteome of Staphylococcus aureus Biofilm Changes Significantly with Aging. Int. J. Mol. Sci. 2022, 23, 6415. [Google Scholar] [CrossRef]
- Kawamura-Sato, K.; Iinuma, Y.; Hasegawa, T.; Horii, T.; Yamashino, T.; Ohta, M. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob. Agents Chemother. 2000, 44, 2869–2872. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Vallet, I.; Olson, J.W.; Lory, S.; Lazdunski, A.; Filloux, A. The chaperone/usher pathways of Pseudomonas aeruginosa: Identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 2001, 98, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Kentache, T.; Ben Abdelkrim, A.; Jouenne, T.; De, E.; Hardouin, J. Global Dynamic Proteome Study of a Pellicle-forming Acinetobacter baumannii Strain. Mol. Cell Proteom. 2017, 16, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Murphy, K.; Krieger, J.R.; Brewer, D.; Taylor, P.; Habash, M.; Khursigara, C.M. A temporal examination of the planktonic and biofilm proteome of whole cell Pseudomonas aeruginosa PAO1 using quantitative mass spectrometry. Mol. Cell Proteom. 2014, 13, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M.T.; Ingmer, H.; Mulholland, F.; Jorgensen, K.; Wells, J.M.; Brondsted, L. Contribution of conserved ATP-dependent proteases of Campylobacter jejuni to stress tolerance and virulence. Appl. Environ. Microbiol. 2007, 73, 7803–7813. [Google Scholar] [CrossRef]
- Lemos, J.A.; Burne, R.A. Regulation and Physiological Significance of ClpC and ClpP in Streptococcus mutans. J. Bacteriol. 2002, 184, 6357–6366. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Dong, D.; Wang, J.; Ren, J.; Otto, M.; Gao, Q. Role of ClpP in biofilm formation and virulence of Staphylococcus epidermidis. Microbes Infect. 2007, 9, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Breidenstein, E.B.; Song, D.; Hancock, R.E. Role of intracellular proteases in the antibiotic resistance, motility, and biofilm formation of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, Y.; Li, G.; Zhou, L.; Liu, S.; Wang, C. The ClpP protease is required for the stress tolerance and biofilm formation in Actinobacillus pleuropneumoniae. PLoS ONE 2013, 8, e53600. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Cao, Q.; Feng, F.; Xu, X.; Cai, X. ClpP participates in stress tolerance and negatively regulates biofilm formation in Haemophilus parasuis. Vet. Microbiol. 2016, 182, 141–149. [Google Scholar] [CrossRef]
