Molecular Regulatory Mechanisms Affecting Fruit Aroma
Abstract
:1. Introduction
2. Main Volatile Components
3. Main Synthetic Pathways
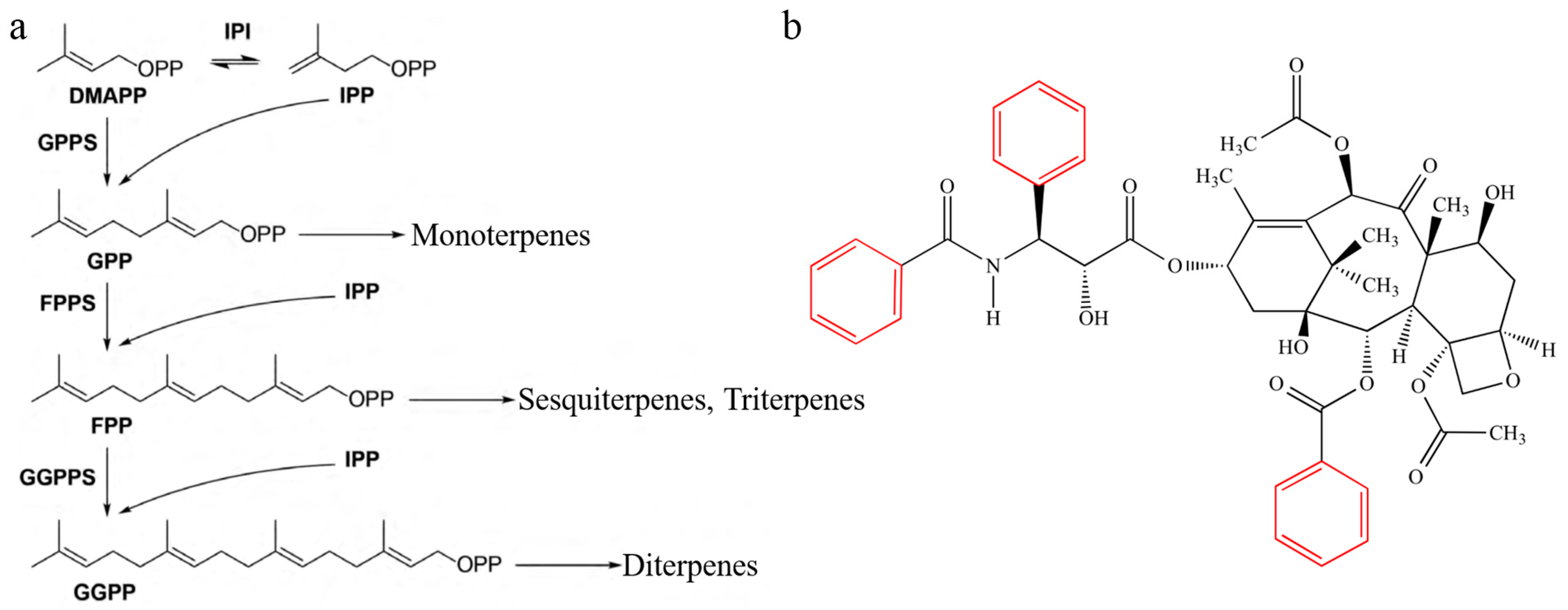
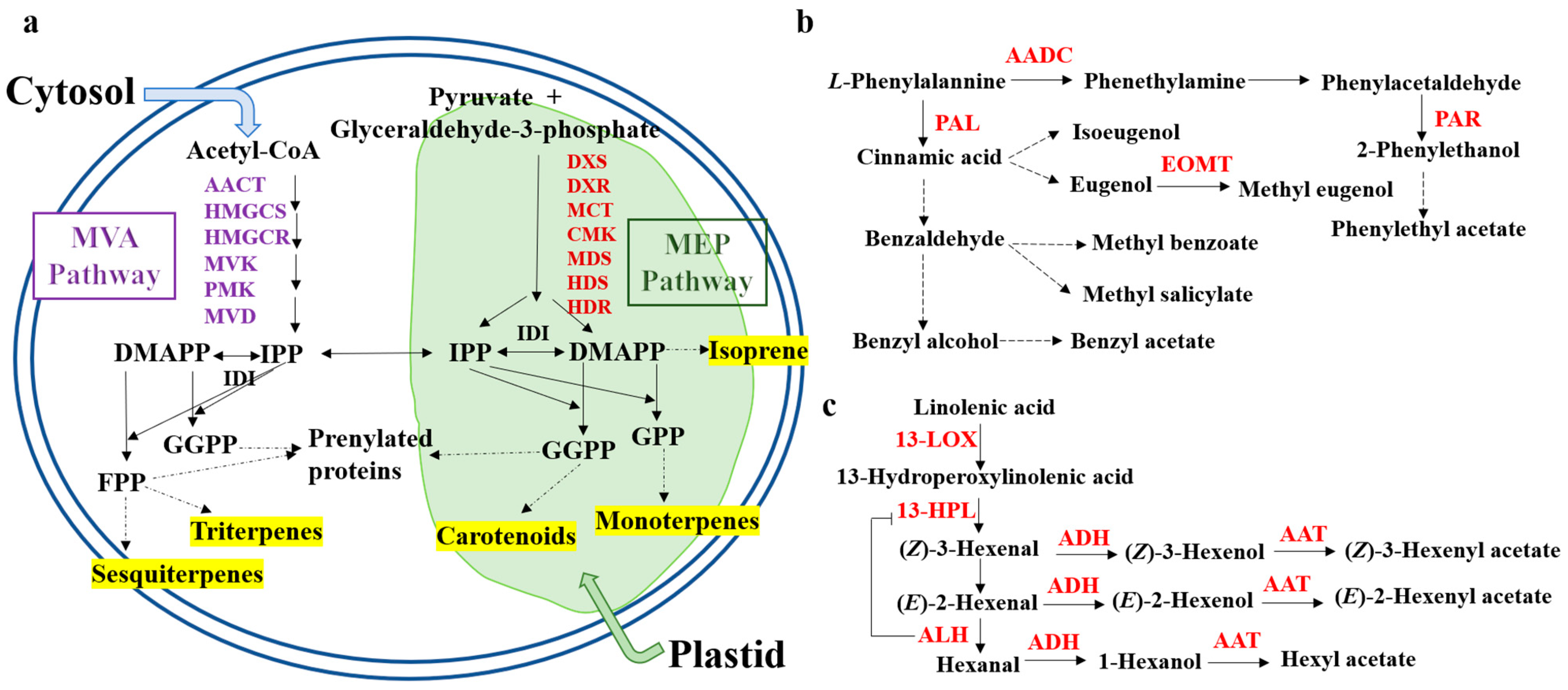
3.1. Terpenoid Synthesis Pathway
3.2. Phenylpropane Synthesis Pathway
3.3. Fatty Acid Synthesis Pathway
4. Regulation of Key Genes Involved in Aroma Formation
4.1. Structural Genes
4.2. Transcription Factors (TFs)
4.3. Noncoding RNAs
5. Epigenetic Factors
6. Main Factors Affecting Fruit Aroma Synthesis
7. Research on Flavor Quality Improvement
8. Issues and Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Jiang, J.; Luo, C.; Rehman, L.; Li, X.; Xie, X. Advances in detecting fruit aroma compounds by combining chromatography and spectrometry. J. Sci. Food Agric. 2023, 103, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Wang, L.; Wu, W.J.; Guo, J.X.; Shen, Y.Y.; Wu, G.L. Advances in aroma compounds biosynthesis and hormone regulation of fruit. Mol. Plant Breed. 2021, 1–11. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20211029.1846.006.html (accessed on 2 November 2021). (In Chinese).
