Nutritional Quality Assessment of Miscellaneous Cassava Tubers Using Principal Component Analysis and Cluster Analysis
Abstract
:1. Introduction
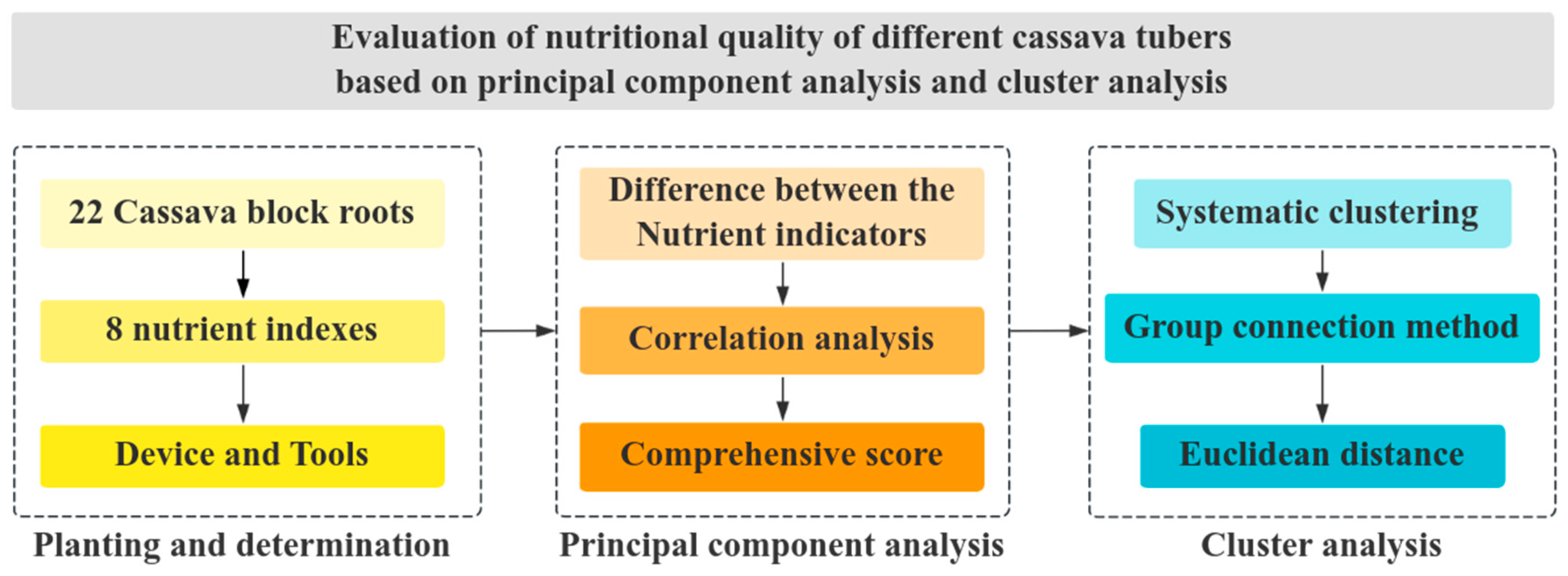
2. Materials and Methodological Framework
2.1. Devices and Materials
2.2. Determination of Indicators
2.3. Analytical Methods
3. Results and Analysis
3.1. Comparative Analysis
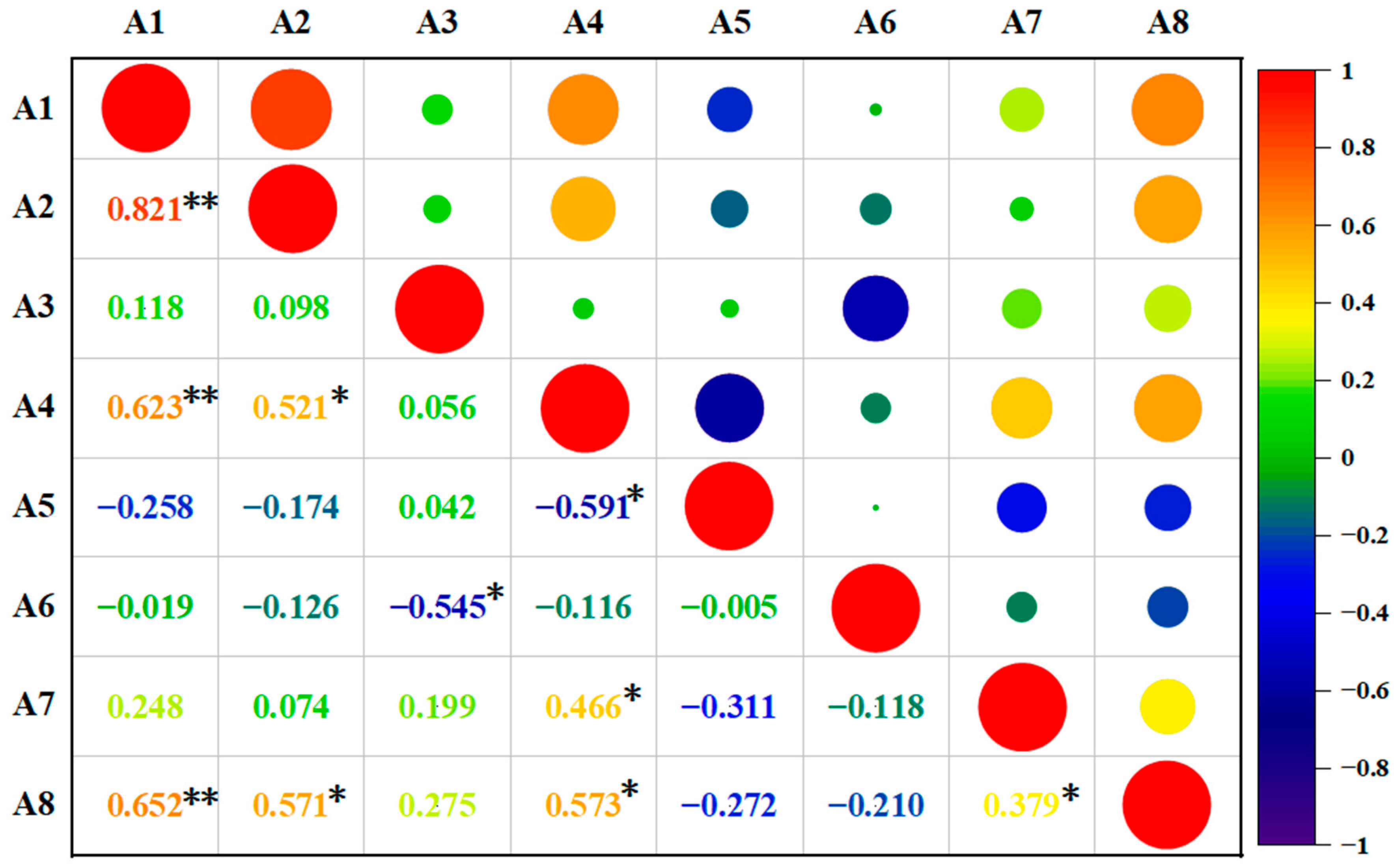
3.2. Correlation Analysis
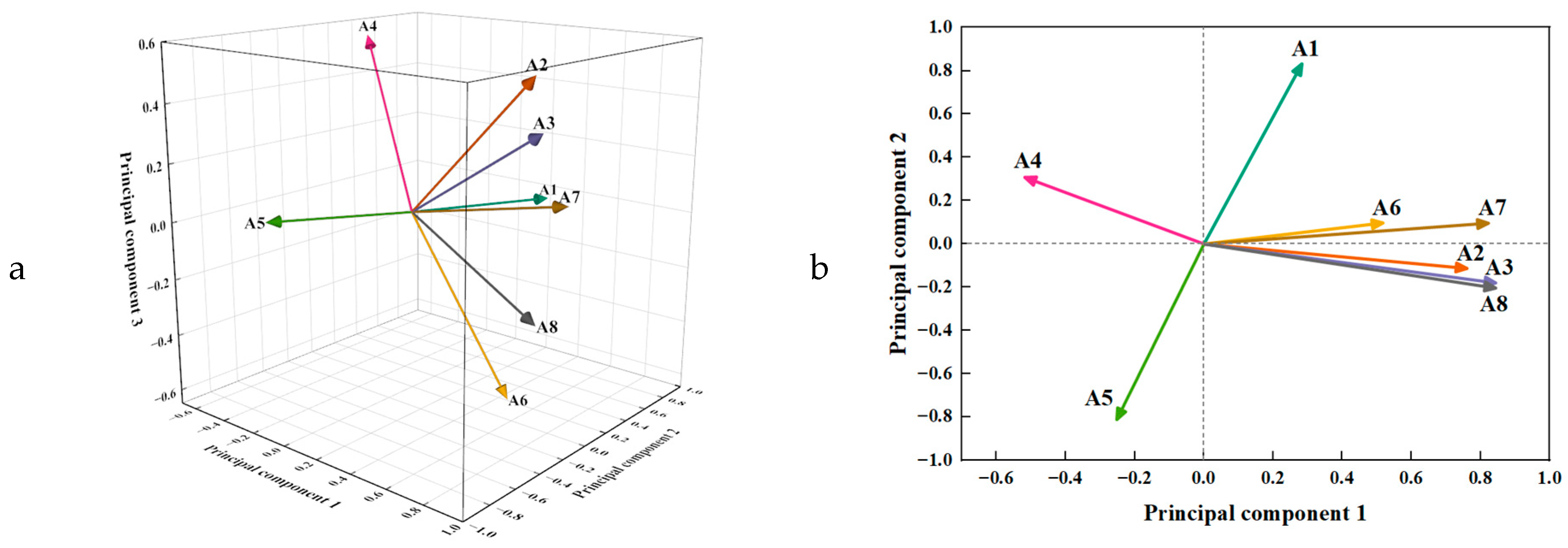
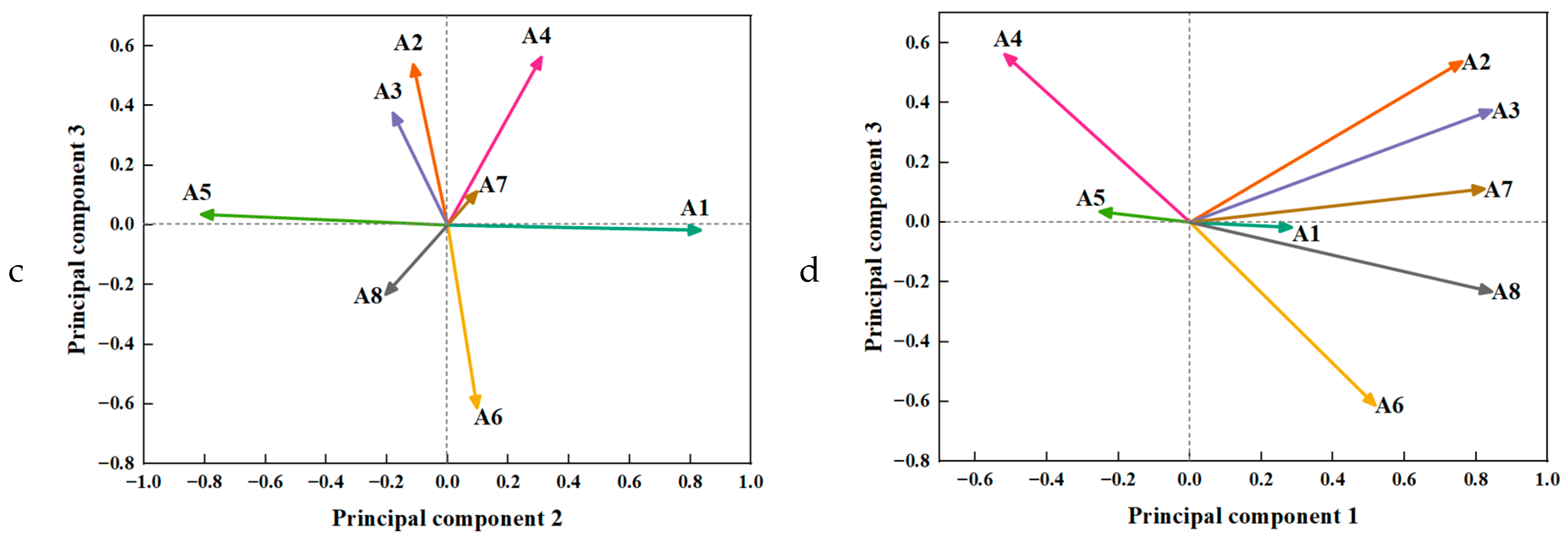
3.3. Principal Component Analysis
3.3.1. Standardized Data Processing
3.3.2. Appropriability Test
3.3.3. Selection of PCA
3.3.4. Comprehensive Evaluation
3.4. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, M.; Liu, G.; Song, J.; Duan, C.; Jiang, T.; Zhang, L.; Yan, W.; Shen, S.; Zhou, Y.; Xiong, X.; et al. Analysis of Agronomic Characteristics of Different Cassava Varieties and Screening of High-Yielding Varieties. Crops 2022, 4, 77–82. [Google Scholar]
- Song, J.M.; Zhang, L.H.; Duan, C.F.; Jiang, T.; Liu, Q.; Xiong, X.; Chang, R.; Liu, G. Analysis and Comprehensive Evaluation of Main Agronomic Traits of Ten New Lines of Cassava. Guangdong Agric. Sci. 2020, 47, 8–15. [Google Scholar] [CrossRef]
- Zainuddin, I.M.; Lecart, B.; Sudarmonowati, E.; Vanderschuren, H. A method for rapid and homogenous initiation of post-harvest physiological deterioration in cassava storage roots identifies Indonesian cultivars with improved shelf-life performance. Plant Methods 2023, 19, 4. [Google Scholar] [CrossRef]