- Capestany, C.A.; Tribble, G.D.; Maeda, K.; Demuth, D.R.; Lamont, R.J. Role of the Clp system in stress tolerance, biofilm formation, and intracellular invasion in Porphyromonas gingivalis. J. Bacteriol. 2008, 190, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Qin, J.; Cheng, S.; Yeo, W.S.; He, L.; Ma, X.; Liu, X.; Li, M.; Bae, T. The ATP-Dependent Protease ClpP Inhibits Biofilm Formation by Regulating Agr and Cell Wall Hydrolase Sle1 in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2017, 7, 181. [Google Scholar] [CrossRef]
- Gundogdu, O.; da Silva, D.T.; Mohammad, B.; Elmi, A.; Mills, D.C.; Wren, B.W.; Dorrell, N. The Campylobacter jejuni MarR-like transcriptional regulators RrpA and RrpB both influence bacterial responses to oxidative and aerobic stresses. Front. Microbiol. 2015, 6, 724. [Google Scholar] [CrossRef] [PubMed]

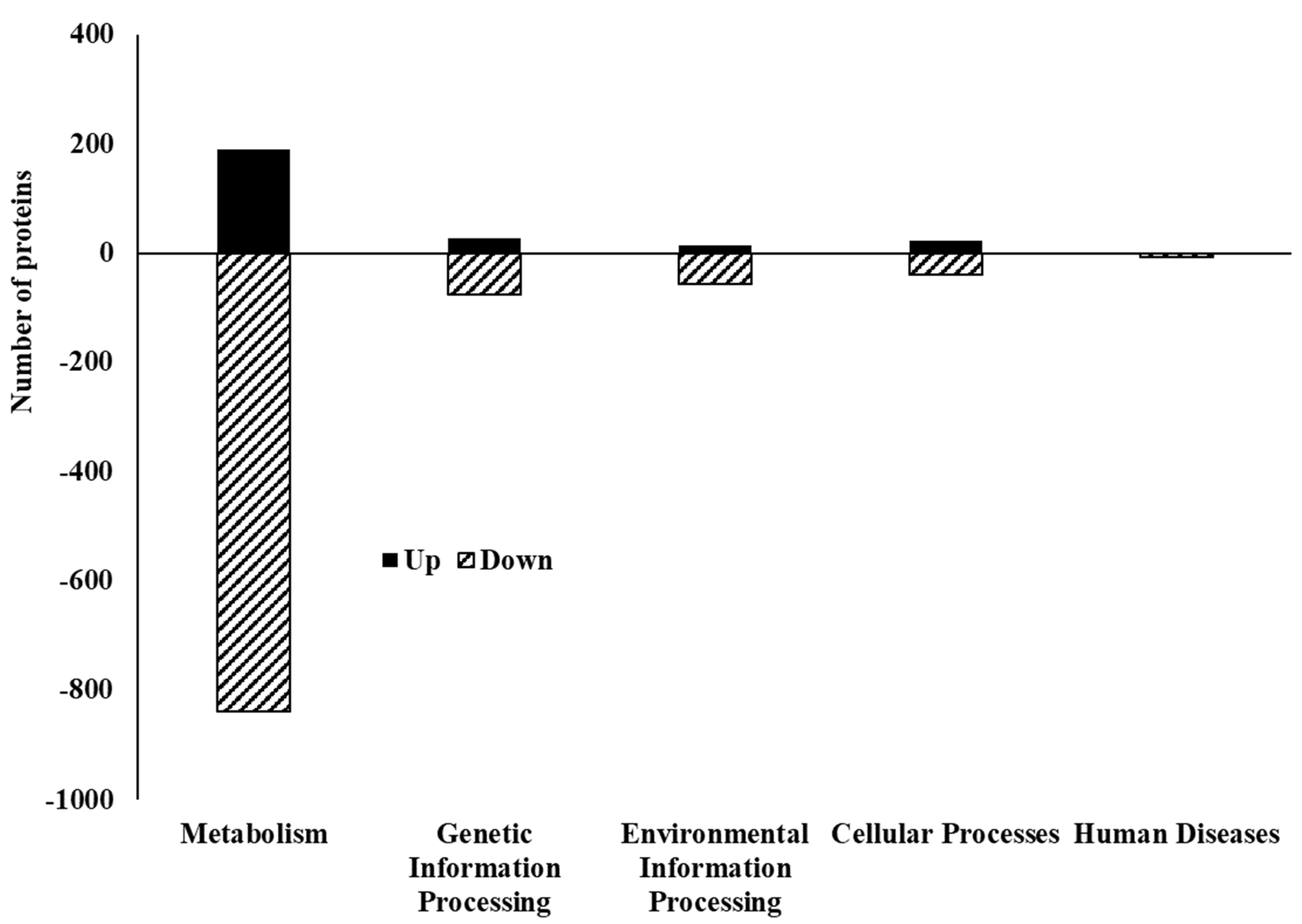


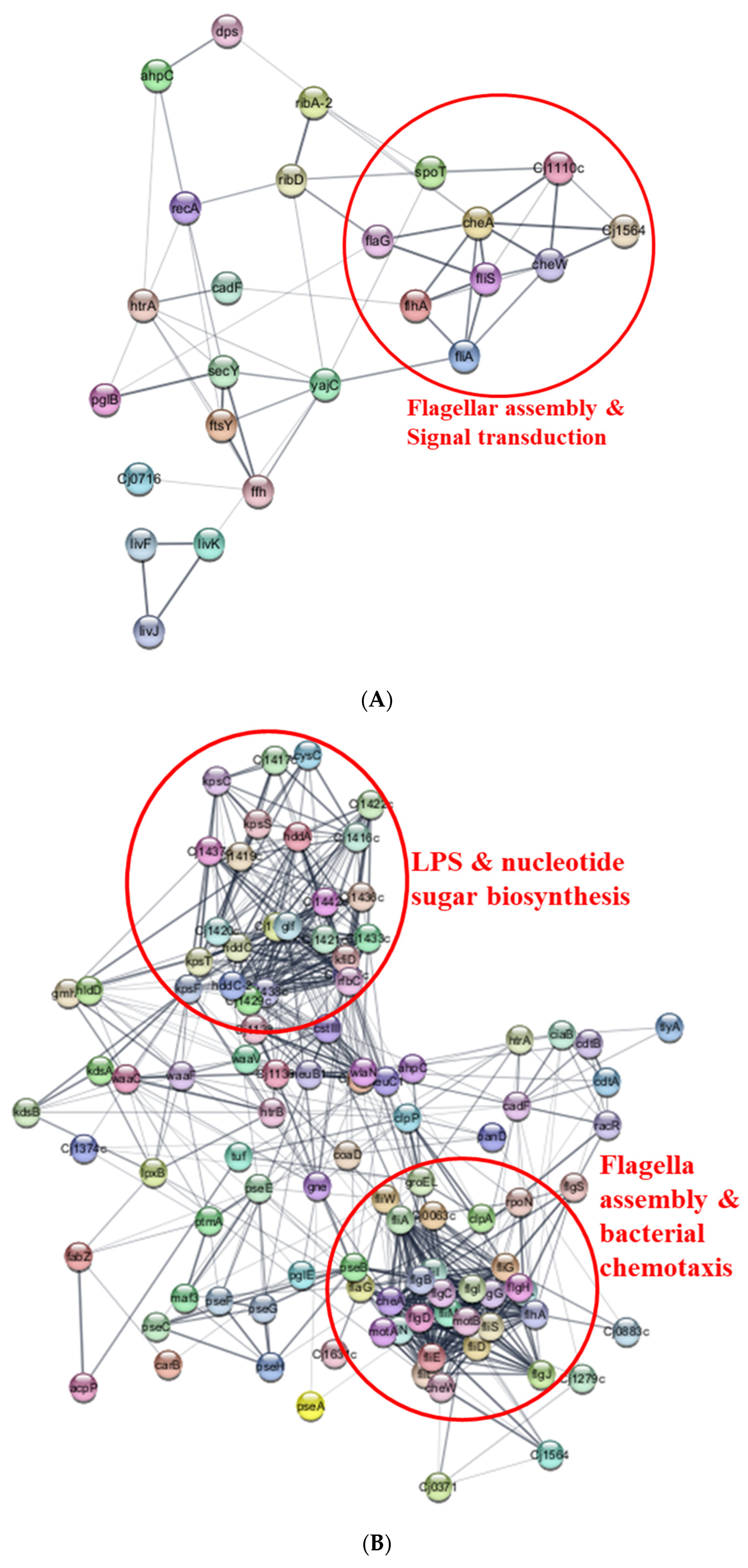
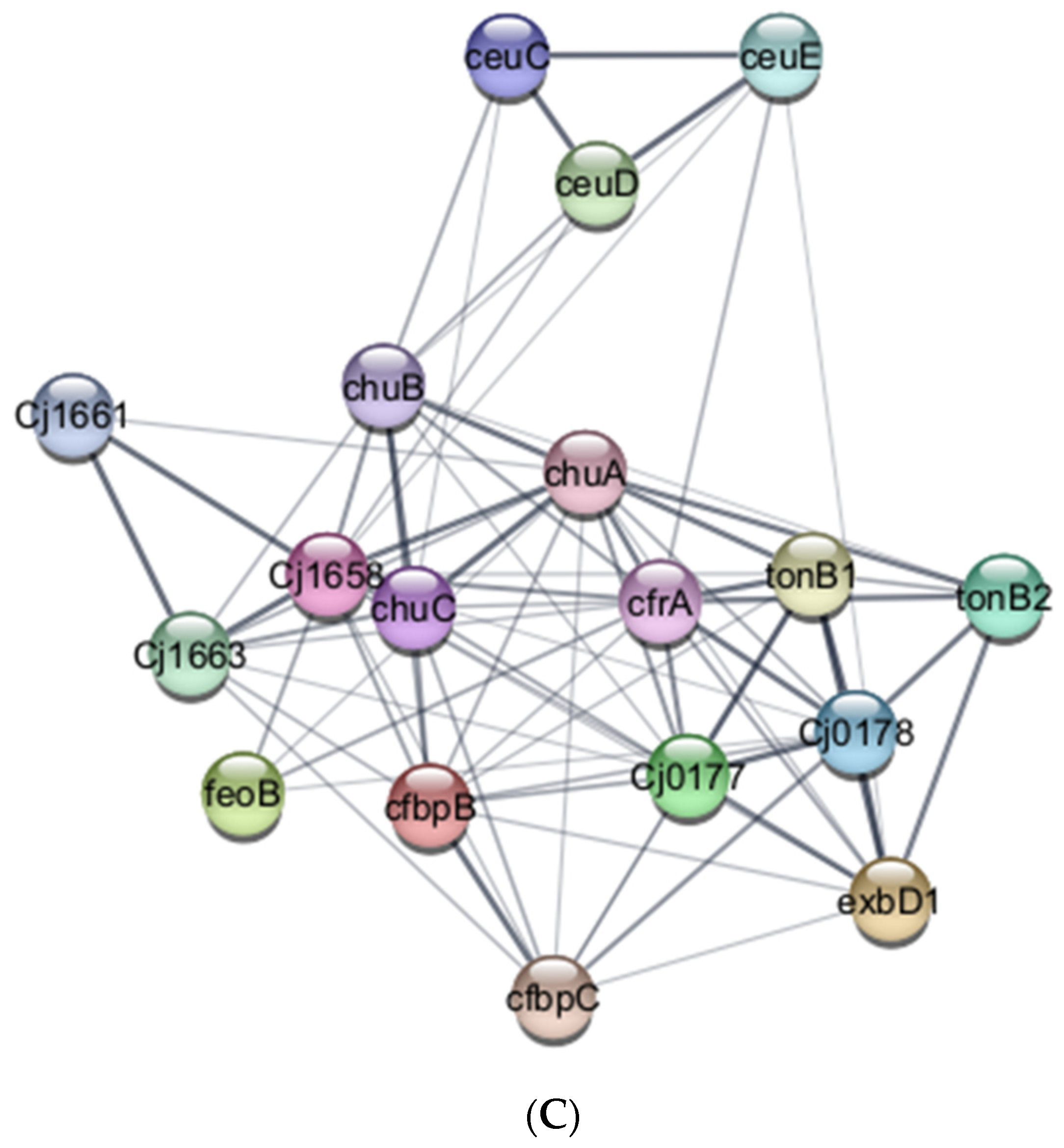
| Locus Tag | Genes | Description | COG | Log2 (Fold Ratio) |
|---|---|---|---|---|
| Cj0061c | fliA | Sigma factor 28 | K | −2.85 |
| Cj0145 | phoX | Alkaline phosphatase | S | 3.14 |
| Cj0283c | cheW | Phosphotransferase | NT | −1.01 |
| Cj0284c | cheA | Histidine kinase sensor | T | −1.40 |
| Cj0334 | ahpC | Alkyl hydroperoxide reductase | O | −1.18 |
| Cj0547 | flaG | Flagellar filament length control | N | −2.04 |
| Cj0549 | fliS | Flagellar secretion chaperon | N | −2.94 |
| Cj0604 | ppk-2 | Polyphosphate kinase | S | −1.28 |
| Cj0709 | ffh | Signal recognition particle protein | U | −2.15 |
| Cj0716 | Phospho-2-dehydro-3-deoxyheptonate aldolase | E | −1.11 | |
| Cj0732 | ABC transporter ATP-binding protein | P | 24.90 | |
| Cj0882c | flhA | Flagellar biosynthesis protein | N | −3.48 |
| Cj0996 | ribA | GTP cyclohydrolase II | F | −23.69 |