- Li, X.; Gao, P.; Zhang, C.; Xiao, X.; Chen, C.; Song, F. Aroma of peach fruit: A review on aroma volatile compounds and underlying regulatory mechanisms. Int. J. Food Sci. Technol. 2023, 58, 4965–4979. [Google Scholar] [CrossRef]
- Chen, X.; Quek, S.Y. Free and glycosidically bound aroma compounds in fruit: Biosynthesis, transformation, and practical control. Crit. Rev. Food Sci. Nutr. 2023, 63, 9052–9073. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zhang, J.; Wang, Z.; Qi, K.; Li, H.; Tian, R.; Wu, X.; Qiao, X.; Zhang, S.; et al. Comparative analysis of volatile aromatic compounds from a wide range of pear (Pyrus L.) germplasm resources based on HS-SPME with GC-MS. Food Chem. 2023, 418, 135963. [Google Scholar] [CrossRef]
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors—Carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J. Plant Physiol. 2014, 171, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Zhang, Y.L.; Shan, W.; Cai, Y.J.; Liang, S.M.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. Identification of two transcriptional activators MabZIP4/5 in controlling aroma biosynthetic genes during banana ripening. J. Agric. Food Chem. 2018, 66, 6142–6150. [Google Scholar] [CrossRef] [PubMed]
- Forney, C.F.; Qiu, S.; Jordan, M.A.; McCarthy, D.; Fillmore, S. Comparison of volatile compounds contributing to flavor of wild lowbush (Vaccinium augustifolium) and cultivated highbush (Vaccinium corymbosum) blueberry fruit using gas chromatography-olfactometry. Foods 2022, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wei, Q.P.; Kang, W.H.; Zhang, Q.; Sun, J.; Liu, S.Z. Comparison of volatile compounds in ‘Fuji’apples in the different regions in China. Food Sci. Technol. Res. 2017, 23, 79–89. [Google Scholar] [CrossRef]
- Pino, J.A.; Febles, Y. Odour-active compounds in banana fruit cv. Giant Cavendish. Food Chem. 2013, 141, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography–olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]
- Li, L.; Ma, X.W.; Zhan, R.L.; Wu, H.X.; Yao, Q.S.; Xu, W.T.; Luo, C.; Zhou, Y.G.; Liang, Q.Z.; Wang, S.B. Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS ONE 2017, 12, e0187487. [Google Scholar] [CrossRef]
- Buttery, R.G.; Takeoka, G.R. Some unusual minor volatile components of tomato. J. Agric. Food Chem. 2004, 52, 6264–6266. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.K.; Liu, G.F.; Rana, M.M.; Zhu, L.W.; Jiang, S.L.; Huang, Y.F.; Lu, W.M.; Wei, S. Volatile profiling of two pear genotypes with different potential for white pear aroma improvement. Sci. Hortic. 2016, 209, 221–228. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Structure and dynamics of the isoprenoid pathway network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, P.; Zhan, P.; Yan, H.; Zhou, W.; Zhang, F. Effects of β-glucosidase on the aroma characteristics of flat peach juice as assessed by descriptive sensory analysis and gas chromatography and compared by partial least squares regression. LWT Food Sci. Technol. 2017, 82, 113–120. [Google Scholar] [CrossRef]
- Wei, C.; Liu, H.; Cao, X.; Zhang, M.; Li, X.; Chen, K.; Zhang, B. Synthesis of flavour-related linalool is regulated by PpbHLH1 and associated with changes in DNA methylation during peach fruit ripening. Plant Biotechnol. J. 2021, 19, 2082–2096. [Google Scholar] [CrossRef]
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Sun, Q.; He, L.; Sun, L.; Xu, H.Y.; Fu, Y.Q.; Sun, Z.Y.; Zhu, B.Q.; Duan, C.Q.; Pan, Q.H. Identification of SNP loci and candidate genes genetically controlling norisoprenoids in grape berry based on genome-wide association study. Front. Plant Sci. 2023, 14, 1142139. [Google Scholar] [CrossRef]