- Cao, S.; Chen, J.-F.; Huang, F.-Y.; Yan, H.-B.; Wei, C.-N.; Li, F.-S.; Lu, L.-Y.; Qin, X.-Y.; Chen, H.-X.; Li, H.-R. Development status and countermeasures of cassava industry in Guangxi. J. South. Agric. 2021, 52, 1468–1476. [Google Scholar]
- Ning, H. Research and Application of Functional Principal Component Analysis. Jilin Univ. 2021, 6–7. [Google Scholar] [CrossRef]
- Zhao, X.H. Research on Feature Extraction Based on Principal Component Analysis. Xidian Univ. 2016, 5–8. [Google Scholar] [CrossRef]
- Sun, J.G.; Liu, J.; Zhao, L.Y. Clustering Algorithms Research. J. Softw. 2008, 1, 48–61. [Google Scholar] [CrossRef]
- Gonçalves, M.G.M.; Avalos, F.A.P.; dos Reis, J.V.; Costa, M.V.; Silva, S.H.G.; Poggere, G.C.; Curi, N.; de Menezes, M.D. Pedology-based management class establishment: A study case in Brazilian coffee crops. Precis. Agric. 2022, 12, 1027–1050. [Google Scholar] [CrossRef]
- Ephzibah, E.P.; Remya, L. Dimensionality Reduction Using Principal Component Analysis and Multi-Label Fuzzy Classification for Rice Crop Disease in Rural Areas of India. Electrochem. Soc. Trans. 2022, 107, 16451. [Google Scholar] [CrossRef]
- Mengistu, S.; Asefa, M. Genetic Diversity Based on Cluster and Principal Component Analyses for Agro-morphological Traits of Wheat Germplasm. Int. J. Genet. Genom. 2022, 10, 79–80. [Google Scholar]
