Nanobody and CuS Nanoflower-Au-Based Lateral Flow Immunoassay Strip to Enhance the Detection of Aflatoxin B1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
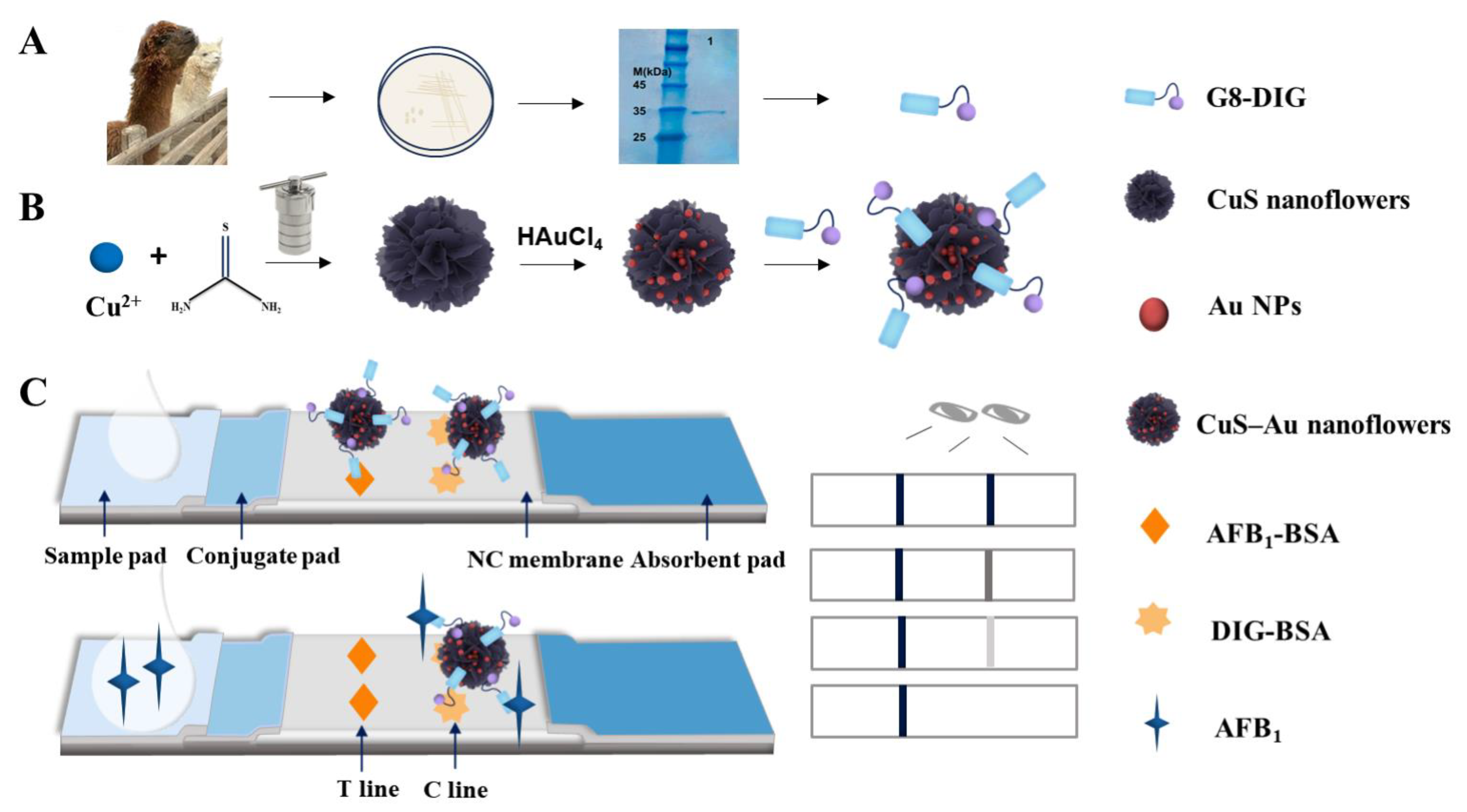
2.2. Synthesis of CuS NFs
2.3. Synthesis of CuS–Au Nanoflowers
2.4. Expression of the Nanobody G8-DIG
2.5. Preparation of G8-DIG@CuS-Au (CuS) Nanoflower Probes
2.6. LFIA Preparation
2.7. Principle of the LFIA Based on CuS-Au
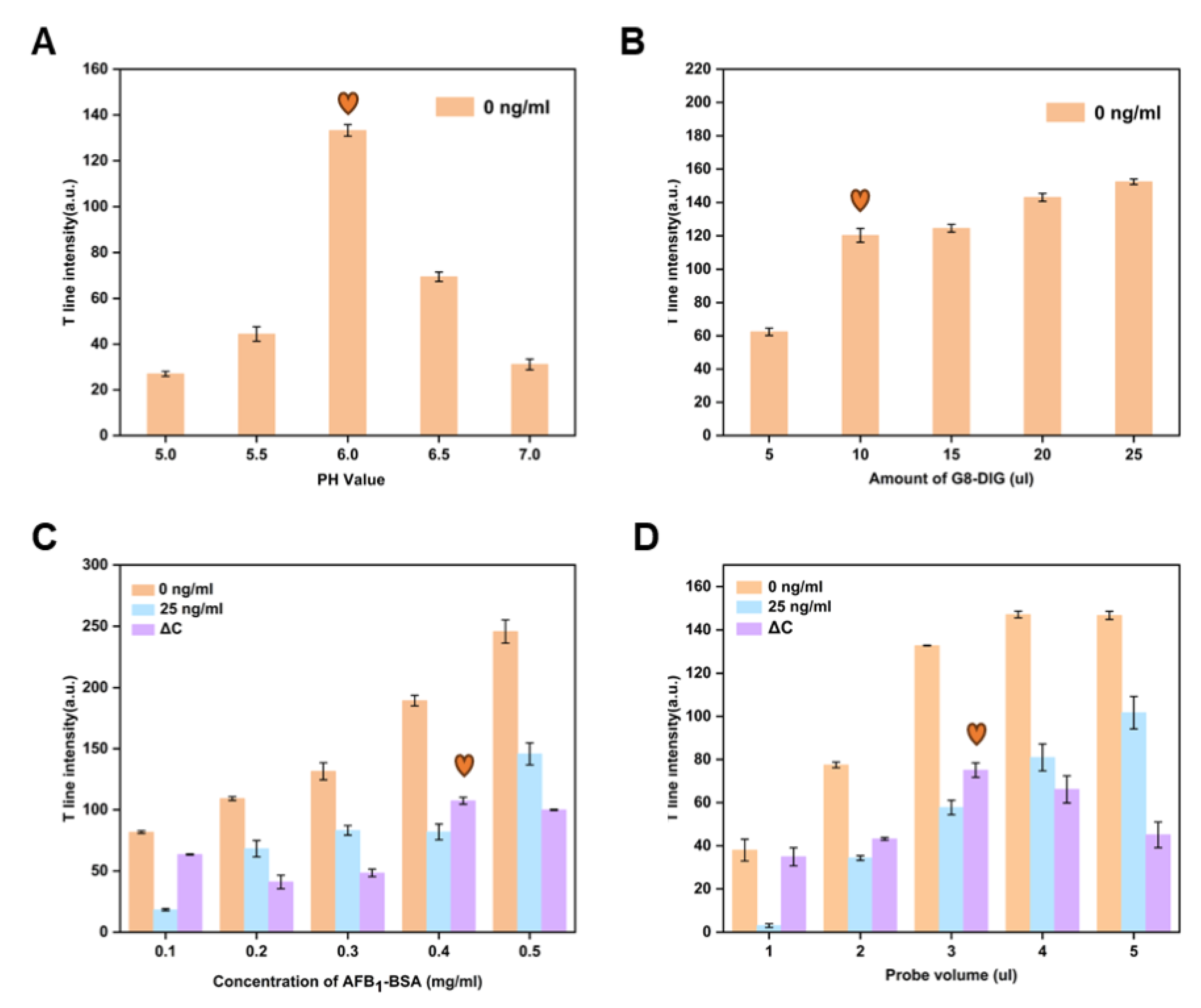
2.8. Key Parameter Optimization
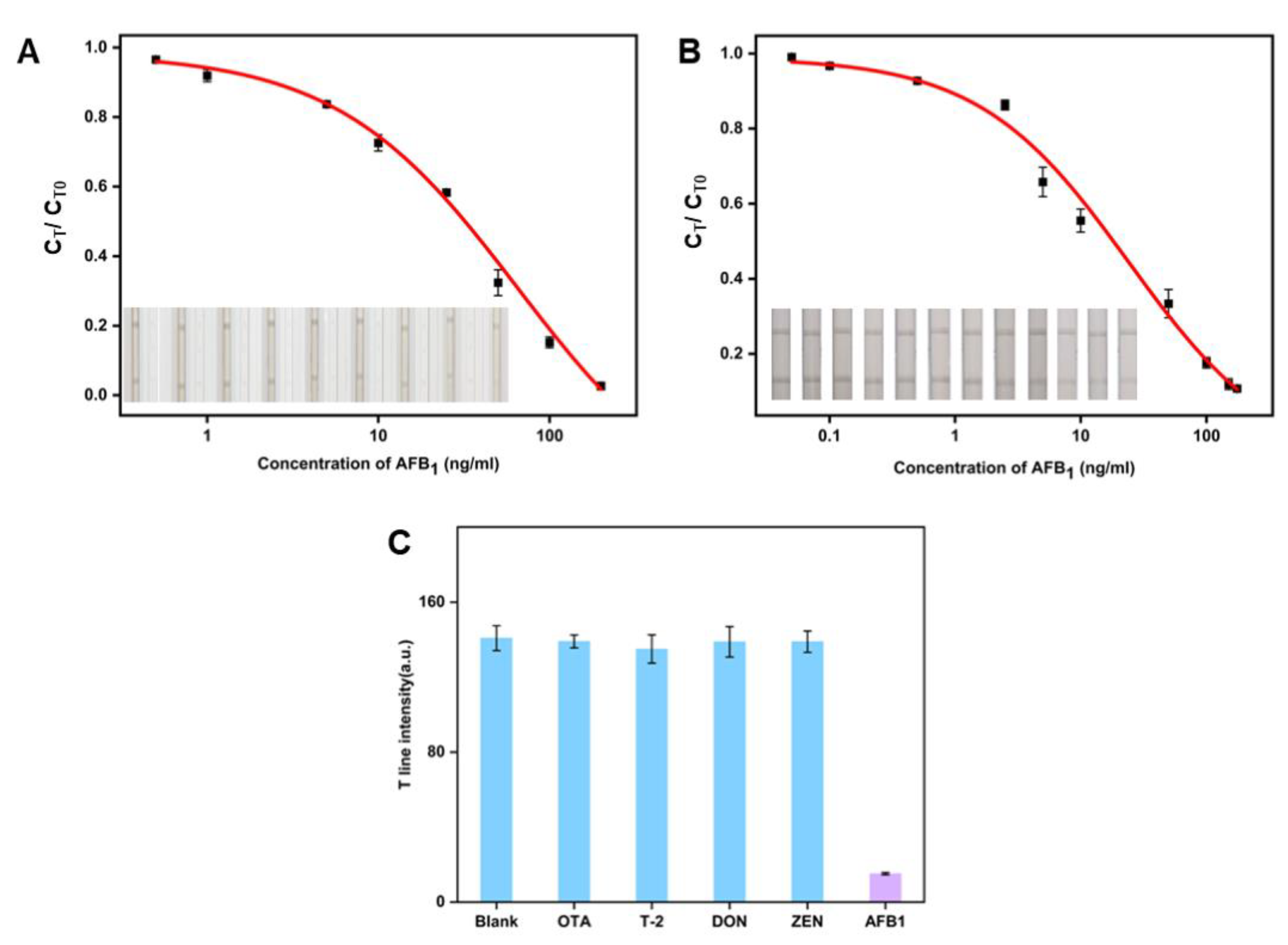
2.9. Sensitivity Analysis of the CuS NFs-Au LFIA Strip
2.10. Specificity Experiment for the CuS NFs-Au-LFIA
2.11. Detection of Actual Samples
3. Results and Discussion
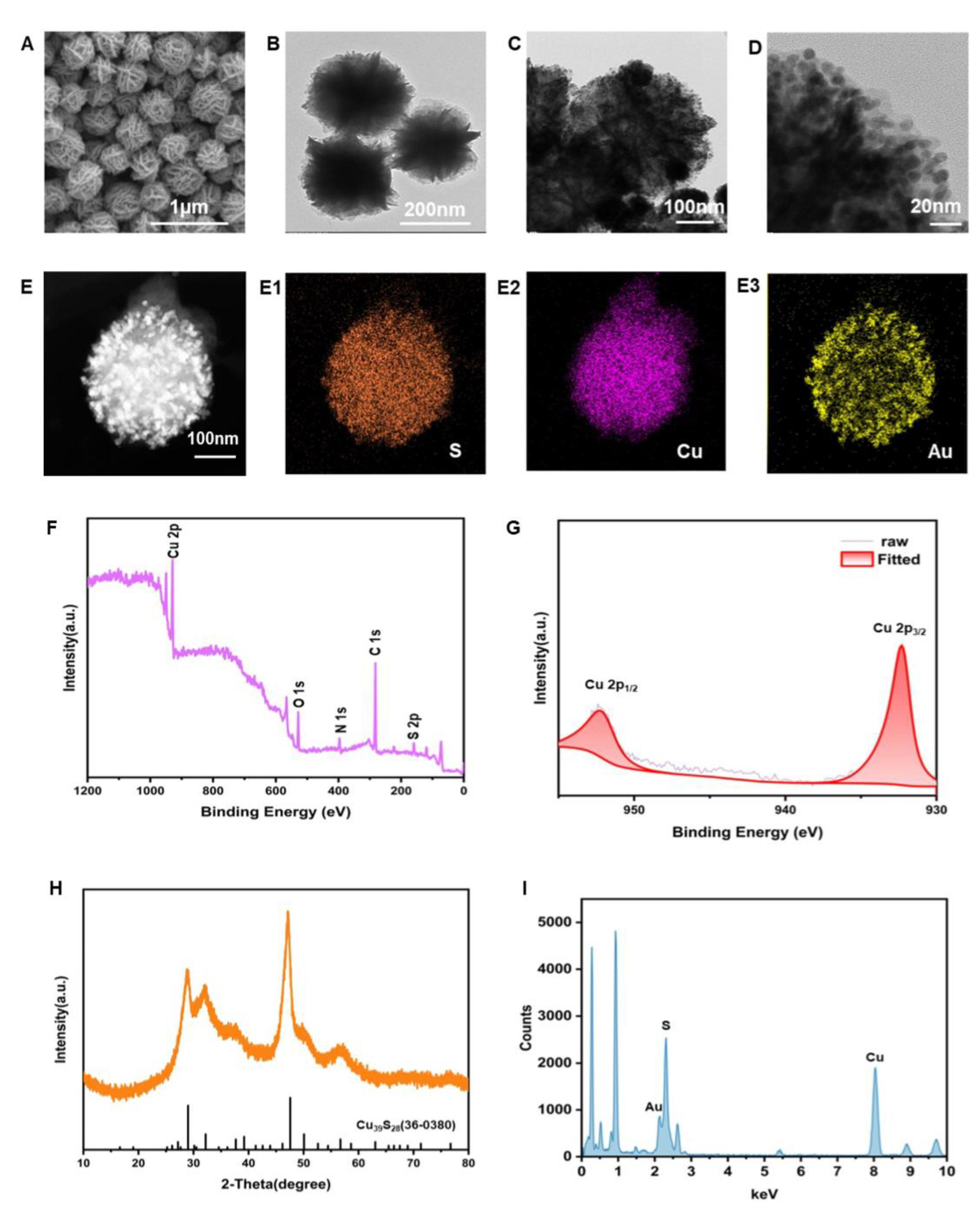
3.1. Characterization of CuS and CuS-Au
3.2. Optimization of the Key Parameters for an LFIA Strip Based on CuS NFs-Au
3.3. Comparison of Sensitivity of the CuS-Au-LFIA and CuS-LFIA
3.4. Specificity Experiment
3.5. Practical Application in Actual Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research progress in toxicological effects and mechanism of aflatoxin B1 toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, J.; Wang, Y.; Ding, G.; Guo, L.; Qin, J. Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, K.I.; Sulyok, M.; Krska, R.; Warth, B.; Ezekiel, C.N. Mycotoxins in complementary foods consumed by infants and young children within the first 18 months of life. Food Control 2023, 144, 109328. [Google Scholar] [CrossRef]