| Cj1014c | livF | ABC transporter ATP-binding protein | E | −2.88 |
| Cj1018c | livK | ABC transporter substrate-binding protein | E | −28.81 |
| Cj1019c | livJ | ABC transporter substrate-binding protein | E | 1.80 |
| Cj1094c | yajC | Protein translocase subunit | U | −27.29 |
| Cj1110c | tlp8 | Transducer-like protein-8 | NT | −1.26 |
| Cj1126c | pglB | Oligosaccharyltransferase | S | −31.08 |
| Cj1148 | waaF | Heptosyltransferase II | M | −27.57 |
| Cj1206c | ftsY | Signal recognition particle protein | U | −28.20 |
| Cj1228c | htrA | Serine protease | M | −1.12 |
| Cj1272c | spoT | Guanosine-3′,5′-bis(Diphosphate) 3′-pyrophosphohydrolase | KT | −2.29 |
| Cj1478c | cadF | Outer membrane fibronectin-binding protein | M | −1.62 |
| Cj1534c | dps | DNA protection during starvation protein | P | −1.97 |
| Cj1564 | tlp3 | Transducer-like protein-3 | NT | −1.38 |
| Cj1565c | pflA | Paralyzed flagellum protein | N | −2.01 |
| Cj1622 | ribD | Riboflavin-specific deaminase/reductase | H | −3.22 |
| Cj1673c | recA | Recombinase A | L | −1.46 |
| Cj1688c | secY | Protein translocase subunit SecY | U | −26.61 |
| Locus Tag | Genes | Description | COG | Log2 (Fold Ratio) |
|---|---|---|---|---|
| Cj0042 | flgD | Flagellar basal-body rod modification protein | N | 2.24 |
| Cj0060c | fliM | Flagellar motor switch protein | N | −1.85 |
| Cj0061c | fliA | RNA polymerase sigma factor for flagellar operon | K | −2.85 |
| Cj0063c | Flagellar synthesis regulator | D | −3.43 | |
| Cj0078c | cdtB | Cytolethal distending toxin B | S | −28.24 |
| Cj0079c | cdtA | Cytolethal distending toxin subunit A | M | −26.86 |
| Cj0192c | clpP | ATP-dependent Clp protease proteolytic subunit | O | −27.93 |
| Cj0195 | fliI | Flagellum-specific ATP synthase | NU | 1.83 |
| Cj0273 | fabZ | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase | I | 25.14 |
| Cj0279 | carB | Carbamoyl-phosphate synthase large chain | F | −1.10 |
| Cj0283c | cheW | Positive regulator of CheA protein activity | NT | −1.01 |
| Cj0284c | cheA | Signal transduction histidine kinase | T | −1.40 |
| Cj0288c | lpxB | Lipid-A-disaccharide synthase | M | 1.07 |