- Seid, E.; Mohammed, W.; Abebe, T. Genetic Diversity Based on Cluster and Principal Component Analyses in Potato (Solanum Tuberosum L.) for Yield and Processing Attributes. J. Hortic. 2021, 8, 1–6. [Google Scholar]
- He, W.; Zhang, X.F.; Guo, S.Y.; Yang, J.Y.; Li, H.R.; Liu, L.J. Comprehensive Evaluation of 22 Potato Germplasms Based on Principal Component Analysis and Cluster Analysis. Seed 2021, 40, 80–86. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Yu, J.; Zhu, D.W.; Zheng, X.L.; Meng, L.Q.; Zhu, Z.W.; Shao, Y.F. Nutritional Quality Evaluation of Different Rice Varieties Based on Principal Component Analysis and Cluster Analysis. China Rice 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Z.; Gao, A.; Luo, R. Quality Evaluation of 23 Species of Clausena lansium (Lour.) Skeels Germplasm Resources Based on Principal Component Analysis and Cluster Analysis. Chin. J. Trop. Crops 2022, 43, 1357–1364. [Google Scholar] [CrossRef]
- GB 5009.9-2016; National Standard for Food Safety, the Determination of Starch in Food. The National Health and Family Planning Commission of the People’s Republic of China: Beijing, China; The China Food and Drug Administration, and the Standardization Administration of the People’s Republic of China. China Standards Press: Beijing, China, 2016.
