Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles
Abstract
:1. Introduction
2. Materials and Methods
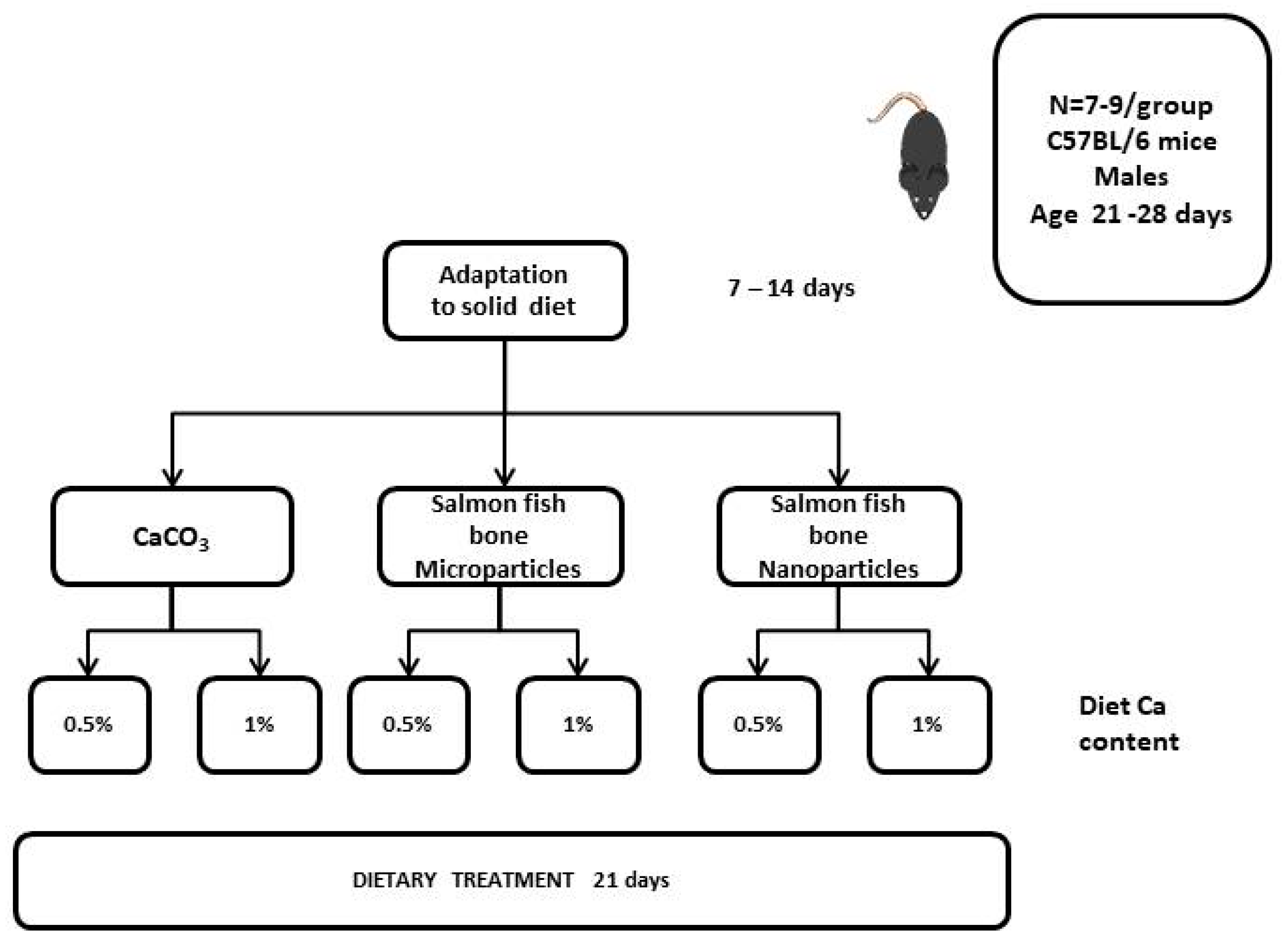
2.1. Animals
2.2. Procedures and Methods
2.3. Determinations in Experimental Animals
3. Results
3.1. General Characteristics of Experimental Animals
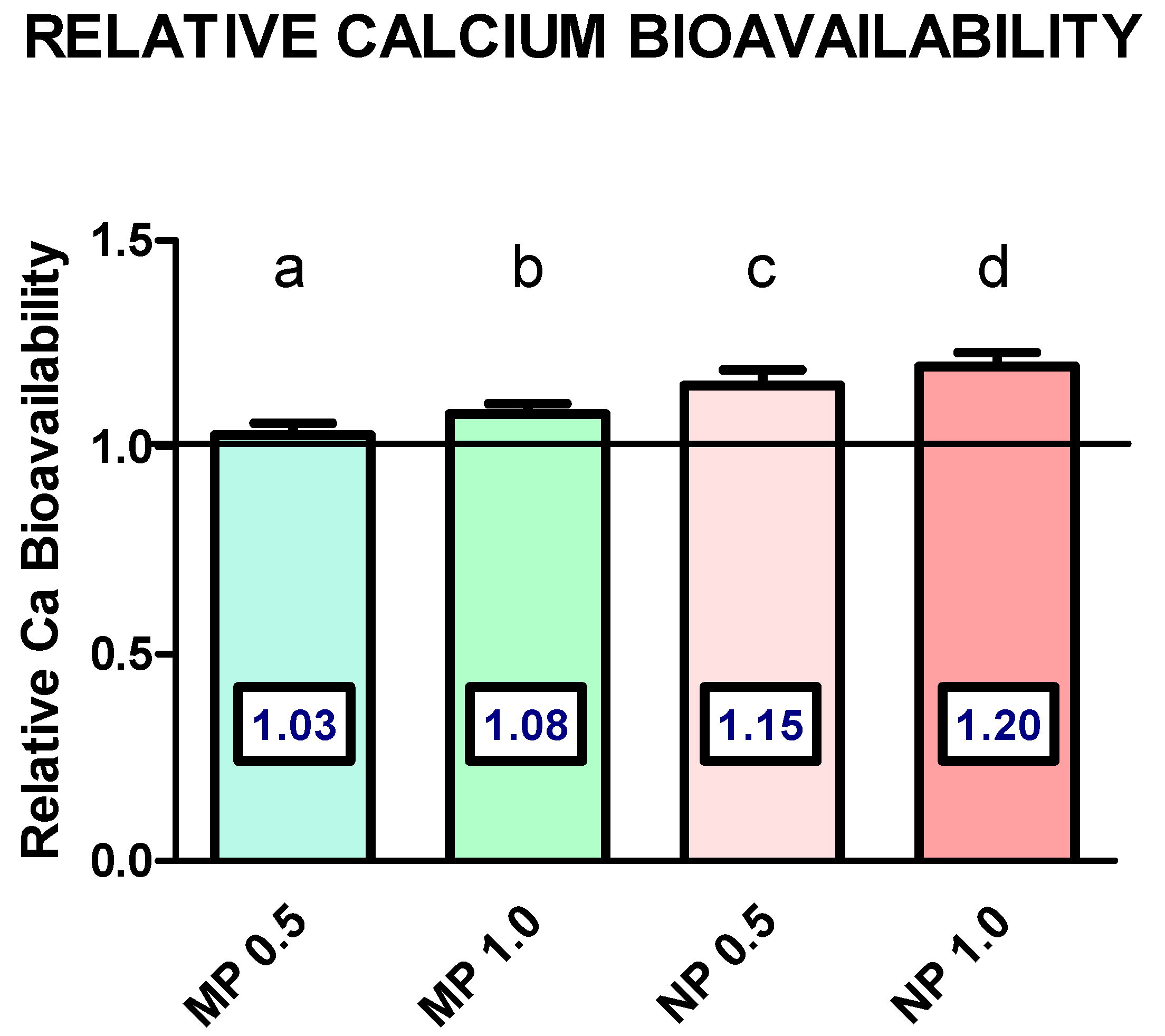
3.2. Results in Mouse Bone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Van Buren, N.; Demmers, M.; Van Der Heijden, R.; Witlox, F. Towards a Circular Economy: The Role of Dutch Logistics Industries and Governments. Sustainability 2016, 8, 647. [Google Scholar] [CrossRef]
- FAO (Ed.) Towards Blue Transformation. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the Utilisation of Marine By-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish Industry Waste: Treatments, Environmental Impacts, Current and Potential Uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Liaset, B.; Julshamn, K.; Espe, M. Chemical Composition and Theoretical Nutritional Evaluation of the Produced Fractions from Enzymic Hydrolysis of Salmon Frames with ProtamexTM. Process Biochem. 2003, 38, 1747–1759. [Google Scholar] [CrossRef]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Ruz, M.; García, P.; Jiménez, P.; Valencia, P.; Ramírez, C.; Pinto, M.; Nuñez, S.M.; Park, J.W.; Almonacid, S. Nutritional Properties of Fish Bones: Potential Applications in the Food Industry. Food Rev. Int. 2024, 40, 79–91. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Tucker, K.; Rosen, C. Prevention and Management of Osteoporosis. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; ISBN 978-1-60547-461-8. [Google Scholar]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global Dietary Calcium Intake among Adults: A Systematic Review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud; Chile Encuesta Nacional de Consumo Alimentario. Informe Final. Available online: https://www.minsal.cl/sites/default/files/ENCA-INFORME_FINAL.pdf (accessed on 6 June 2024).