- Cai, X.; Ma, F.; Jiang, J.; Yang, X.; Zhang, Z.; Jian, Z.; Liang, M.; Li, P.; Yu, L. Fe-N-C single-atom nanozyme for ultrasensitive, on-site and multiplex detection of mycotoxins using lateral flow immunoassay. J. Hazard. Mater. 2023, 441, 129853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, P.; Zheng, B. A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods 2021, 10, 2109. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, S.; Fotina, H.; Wang, Z. A Novel Lateral Flow Immunochromatographic Assay for Rapid and Simultaneous Detection of Aflatoxin B1 and Zearalenone in Food and Feed Samples Based on Highly Sensitive and Specific Monoclonal Antibodies. Toxins 2022, 14, 615. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, C.; Dai, Y.; Chu, L.; Geng, S.; Zheng, S.; Kang, X. Determination of aflatoxin B1 by novel nanofiber-packed solid-phase extraction coupled with a high performance liquid chromatography-fluorescence detector. Anal. Methods 2023, 15, 472–481. [Google Scholar] [CrossRef]
- Salisu, B.; Anua, S.; Ishak, W.; Mazlan, N. Development and validation of quantitative thin layer chromatographic technique for determination of total aflatoxins in poultry feed and food grains without sample clean-up. J. Adv. Vet. Anim. Res. 2021, 8, 656. [Google Scholar] [CrossRef]
- Wei, W.; Jiao-Jie, M.; Chuan-Chuan, Y.; Xiao-Hui, L.; Hong-Ru, J.; Bing, S.; Feng-Qin, L. Simultaneous Determination of Masked Deoxynivalenol and Some Important Type B Trichothecenes in Chinese Corn Kernels and Corn-Based Products by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 11638–11646. [Google Scholar] [CrossRef]