- Li, Z.X.; Nie, J.Y.; Li, J.; Kuang, L.X.; Shen, Y.M. Optimization of 3,5-Dinitrosalicylic Acid Colorimetry Determination Conditions of Reducing Sugar in Fruits by Response Surface Method. J. Instrum. Anal. 2016, 35, 1283–1288. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.L.; Bai, L.L.; Zhao, D. Optimization of ultrasonic-assisted extraction of anthocyanins from Lonicera edulis by response surface methodology and anti-inflammatory activity studies. Biotechnology 2020, 30, 473–480. [Google Scholar] [CrossRef]
- Wu, P.; Jia, C.S.; Fan, S.Y.; Sun, Y. Principal component analysis and fuzzy comprehensive evaluation of fruit quality in cultivars of cherry. Trans. Chin. Soc. Agric. Eng. 2018, 34, 291–300. [Google Scholar] [CrossRef]
- GB 5009.88-2014; National Standard for Food Safety, the Determination of Dietary Fiber in Food; The National Health and Family Planning Commission of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China; China Standards Press: Beijing, China, 2014.
- Yao, G.F.; Zhang, S.L.; Wu, J.; Cao, Y.F.; Liu, J.; Han, K.; Yang, Z.J. Analysis of components and contents of soluble sugars and organic acids in ten cultivars of pear by high performance liquid chromatography. J. Nanjing Agric. Univ. 2011, 34, 25–31. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, Y.; Zhang, Y.X.; Du, G.Q. Analysis and Evaluation of Fruit Quality and Aroma Components of ‘Yali’ Pear (Pyrus bretschneideri Rehd.) Pollinated with Eighteen Pollinizers. Food Sci. 2022, 43, 294–302. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Chen, M.; Lin, X.; Li, X.; Liu, X.; Gao, H.; Ming, J.; Dai, M.; Zhou, Z. Comprehensive Evaluation of Durian Quality Based on Principal Component Analysis and Cluster Analysis. Sci. Technol. Food Ind. 2023, 44, 278–286, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Fujikoshi, Y.; Satoh, T.; Sugiyama, T. Asymptotic Distribution of the Contribution Ratio in High-Dimensional Principal Component Analysis. Am. J. Math. Manag. Sci. 2011, 31, 39–54. [Google Scholar] [CrossRef]
- Jiang, L.; Bao, Y.; Jia, Y.; Lai, Z.; Xu, F. Nutritional component analysis and comprehensive quality evaluation of 18 different varieties of Lonicera caerulea. Trans. Chin. Soc. Agric. Eng. 2022, 38, 326–335. [Google Scholar] [CrossRef]



| Breed | Character | |||||||
|---|---|---|---|---|---|---|---|---|