| Cj0296c | panD | Aspartate 1-decarboxylase | H | −28.06 |
| Cj0319 | fliG | Flagellar motor switch protein | N | −7.39 |
| Cj0334 | ahpC | Alkyl hydroperoxide reductase subunit C-like protein | O | −1.18 |
| Cj0336c | motB | Flagellar motor rotation protein MotB | N | 10.41 |
| Cj0337c | motA | Flagellar motor rotation protein | N | −29.44 |
| Cj0351 | fliN | Flagellar motor switch protein | N | 23.96 |
| Cj0371 | Putative flagellar motility protein | - | −1.70 | |
| Cj0384c | kdsA | 2-dehydro-3-deoxyphosphooctonate aldolase | M | −1.02 |
| Cj0441 | acpP | Acyl carrier protein | IQ | 1.53 |
| Cj0470 | tuf | Translation elongation factor Tu | J | 1.16 |
| Cj0526c | fliE | Flagellar hook-basal body complex protein | N | −29.45 |
| Cj0527c | flgC | Flagellar basal-body rod protein | N | 2.07 |
| Cj0528c | flgB | Flagellar basal-body rod protein | N | −3.39 |
| Cj0547 | flaG | Flagellar protein | N | −2.04 |
| Cj0548 | fliD | Flagellar cap protein | N | −1.74 |
| Cj0549 | fliS | Flagellar biosynthesis protein | N | −2.94 |
| Cj0588 | tlyA | RNA binding methyltransferase FtsJ like | J | −24.14 |
| Cj0670 | rpoN | RNA polymerase sigma-54 factor | K | −1.62 |
| Cj0687c | flgH | Flagellar L-ring protein | N | −25.29 |
| Cj0698 | flgG | Flagellar basal-body rod protein | N | −1.81 |
| Cj0767c | coaD | Phosphopantetheine adenylyltransferase | H | −3.83 |
| Cj0788 | ciaD | 2-oxoglutarate:acceptor oxidoreductase | - | −3.63 |
| Cj0793 | flgS | Flagellar sensory histidine kinase | T | 1.07 |
| Cj0813 | kdsB | 3-deoxy-manno-octulosonate cytidylyltransferase | M | −1.34 |
| Cj0882c | flhA | Flagellar biosynthesis protein | N | −3.48 |
| Cj0883c | Rrf2 family transcriptional regulator | K | −1.03 | |
| Cj0914c | ciaB | Campylobacter invasion antigen B | G | −1.51 |
| Cj1024c | flgR | Signal-transduction regulatory protein | T | −2.38 |
| Cj1075 | fliW | Flagellar assembly factor | N | −1.26 |
| Cj1108 | clpA | ATP-dependent Clp protease ATP-binding subunit | O | −2.27 |
| Cj1121c | pglE | UDP-N-acetylbacillosamine transaminase | E | −2.45 |
| Cj1131c | gne | UDP-glucose 4-epimerase | M | −1.85 |