- Hunt, C.D.; Johnson, L.K. Calcium Requirements: New Estimations for Men and Women by Cross-Sectional Statistical Analyses of Calcium Balance Data from Metabolic Studies. Am. J. Clin. Nutr. 2007, 86, 1054–1063. [Google Scholar] [CrossRef]
- Charles, P. Calcium Absorption and Calcium Bioavailability. J. Intern. Med. 1992, 231, 161–168. [Google Scholar] [CrossRef]
- Wawrzyniak, N.; Suliburska, J. Nutritional and Health Factors Affecting the Bioavailability of Calcium: A Narrative Review. Nutr. Rev. 2021, 79, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Gulshad, L.; Haq, I.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A Novel Tool to Enhance the Bioavailability of Micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An Approach to Overcome Bioavailability Challenges of Nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of Nanoparticles in Nutrient and Nutraceutical Delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- National Academies Press. Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, DC, USA, 1995; p. 4758. ISBN 978-0-309-05126-2. [Google Scholar]
- Valencia, P.; Valdivia, S.; Nuñez, S.; Ovissipour, R.; Pinto, M.; Ramirez, C.; Perez, A.; Ruz, M.; Garcia, P.; Jimenez, P.; et al. Assessing the Enzymatic Hydrolysis of Salmon Frame Proteins through Different By-Product/Water Ratios and pH Regimes. Foods 2021, 10, 3045. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.; Nuñez, S.; Valdivia, S.; Ramírez, C.; Pinto, M.; Ovissipour, R.; Almonacid, S. Enhancing the Enzymatic Nitrogen Recovery from Salmon Frame Proteins: Sequential Batch Strategy. LWT 2023, 184, 115093. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium Deficiency Worldwide: Prevalence of Inadequate Intakes and Associated Health Outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.H.; Nayan, M.M.; Jubayer, A.; Amin, M.R. A Review of the Dietary Diversity and Micronutrient Adequacy among the Women of Reproductive Age in Low- and Middle-income Countries. Food Sci. Nutr. 2024, 12, 1367–1379. [Google Scholar] [CrossRef]
- Malde, M.K.; Bugel, S.; Kristensen, M.; Malde, K.; Graff, I.E.; Pedersen, J.I. Calcium from Salmon and Cod Bone Is Well Absorbed in Young Healthy Men: A Double-Blinded Randomised Crossover Design. Nutr. Metab. 2010, 7, 61. [Google Scholar] [CrossRef]
- Guéguen, L.; Pointillart, A. The Bioavailability of Dietary Calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, T.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Liu, R.; Li, Y. Mechanism on Releasing and Solubilizing of Fish Bone Calcium during Nano-Milling. J. Food Process Eng. 2020, 43, e13354. [Google Scholar] [CrossRef]
- Erfanian, A.; Mirhosseini, H.; Rasti, B.; Hair-Bejo, M.; Mustafa, S.B.; Manap, M.Y.A. Absorption and Bioavailability of Nano-Size Reduced Calcium Citrate Fortified Milk Powder in Ovariectomized and Ovariectomized-Osteoporosis Rats. J. Agric. Food Chem. 2015, 63, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Erfanian, A.; Rasti, B.; Manap, Y. Comparing the Calcium Bioavailability from Two Types of Nano-Sized Enriched Milk Using in-Vivo Assay. Food Chem. 2017, 214, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Zobitz, M.M.; Pak, C.Y.C. Dose Dependency of Calcium Absorption: A Comparison of Calcium Carbonate and Calcium Citrate. J. Bone Miner. Res. 1988, 3, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Kenny, P.; Poindexter, J.; Pak, C.Y. Superior Calcium Absorption from Calcium Citrate than Calcium Carbonate Using External Forearm Counting. J. Am. Coll. Nutr. 1990, 9, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Barger-Lux, M.J. Absorption of Calcium as the Carbonate and Citrate Salts, with Some Observations on Method. Osteoporos. Int. 1999, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Costa, N.M.B.; Saleeb, F.Z.; Huth, P.J. Absorption of Calcium Fumarate Salts Is Equivalent to Other Calcium Salts When Measured in the Rat Model. J. Agric. Food Chem. 2002, 50, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, Q.; Wastney, M.E.; Weaver, C.M. Calcium Bioavailability and Kinetics of Calcium Ascorbate and Calcium Acetate in Rats. Exp. Biol. Med. 2004, 229, 40–45. [Google Scholar] [CrossRef]
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the Absorption of Calcium Carbonate and Calcium Citrate after Roux-En-Y Gastric Bypass. Obes. Surg. 2009, 19, 1256–1261. [Google Scholar] [CrossRef]
- Wang, H.; Bua, P.; Capodice, J. A Comparative Study of Calcium Absorption Following a Single Serving Administration of Calcium Carbonate Powder versus Calcium Citrate Tablets in Healthy Premenopausal Women. Food Nutr. Res. 2014, 58, 23229. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Bierman, J.; Hale, C.A.; Bendich, A. Absorbability and Cost Effectiveness in Calcium Supplementation. J. Am. Coll. Nutr. 2001, 20, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed]


| Group | Initial Weight (g) | Final Weight (g) | Food Intake (g/d) | Femur Weight (mg) | Femur Length (mm) |
|---|---|---|---|---|---|
| CN 0.5 (n = 8) | 16.6 ± 2.8 a | 25.5 ± 2.8 a | 7.0 ± 0.5 a | 48.9 ± 8.3 a | 14.3 ± 0.5 abc |
| CN 1.0 (n = 8) | 15.2 ± 2.1 a | 24.9 ± 3.0 a | 6.0 ± 0.8 b | 55.1 ± 8.1 ab | 14.1 ± 0.5 a |
| MP 0.5 (n = 7) | 20.2 ± 1.6 b | 26.0 ± 2.5 ab | 6.1 ± 0.3 b | 62.3 ± 11.8 bc | 14.8 ± 0.4 bc |
| MP 1.0 (n = 9) | 20.2 ± 2.1 b | 27.2 ± 2.5 abc | 6.2 ± 0.2 b | 61.6 ± 5.3 abc | 14.7 ± 0.2 abc |
| NP 0.5 (n = 8) | 24.7 ± 1.6 c | 30.2 ± 1.0 c | 6.2 ± 0.4 b | 75.7 ± 9.6 d | 15.4 ± 0.4 d |
| NP 1.0 (n = 8) | 23.9 ± 1.6 c | 29.6 ± 3.5 bc | 6.6 ± 0.1 ab | 62.7 ± 4.9 c | 14.9 ± 0.2 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, B.; Pérez, A.; García, P.; Jiménez, P.; Abrigo, K.; Valencia, P.; Ramírez, C.; Pinto, M.; Almonacid, S.; Ruz, M. Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles. Foods 2024, 13, 1840. https://doi.org/10.3390/foods13121840
Torres B, Pérez A, García P, Jiménez P, Abrigo K, Valencia P, Ramírez C, Pinto M, Almonacid S, Ruz M. Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles. Foods. 2024; 13(12):1840. https://doi.org/10.3390/foods13121840
Chicago/Turabian StyleTorres, Benjamín, Alvaro Pérez, Paula García, Paula Jiménez, Karen Abrigo, Pedro Valencia, Cristian Ramírez, Marlene Pinto, Sergio Almonacid, and Manuel Ruz. 2024. "Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles" Foods 13, no. 12: 1840. https://doi.org/10.3390/foods13121840
APA StyleTorres, B., Pérez, A., García, P., Jiménez, P., Abrigo, K., Valencia, P., Ramírez, C., Pinto, M., Almonacid, S., & Ruz, M. (2024). Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles. Foods, 13(12), 1840. https://doi.org/10.3390/foods13121840