| A1/(g·kg−1) | A2/(mg·kg−1) | A3/ (mg·kg−1) | A4/(g·kg−1) | A5/(g·100 g−1) | A6/(mg·g−1) | A7/(mg·100 g−1) | A8/ % | |
| C1115 | 680 | 36.25 | 196.49 | 8.31 | 1.4 | 0.24 | 41.62 | 19.61 |
| SC9 | 676 | 36.13 | 276.81 | 5.82 | 2.17 | 0.29 | 24.1 | 18.67 |
| Nanzhi 199 | 671 | 35.31 | 263.24 | 6.09 | 1.62 | 0.18 | 32.21 | 18.46 |
| GR 4 | 664 | 26.49 | 120.43 | 5.32 | 1.5 | 0.26 | 35.76 | 16.81 |
| SC6068 | 672 | 36.02 | 249.45 | 6.09 | 1.8 | 0.22 | 42.23 | 18.96 |
| Dongguan Hongwei | 684 | 45.81 | 312.92 | 7.87 | 1.23 | 0.16 | 20.45 | 18.36 |
| Glutinous Rice Cassava | 693 | 43.57 | 335.46 | 7.75 | 1.84 | 0.18 | 35.6 | 19.82 |
| Qiongzhong 1 | 663 | 20.95 | 167.44 | 7.12 | 1.93 | 0.21 | 34.25 | 16.79 |
| Guangdong 1 | 678 | 36.76 | 416.33 | 4.5 | 2.42 | 0.16 | 32.12 | 18.86 |
| Colombia 8H | 662 | 37.88 | 12.67 | 4.92 | 2.24 | 0.18 | 25.68 | 15.56 |
| Switzerland D32 | 679 | 39.46 | 146.32 | 5.53 | 2.38 | 0.22 | 28.44 | 19.43 |
| Hainan Hongxin | 684 | 36.45 | 101.53 | 7.13 | 1.34 | 0.23 | 35.38 | 19.68 |
| Xinglong 1 | 685 | 44.69 | 194.16 | 6.1 | 2.01 | 0.29 | 33.88 | 16.34 |
| Huifeng 60 | 669 | 38.65 | 414.62 | 7.04 | 1.86 | 0.15 | 38.98 | 19.61 |
| Guangxi Muci | 709 | 49.62 | 536.32 | 8.02 | 1.68 | 0.15 | 41.17 | 21.06 |
| Huixian Baipi | 670 | 37.55 | 237.04 | 7.58 | 2.47 | 0.19 | 39.01 | 18.71 |
| Rongyang1 | 674 | 38.68 | 235.96 | 7.64 | 1.69 | 0.2 | 37.53 | 17.56 |
| Swiss P11 | 680 | 43.13 | 227.91 | 6.44 | 2.13 | 0.24 | 25.85 | 17.98 |
| Gui Cassava 4 | 728 | 53.24 | 190.98 | 8.64 | 1.86 | 0.21 | 39.43 | 19.73 |
| Shatian Bread | 671 | 40.29 | 340.29 | 6.2 | 1.99 | 0.17 | 36.73 | 18.21 |
| TMS60444 | 677 | 37.56 | 133.74 | 5.34 | 2.65 | 0.22 | 24.48 | 18.68 |
| BRA900 | 653 | 20.71 | 482.57 | 4.23 | 2.5 | 0.18 | 29.67 | 15.26 |
| Item | Minimum | Maximum | Average | Standard Deviations | Coefficient of Variation/% |
|---|---|---|---|---|---|
| A1/(g·kg−1) | 653.00 | 728.00 | 685.2346 | 21.2674 | 3.10 |
| A2/(mg·kg−1) | 2.71 | 53.24 | 14.2679 | 15.2364 | 106.79 |
| A3/(mg·kg−1) | 12.67 | 1198.32 | 242.3659 | 295.6314 | 121.98 |
| A4/(g·kg−1) | 4.23 | 8.64 | 7.1265 | 1.2697 | 17.82 |
| A5/(g·100 g−1) | 1.21 | 2.65 | 1.6984 | 1.3659 | 80.42 |
| A6/(mg·g−1) | 0.14 | 0.29 | 0.2059 | 0.04102 | 19.92 |
| A7/(mg·100 g−1) | 20.60 | 42.30 | 35 | 26.3658 | 75.33 |
| A8/% | 15.26 | 21.06 | 6.2277 | 1.2685 | 20.37 |