| Cj1133 | waaC | Lipopolysaccharide core heptosyltransferase I | M | −1.94 |
| Cj1134 | htrB | Lipid A biosynthesis lauroyl acyltransferase | M | 2.41 |
| Cj1136 | Glycosyltransferase | M | −29.66 | |
| Cj1137c | Hypothetical protein | M | 22.54 | |
| Cj1138 | Glycosyltransferase | M | 2.66 | |
| Cj1139c | wlaN | β-1,3 galactosyltransferase | S | 29.18 |
| Cj1140 | cstIII | α-2,3 sialyltransferase | G | 25.08 |
| Cj1141 | neuB1 | N-acetylneuraminate synthase | M | −1.85 |
| Cj1142 | neuC1 | UDP-N-acetylglucosamine 2-epimerase | M | −2.85 |
| Cj1146c | waaV | Glucosyltransferase | S | 24.23 |
| Cj1148 | waaF | ADP-heptose--lipooligosaccharide heptosyltransferase II | M | −27.57 |
| Cj1151c | hldD | ADP-L-glycero-D-manno-heptose-6-epimerase | M | −1.73 |
| Cj1152c | gmhB | D-glycero-α-D-manno-heptose-1,7-bisphosphate 7-phosphatase | E | −24.54 |
| Cj1221 | groEL | Heat shock protein 60 kDa family chaperone | O | −1.32 |
| Cj1228c | htrA | Serine protease | M | −1.12 |
| Cj1239 | pdxA | 4-hydroxythreonine-4-phosphate dehydrogenase | H | −3.27 |
| Cj1242 | ciaC | Invasion antigen | - | −23.59 |
| Cj1261 | racR | Two-component system response regulator | K | 3.63 |
| Cj1279c | Putative fibronectin domain-containing lipoprotein | S | 1.27 | |
| Cj1293 | pseB | UDP-N-acetylglucosamine 4,6-dehydratase (inverting) | M | −2.76 |
| Cj1294 | pseC | UDP-4-amino-4,6-dideoxy-N-acetyl-β-L-altrosamine transaminase | E | −2.85 |
| Cj1311 | pseF | Pseudaminic acid cytidylyltransferase | M | 2.42 |
| Cj1312 | pseG | UDP-2,4-diacetamido-2,4,6-trideoxy-β-L-altropyranose hydrolase | M | −27.16 |
| Cj1313 | pseH | Acetyltransferase | J | 35.25 |
| Cj1316c | pseA | Flagellin modification protein | D | −2.06 |
| Cj1332 | ptmA | Oxidoreductase (Flagellin modification) | N | −2.35 |
| Cj1334 | maf3 | Motility accessory factor | S | −2.52 |
| Cj1337 | pseE | Motility accessory factor | S | −1.14 |
| Cj1374c | dITP/XTP pyrophosphatase | F | 3.84 | |
| Cj1408 | fliL | Flagellar basal body-associated protein | N | −3.32 |
| Cj1413c | kpsS | Capsular polysaccharide export system protein | M | −26.38 |