- Wang, X.; Sun, T.; Shen, W.; Liu, M.; Liu, W.; Zuo, H.; Zhang, Y.; Geng, L.; Wang, W.; Shao, C.; et al. A lateral flow immunochromatographic assay based on nanobody-oriented coupling strategy for aflatoxin B1 detection. Sens. Actuators B Chem. 2023, 394, 134419. [Google Scholar] [CrossRef]
- Tong, L.; Li, D.; Huang, M.; Huang, L.; Wang, J. Gold–Silver Alloy Nanoparticle-Incorporated Pitaya-Type Silica Nanohybrids for Sensitive Competitive Lateral Flow Immunoassay. Anal. Chem. 2023, 95, 17318–17327. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Xu, Q.; Long, N.; Song, P.; Wang, J.; Zhou, L.; Fu, B.; Kong, W. Polydopamine-coated HKUST MOFs-based strip lateral flow immunoassay for on-site ultrasensitive detection of aflatoxin B1 in foods. Food Control 2023, 152, 109864. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, T.; Tian, Y.; Sun, X.; Wang, Q.; Liu, Y.; Bai, F.; Zhao, S.; Wang, L. Fe3O4@CuS-based immunochromatographic test strips and their application to label-free and dual-readout detection of Escherichia coli O157:H7 in food. Food Chem. 2020, 332, 127398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xiong, J.; Duan, L.; Chen, S.; Peng, Z.; Liao, X.; Ning, Z.; Wang, D. Polydopamine@ZIFs with enhanced electrochemiluminescence quenching performance for mycotoxin detection. Food Chem. 2024, 439, 138058. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, M.; Jiang, M.; Lai, W.; Li, J.; Liu, K.; Li, H.; Hong, C. Application of a CuS/Au heterostructure with peroxidase-like activity in immunosensors. New J. Chem. 2022, 46, 13963–13970. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Xu, J.; Wang, S.; Ma, Y.; Chen, Y.; Li, Y.; Sun, J.; Zhang, D.; Wang, J. Galvanic replacement inspired signal amplification: Background-free and antibody-thrift in-situ growth immunochromatography. Chem. Eng. J. 2022, 437, 135362. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, N.; Li, X.; Yu, W.; Han, S.; Ren, X.; Yin, S.; Li, M.; Chen, Z. Galvanic exchange-induced growth of Au nanocrystals on CuS nanoplates for imaging guided photothermal ablation of tumors. Chem. Eng. J. 2020, 381, 122613. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Wang, M.; Zhang, M.; Wang, B.; Wang, K.; Darwish, I.A.; Wang, J.; Zhang, D. Dual-plasmonic CuS@Au heterojunctions synergistic enhanced photothermal and colorimetric dual signal for sensitive multiplexed LFIA. Biosens. Bioelectron. 2024, 255, 116235. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, H.; Deng, S.; Xiao, X.; Xiong, Y.; Peng, J.; Lai, W. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag–Au urchin-like hollow structures for the sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2023, 225, 115090. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.-l.; Lu, K.; Zhang, C.; Shi, W.; Zhao, W. Structural Engineering of Hollow Microflower-like CuS@C Hybrids as Versatile Electrochemical Sensing Platform for Highly Sensitive Hydrogen Peroxide and Hydrazine Detection. ACS Appl. Mater. Interfaces 2021, 13, 40942–40952. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, C.; Meng, H.; Mo, F.; Fu, Y. A novel signal self-enhancement photoelectrochemical immunosensor without addition of a sacrificial agent in solution based on Ag2S/CuS/α-Fe2O3 n–p–n heterostructure films. Chem. Commun. 