| Breed | Character | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| C1115 | 0.10686 | −0.21925 | −0.44885 | 1.40851 | −1.35883 | 0.83112 | 1.30117 | 0.84253 |
| SC9 | −0.14192 | −0.23461 | 0.17571 | −0.56283 | 0.57388 | 2.05010 | −1.46860 | 0.20360 |
| Nanzhi 199 | −0.45290 | −0.33952 | 0.07020 | −0.34907 | −0.80663 | −0.63165 | −0.18648 | 0.06086 |
| GR 4 | −0.88827 | −1.46802 | −1.04028 | −0.95868 | −1.10783 | 1.31871 | 0.37475 | −10.06066 |
| SC6068 | −0.39070 | −0.24868 | −0.03703 | −0.34907 | −0.35482 | 0.34353 | 1.39760 | 0.40072 |
| Dongguan Hongwei | 0.35565 | 1.00392 | 0.45650 | 1.06016 | −1.78553 | −1.11925 | −2.04564 | −0.00711 |
| Glutinous Rice Cassava | 0.91541 | 0.71732 | 0.63177 | 0.96516 | −0.25442 | −0.63165 | 0.34945 | 0.98527 |
| Qiongzhong 1 | −0.95047 | −2.17684 | −0.67474 | 0.46639 | −0.02852 | 0.09973 | 0.13603 | −1.07425 |
| Guangdong 1 | −0.01753 | −0.15400 | 1.26061 | −1.60788 | 1.20138 | −1.11925 | −0.20070 | 0.33275 |
| Colombia 8H | −1.01266 | −0.01070 | −1.87822 | −1.27536 | 0.74958 | −0.63165 | −1.21882 | −1.91029 |
| Switzerland D32 | 0.04467 | 0.19146 | −0.83896 | −0.79242 | 1.10098 | 0.34353 | −0.78248 | 0.72018 |
| Hainan Hongxin | 0.35565 | −0.19367 | −1.18725 | 0.47430 | −1.50943 | 0.58733 | 0.31467 | 0.89011 |
| Xinglong 1 | 0.41784 | 0.86062 | −0.46696 | −0.34115 | 0.17228 | 2.05010 | 0.07754 | −1.38012 |
| Huifeng 60 | −0.57729 | 0.08782 | 1.24731 | 0.40305 | −0.20422 | −1.36304 | 0.88381 | 0.84253 |
| Guangxi Muci | 1.92299 | 1.49140 | 2.19364 | 1.17892 | −0.65602 | −1.36304 | 1.23003 | 1.82811 |
| Huixian Baipi | −0.51510 | −0.05292 | −0.13353 | 0.83057 | 1.32688 | −0.38786 | 0.88855 | 0.23079 |
| Rongyang1 | −0.26631 | 0.09166 | −0.14193 | 0.87807 | −0.63092 | −0.14406 | 0.65457 | −0.55087 |
| Swiss P11 | 0.10686 | 0.66102 | −0.20453 | −0.07197 | 0.47348 | 0.83112 | −1.19194 | −0.26540 |
| Gui Cassava 4 | 3.09227 | 1.95456 | −0.49169 | 1.66978 | −0.20422 | 0.09973 | 0.95495 | 0.92410 |
| Shatian Bread | −0.45290 | 0.29765 | 0.66933 | −0.26198 | 0.12208 | −0.87545 | 0.52810 | −0.10906 |
| TMS60444 | −0.07972 | −0.05164 | −0.93679 | −0.94285 | 1.77868 | 0.34353 | −1.40853 | 0.21040 |
| BRA900 | −1.57243 | −2.20755 | 1.77569 | −1.82164 | 1.40218 | −0.63165 | −0.58803 | −2.11420 |
| Ingredient | Initial Eigenvalue | Extracted Sum of Load Squares | ||||