- Barja, M.V.; Ezquerro, M.; Beretta, S.; Diretto, G.; Florez-Sarasa, I.; Feixes, E.; Fiore, A.; Karlova, R.; Fernie, A.R.; Beekwilder, J.; et al. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol. 2021, 231, 255–272. [Google Scholar] [CrossRef]
- Peled-Zehavi, H.; Oliva, M.; Xie, Q.; Tzin, V.; Oren-Shamir, M.; Aharoni, A.; Galili, G. Metabolic engineering of the phenylpropanoid and its primary, precursor pathway to enhance the flavor of fruits and the aroma of flowers. Bioengineering 2015, 2, 204–212. [Google Scholar] [CrossRef]
- Muto, A.; Müller, C.T.; Bruno, L.; McGregor, L.; Ferrante, A.; Chiappetta, A.A.C.; Bitonti, M.B.; Rogers, H.J.; Spadafora, N.D. Fruit volatilome profiling through GC × GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10, 18333. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Venegas-Calerón, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genom. 2013, 14, 343. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Gao, L.; Qi, Y.; Fu, W.; Li, X.; Zhou, X.; Gao, Q.; Gao, Z.; Jia, H. Acyl-CoA oxidase 1 is involved in γ-decalactone release from peach (Prunus persica) fruit. Plant Cell Rep. 2017, 36, 829–842. [Google Scholar] [CrossRef]
- Peng, B.; Yu, M.; Zhang, B.; Xu, J.; Ma, R. Differences in PpAAT1 activity in high- and low-aroma peach varieties affect γ-decalactone production. Plant Physiol. 2020, 182, 2065–2080. [Google Scholar] [CrossRef]
- Li, X.; Qi, L.; Zang, N.; Zhao, L.; Sun, Y.; Huang, X.; Wang, H.; Yin, Z.; Wang, A. Integrated metabolome and transcriptome analysis of the regulatory network of volatile ester formation during fruit ripening in pear. Plant Physiol. Biochem. 2022, 185, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R. Some advances in the knowledge of grape, wine and distillates chemistry as achieved by mass spectrometry. J. Mass Spectrom. 2005, 40, 705–713. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Monteiro, M. HS-GC-MS-O analysis and sensory acceptance of passion fruit during maturation. J. Food Sci. Technol. 2017, 54, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ma, F.; Wu, B.; Lv, W.; Xu, Y.; Xing, W.; Chen, D.; Xu, B.; Song, S. Genome-wide association and expression analysis of the lipoxygenase gene family in Passiflora edulis revealing PeLOX4 might be involved in fruit ripeness and ester formation. Int. J. Mol. Sci. 2022, 23, 12496. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Jiang, J.; Zhang, L.P.; Yu, Y.; Ye, Z.W.; Wang, X.M.; Zhou, J.Y.; Chai, M.L.; Zhang, H.Q.; Arús, P.; et al. Identification of volatile and softening-related genes using digital gene expression profiles in melting peach. Tree Genet. Genomes 2015, 11, 71. [Google Scholar] [CrossRef]
- Wang, A.H.; Ma, H.Y.; Zhang, B.H.; Mo, C.Y.; Li, E.H.; Li, F. Transcriptomic and metabolomic analyses provide insights into the formation of the peach-like aroma of Fragaria nilgerrensis Schlecht. Fruits. Genes 2022, 13, 1285. [Google Scholar] [CrossRef]
- Feng, S.; Yan, C.; Zhang, T.; Ji, M.; Tao, R.; Gao, H. Comparative study of volatile compounds and expression of related genes in fruit from two apple cultivars during different developmental stages. Molecules 2021, 26, 1553. [Google Scholar] [CrossRef]
- Li, L.X.; Fang, Y.; Li, D.; Zhu, Z.H.; Zhang, Y.; Tang, Z.Y.; Li, T.; Chen, X.S.; Feng, S.Q. Transcription factors MdMYC2 and MdMYB85 interact with ester aroma synthesis gene MdAAT1 in apple. Plant Physiol. 2023, 193, 2442–2458. [Google Scholar] [CrossRef]
- Wei, C.; Li, M.; Cao, X.; Jin, Z.; Zhang, C.; Xu, M.; Chen, K.; Zhang, B. Linalool synthesis related PpTPS1 and PpTPS3 are activated by transcription factor PpERF61 whose expression is associated with DNA methylation during peach fruit ripening. Plant Sci. 2022, 317, 111200. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hidalgo, F.J.; Medina-Puche, L.; Cañete-Gómez, C.; Franco-Zorrilla, J.M.; López-Vidriero, I.; Solano, R.; Caballero, J.L.; Rodríguez-Franco, A.; Blanco-Portales, R.; Muñoz-Blanco, J.; et al. The fruit-specific transcription factor FaDOF2 regulates the production of eugenol in ripe fruit receptacles. J. Exp. Bot. 2017, 68, 4529–4543. [Google Scholar] [CrossRef]