| Cj1414c | kpsC | Capsular polysaccharide export system protein | M | 2.53 |
| Cj1415c | cysC | Cytidine diphosphoramidate kinase | P | −29.06 |
| Cj1416c | CTP:phosphoglutamine cytidylyltransferase | M | −1.20 | |
| Cj1417c | Gamma-glutamyl-CDP-amidate hydrolase | S | −23.42 | |
| Cj1419c | Methyltransferase | Q | 4.62 | |
| Cj1420c | Methyltransferase | Q | −1.63 | |
| Cj1421c | Possible sugar transferase | S | −2.48 | |
| Cj1422c | Hypothetical protein | S | −5.72 | |
| Cj1423c | hddC | D-glycero-α-D-manno-heptose 1-phosphate guanylyltransferase | JM | 25.59 |
| Cj1425c | hddA | D-glycero-alpha-D-manno-heptose 7-phosphate kinase | S | 1.31 |
| Cj1426c | Hypothetical protein | J | −2.00 | |
| Cj1429c | Hypothetical protein | S | 23.07 | |
| Cj1430c | rfbC | dTDP-4-dehydrorhamnose 3,5-epimerase | M | −2.74 |
| Cj1431c | hddC | Hypothetical protein | S | −1.81 |
| Cj1433c | Hypothetical protein | H | −3.57 | |
| Cj1435c | Hypothetical protein | E | −1.77 | |
| Cj1436c | Hypothetical protein | E | −1.14 | |
| Cj1437c | Aminotransferase | E | −3.58 | |
| Cj1438c | Putative sugar transferase | S | −1.39 | |
| Cj1439c | glf | UDP-galactopyranose mutase | M | 1.87 |
| Cj1441c | kfiD | UDP-glucose 6-dehydrogenase | C | −2.80 |
| Cj1442c | Sugar transferase | M | −2.63 | |
| Cj1443c | kpsF | D-arabinose-5-phosphate isomerase | M | −2.90 |
| Cj1447c | kpsT | Capsule polysaccharide export ATP-binding protein | GM | 3.58 |
| Cj1462 | flgI | Flagellar P-ring protein | N | 2.20 |
| Cj1463 | flgJ | Hypothetical protein | MNO | 1.80 |
| Cj1478c | cadF | Outer membrane fibronectin-binding protein | M | −1.62 |
| Cj1564 | Methyl-accepting chemotaxis signal transduction protein | NT | −1.38 | |
| Cj1565c | pflA | Paralyzed flagella protein | N | −2.01 |
| Cj1631c | DUF773 domain-containing protein | - | 3.36 |
| Locus Tag | Genes | Description | COG | Log2 (Fold Ratio) |
|---|---|---|---|---|
| Cj0173c | cfbpC | Putative iron-uptake ABC transport system ATP-binding protein | E | −28.05 |
| Cj0174c | cfbpB | Ferric iron ABC transporter, permease protein | P | −27.34 |
| Cj0177 | Hypothetical protein | S | −28.21 | |