|---|---|---|---|---|---|---|
| Total | Variance % | Accumulation % | Total | Variance % | Accumulation % | |
| 1 | 3.354 | 41.921 | 41.921 | 3.354 | 41.921 | 41.921 |
| 2 | 1.550 | 19.369 | 61.290 | 1.550 | 19.369 | 61.290 |
| 3 | 1.188 | 14.852 | 76.142 | 1.188 | 14.852 | 76.142 |
| 4 | 0.700 | 8.748 | 84.891 | |||
| 5 | 0.452 | 5.653 | 90.544 | |||
| 6 | 0.368 | 4.601 | 95.145 | |||
| 7 | 0.247 | 3.082 | 98.228 | |||
| 8 | 0.142 | 1.772 | 100.000 | |||
| Item | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A3 | 0.843 | −0.180 | 0.375 |
| A8 | 0.843 | −0.204 | −0.232 |
| A7 | 0.822 | 0.096 | 0.112 |
| A2 | 0.760 | −0.113 | 0.538 |
| A1 | 0.283 | 0.832 | −0.017 |
| A5 | −0.252 | −0.811 | 0.036 |
| A6 | 0.517 | 0.097 | −0.612 |
| A4 | −0.518 | 0.308 | 0.562 |
| Item | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A3 | 0.251 | −0.116 | 0.316 |
| A8 | 0.227 | −0.073 | 0.453 |
| A7 | 0.084 | 0.537 | −0.014 |
| A2 | 0.251 | −0.132 | −0.196 |
| A1 | −0.154 | 0.199 | 0.473 |
| A5 | −0.075 | −0.523 | 0.031 |
| A6 | 0.154 | 0.063 | −0.515 |
| A4 | 0.245 | 0.062 | 0.095 |
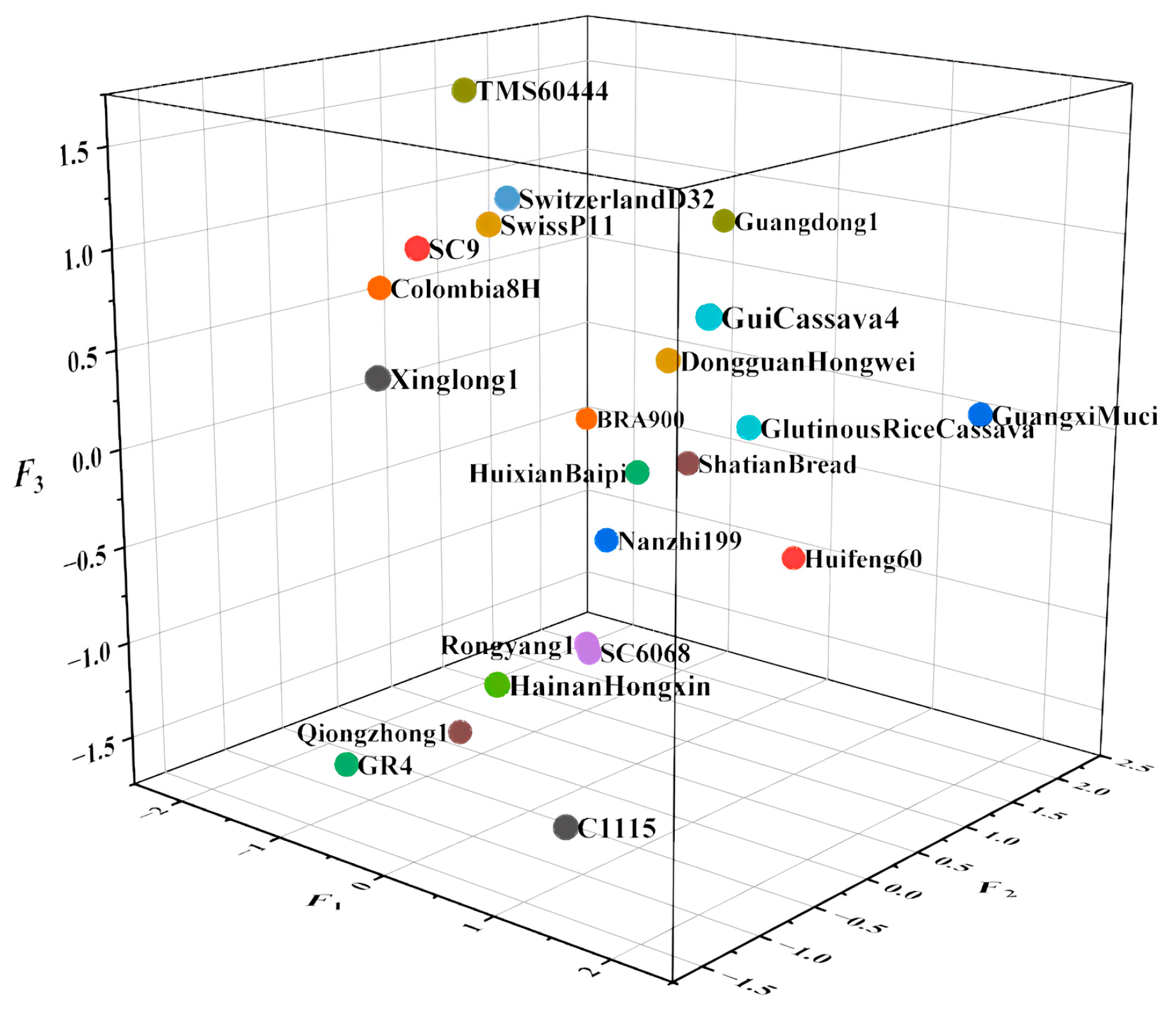
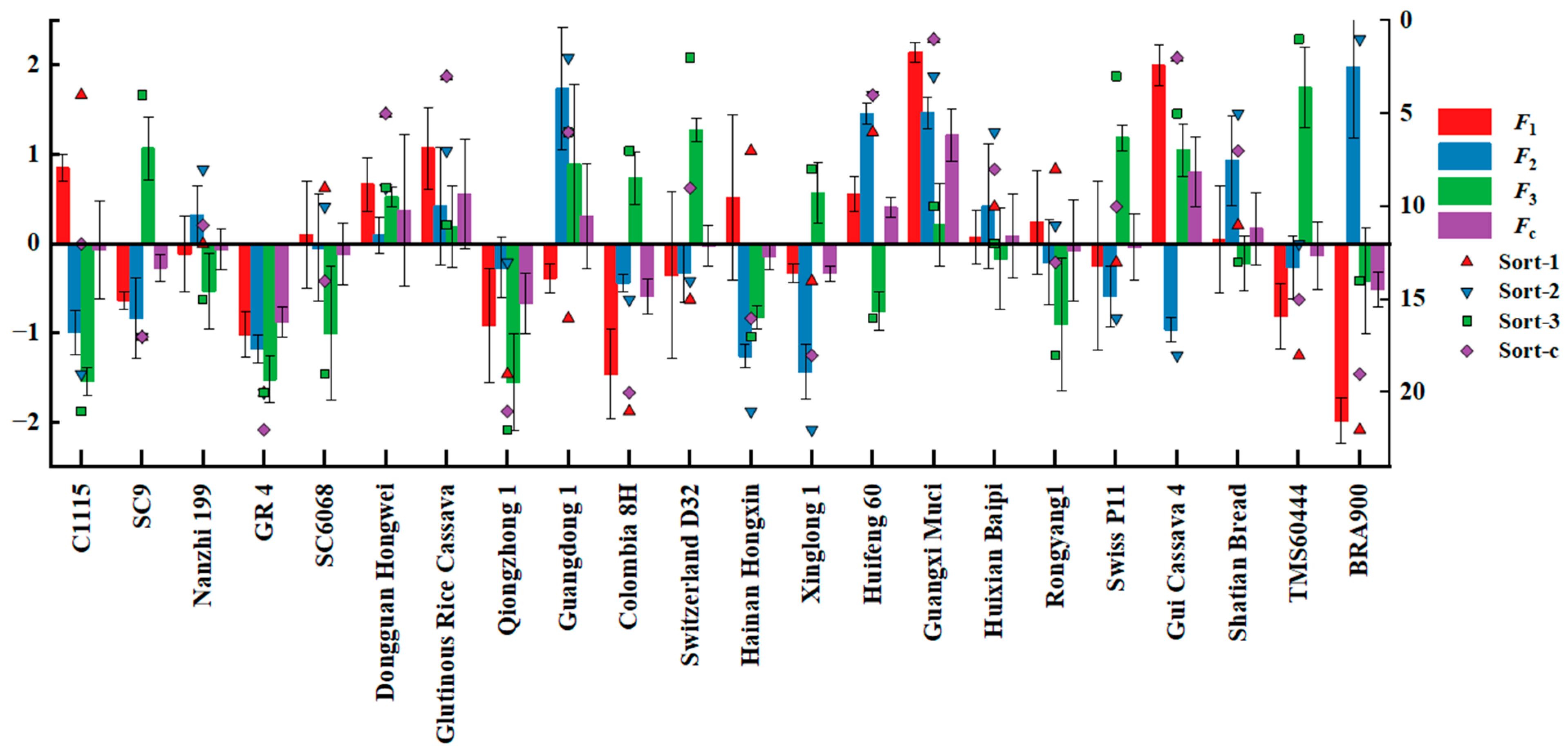
| Breed | F1 | Sort-1 | F2 | Sort-2 | F3 | Sort-3 | Comprehensive Score (Fc) | Sort-c |
|---|---|---|---|---|---|---|---|---|
| C1115 | 0.84661057 | 4 | −0.99421864 | 19 | −1.54236062 | 21 | −0.066733991 | 12 |
| SC9 | −0.6338065 | 17 | −0.8356597 | 17 | 1.06739903 | 4 | −0.269026846 | 17 |
| Nanzhi 199 | −0.11468116 | 12 | 0.32295466 | 8 | −0.52878228 | 15 | −0.064057145 | 11 |
| GR 4 | −1.01465614 | 20 | −1.17417499 | 20 | −1.52012371 | 20 | −0.878548728 | 22 |
| SC6068 | 0.09704118 | 9 | −0.04771496 | 10 | −1.00605313 | 19 | −0.117980288 | 14 |
| Dongguan Hongwei | 0.66374901 | 5 | 0.09138896 | 9 | 0.52655551 | 9 | 0.374155374 | 5 |
| Glutinous Rice Cassava | 1.06968427 | 3 | 0.41613291 | 7 | 0.18991147 | 11 | 0.557228778 | 3 |
| Qiongzhong 1 | −0.91765522 | 19 | −0.2706029 | 13 | −1.55093065 | 22 | −0.667447541 | 21 |
| Guangdong 1 | −0.38749749 | 16 | 1.73489198 | 2 | 0.8907222 | 6 | 0.305878466 | 6 |
| Colombia 8H | −1.4582733 | 21 | −0.43771123 | 15 | 0.73260292 | 7 | −0.587296853 | 20 |
| Switzerland D32 | −0.35406996 | 15 | −0.33029663 | 14 | 1.27071413 | 2 | −0.02367836 | 9 |
| Hainan Hongxin | 0.51956396 | 7 | −1.25981747 | 21 | −0.82493617 | 17 | −0.148727158 | 16 |