2020, 56, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Ren, X.; Li, Y.; Wang, H.; Ma, H.; Wu, D.; Wei, Q. A No-washing Point-of-Care Electrochemical Biosensor Based on CuS Nanoparticles for Rapid and Sensitive Detection of Neuron-specific Enolase. Electroanalysis 2021, 34, 338–344. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Yan, T.; Tang, X.; Zhang, Q.; Li, P. Dual-Modal Immunosensor Made with the Multifunction Nanobody for Fluorescent/Colorimetric Sensitive Detection of Aflatoxin B1 in Maize. ACS Appl. Mater. Interfaces 2023, 15, 2771–2780. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Li, D.; Hu, J.; Wan, D.; Zhang, Z.; Hammock, B.D. Development of nanobody-based flow-through dot ELISA and lateral-flow immunoassay for rapid detection of 3-phenoxybenzoic acid. Anal. Methods 2021, 13, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, X.; Wang, W.; Liu, C.; Yang, W.; Wang, D. Nanobody@Biomimetic mineralized MOF as a sensing immunoprobe in detection of aflatoxin B1. Biosens. Bioelectron. 2023, 220, 114906. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhu, J.; Li, Y.; He, T.; Yang, Y.; Liu, M. Development of a biotinylated nanobody for sensitive detection of aflatoxin B1 in cereal via ELISA. Talanta 2022, 239, 19359–19371. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Zhang, Q.; Li, P.; Tang, X. Self-Assembly Multivalent Fluorescence-Nanobody Coupled Multifunctional Nanomaterial with Colorimetric Fluorescence and Photothermal to Enhance Immunochromatographic Assay. ACS Nano 2023, 17, 19359–19371. [Google Scholar] [CrossRef]