| Cj0178 | Putative outer membrane siderophore receptor | P | 4.89 | |
| Cj0180 | exbD1 | Ferric siderophore transport system, biopolymer transport protein | U | 24.51 |
| Cj0181 | tonB1 | putative TonB-dependent receptor | M | 3.56 |
| Cj0755 | cfrA | Ferric enterobactin uptake receptor | P | 1.48 |
| Cj1353 | ceuC | Hypothetical protein | P | 2.58 |
| Cj1354 | ceuD | Hypothetical protein | P | −26.47 |
| Cj1355 | ceuE | Enterochelin uptake periplasmic binding protein | P | −1.17 |
| Cj1398 | feoB | Ferrous iron transporter FeoB | P | −2.84 |
| Cj1614 | chuA | Haemin uptake system outer membrane receptor | P | −26.07 |
| Cj1615 | chuB | Haemin uptake system permease protein | P | −27.44 |
| Cj1616 | chuC | Haemin uptake system ATP-binding protein | HP | −9.31 |
| Cj1630 | tonB2 | putative TonB-dependent receptor | U | −1.39 |
| Cj1658 | Iron permease | P | −1.05 | |
| Cj1661 | Fe2+ ABC transporter, permease protein 1 | V | −23.75 | |
| Cj1663 | Fe2+ ABC transporter, ATP-binding subunit | V | −1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, K.; Park, M.; Chon, J.; Kweon, O.; Paredes, A.; Khan, S.A. Chicken Juice Enhances C. jejuni NCTC 11168 Biofilm Formation with Distinct Morphological Features and Altered Protein Expression. Foods 2024, 13, 1828. https://doi.org/10.3390/foods13121828
Sung K, Park M, Chon J, Kweon O, Paredes A, Khan SA. Chicken Juice Enhances C. jejuni NCTC 11168 Biofilm Formation with Distinct Morphological Features and Altered Protein Expression. Foods. 2024; 13(12):1828. https://doi.org/10.3390/foods13121828
Chicago/Turabian StyleSung, Kidon, Miseon Park, Jungwhan Chon, Ohgew Kweon, Angel Paredes, and Saeed A. Khan. 2024. "Chicken Juice Enhances C. jejuni NCTC 11168 Biofilm Formation with Distinct Morphological Features and Altered Protein Expression" Foods 13, no. 12: 1828. https://doi.org/10.3390/foods13121828
APA StyleSung, K., Park, M., Chon, J., Kweon, O., Paredes, A., & Khan, S. A. (2024). Chicken Juice Enhances C. jejuni NCTC 11168 Biofilm Formation with Distinct Morphological Features and Altered Protein Expression. Foods, 13(12), 1828. https://doi.org/10.3390/foods13121828