| Xinglong 1 | −0.33109157 | 14 | −1.43562142 | 22 | 0.56929818 | 8 | −0.332310244 | 18 |
| Huifeng 60 | 0.55717941 | 6 | 1.45730468 | 4 | −0.75307342 | 16 | 0.40399406 | 4 |
| Guangxi Muci | 2.14195962 | 1 | 1.46358485 | 3 | 0.20914306 | 10 | 1.212474569 | 1 |
| Huixian Baipi | 0.07408384 | 10 | 0.41946144 | 6 | −0.16775428 | 12 | 0.087387307 | 8 |
| Rongyang1 | 0.23624327 | 8 | −0.2110466 | 11 | −0.9050739 | 18 | −0.07626365 | 13 |
| Swiss P11 | −0.24220327 | 13 | −0.59298305 | 16 | 1.18853622 | 3 | −0.03986752 | 10 |
| Gui Cassava 4 | 1.99509464 | 2 | −0.96117721 | 18 | 1.0446656 | 5 | 0.805346945 | 2 |
| Shatian Bread | 0.04582152 | 11 | 0.93348237 | 5 | −0.2180308 | 13 | 0.167633105 | 7 |
| TMS60444 | −0.8121248 | 18 | −0.26698425 | 12 | 1.74667524 | 1 | −0.13274681 | 15 |
| BRA900 | −1.98096505 | 22 | 1.97881552 | 1 | −0.41908981 | 14 | −0.509406799 | 19 |
| Item | Mean Value | ||||
|---|---|---|---|---|---|
| Category I (2) | Category II (12) | Category III (1) | Category IV (6) | Category V (1) | |
| A1/(g·kg−1) | 718.60 | 674.08 | 684.00 | 676.50 | 653.00 |
| A2/(mg·kg−1) | 51.43 | 35.58 | 45.81 | 39.81 | 20.71 |
| A3/(mg·kg−1) | 363.65 | 256.52 | 312.92 | 165.27 | 482.57 |
| A4/(g·kg−1) | 8.33 | 6.73 | 7.87 | 5.69 | 4.23 |
| A5/(g·100 g−1) | 1.77 | 1.82 | 1.23 | 2.26 | 2.50 |
| A6/(mg·g−1) | 0.18 | 0.20 | 0.16 | 0.24 | 0.18 |
| A7/(mg·100 g−1) | 40.30 | 36.79 | 20.45 | 27.07 | 29.67 |
| A8/% | 20.39 | 18.59 | 18.36 | 17.78 | 15.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Chen, R.; Atwa, E.M.; Mabrouk, M.; Jiang, H.; Mou, X.; Ma, X. Nutritional Quality Assessment of Miscellaneous Cassava Tubers Using Principal Component Analysis and Cluster Analysis. Foods 2024, 13, 1861. https://doi.org/10.3390/foods13121861
Chen L, Chen R, Atwa EM, Mabrouk M, Jiang H, Mou X, Ma X. Nutritional Quality Assessment of Miscellaneous Cassava Tubers Using Principal Component Analysis and Cluster Analysis. Foods. 2024; 13(12):1861. https://doi.org/10.3390/foods13121861
Chicago/Turabian StyleChen, Lintao, Rui Chen, Elsayed M. Atwa, Mahmoud Mabrouk, Huanyu Jiang, Xiangwei Mou, and Xu Ma. 2024. "Nutritional Quality Assessment of Miscellaneous Cassava Tubers Using Principal Component Analysis and Cluster Analysis" Foods 13, no. 12: 1861. https://doi.org/10.3390/foods13121861