- Kim, J.; Shin, M.-S.; Shin, J.; Kim, H.-M.; Pham, X.-H.; Park, S.-m.; Kim, D.-E.; Kim, Y.J.; Jun, B.-H. Recent Trends in Lateral Flow Immunoassays with Optical Nanoparticles. Int. J. Mol. Sci. 2023, 24, 9600. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Z.; Fan, F.; Dong, X.; Peng, Y.; Hao, S.; Long, L. Facile synthesis of size-tunable Cu39S28 micro/nano-crystals and small-sized configuration enhanced visible-light photocatalytic activity. CrystEngComm 2013, 15, 5792–5798. [Google Scholar] [CrossRef]
| Sample | Spiked (µg/L) | Detected (µg/L) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Corn flour | 0.1 | 0.09 ± 0.01 | 90 | 11.1 |
| 1 | 1.17 ± 0.12 | 117 | 10.3 | |
| 10 | 8.17 ± 0.16 | 81.7 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; He, B.; Li, D.; Gao, L.; Ren, W. Nanobody and CuS Nanoflower-Au-Based Lateral Flow Immunoassay Strip to Enhance the Detection of Aflatoxin B1. Foods 2024, 13, 1845. https://doi.org/10.3390/foods13121845
Zhao Y, He B, Li D, Gao L, Ren W. Nanobody and CuS Nanoflower-Au-Based Lateral Flow Immunoassay Strip to Enhance the Detection of Aflatoxin B1. Foods. 2024; 13(12):1845. https://doi.org/10.3390/foods13121845
Chicago/Turabian StyleZhao, Yiming, Baoshan He, Danyang Li, Leyan Gao, and Wenjie Ren. 2024. "Nanobody and CuS Nanoflower-Au-Based Lateral Flow Immunoassay Strip to Enhance the Detection of Aflatoxin B1" Foods 13, no. 12: 1845. https://doi.org/10.3390/foods13121845