- Sheng, L.; Ni, Y.; Wang, J.; Chen, Y.; Gao, H. Characteristic-aroma-component-based evaluation and classification of strawberry varieties by aroma type. Molecules 2021, 26, 6219. [Google Scholar] [CrossRef]
- Cao, X.; Wei, C.; Duan, W.; Gao, Y.; Kuang, J.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis. Plant J. 2021, 106, 785–800. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Li, S.; Li, D.; Yu, J.; Zhao, Z. Jasmonate-induced MdMYC2 improves fruit aroma during storage of ‘Ruixue’ apple based on transcriptomic, metabolic and functional analyses. LWT Food Sci. Technol. 2023, 185, 115168. [Google Scholar] [CrossRef]
- Moazed, D. Small RNAs in transcriptional gene silencing and genome defence. Nature 2009, 457, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Vaucheret, H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006, 38 (Suppl. 6), S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Lakhwani, D.; Sanchita; Pandey, A.; Sharma, D.; Asif, M.H.; Trivedi, P.K. Novel microRNAs regulating ripening-associated processes in banana fruit. Plant Growth Regul. 2020, 90, 223–235. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, X.; Yao, M.M.; Tan, Z.; Zhou, Q.; Zhang, L.; Ji, S.J. miRNAs play important roles in aroma weakening during the shelf life of ‘Nanguo’ pear after cold storage. Food Res. Int. 2019, 116, 942–952. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, F.; Wang, X.; Qiu, K.; Sheng, Y.; Xie, Q.; Shi, P.; Zhang, J.; Pan, H. Genome-wide identification and characterization of long noncoding RNAs during peach (Prunus persica) fruit development and ripening. Sci. Rep. 2022, 12, 11044. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef]
- DuPont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, X.; Hao, Y.; Sun, H.; Zhou, Q.; Sun, Y.; Ji, S.J. Methyl jasmonate pretreatment improves aroma quality of cold-stored ‘Nanguo’ pears by promoting ester biosynthesis. Food Chem. 2021, 338, 127846. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.J.; Moreno-Rojas, J.M.; Ruiz-Moreno, M.J. Assessing a traceability technique in fresh oranges (Citrus sinensis L. Osbeck) with an HS-SPME-GC-MS method. Towards a volatile characterisation of organic oranges. Food Chem. 2017, 221, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, S.; Rambla, J.L.; Besada, C.; Granell, A.; Salvador, A. Changes in the volatile profile of citrus fruit submitted to postharvest degreening treatment. Postharvest Biol. Technol. 2017, 133, 48–56. [Google Scholar] [CrossRef]
- Cheong, M.W.; Zhu, D.; Sng, J.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Characterisation of calamansi (Citrus microcarpa). Part II: Volatiles, physicochemical properties and non-volatiles in the juice. Food Chem. 2012, 134, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Xiao, Y.; Zhang, Z.; Shi, Y.; Kong, W.; Yang, X.; Jiang, G.; Zhang, B.; et al. Cultivation conditions change aroma volatiles of strawberry fruit. Horticulturae 2021, 7, 81. [Google Scholar] [CrossRef]
- Meucci, A.; Shiriaev, A.; Rosellini, I.; Malorgio, F.; Pezzarossa, B. Se-enrichment pattern, composition, and aroma profile of ripe tomatoes after sodium selenate foliar spraying performed at different plant developmental stages. Plants 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Jiang, K.; Kuang, Y.; Zeng, Y.; Cheng, X.; Liu, Y.; Wang, S.; Shen, W. Preharvest application of hydrogen nanobubble water enhances strawberry flavor and consumer preferences. Food Chem. 2022, 377, 131953. [Google Scholar] [CrossRef]
- Tietel, Z.; Bar, E.; Lewinsohn, E.; Feldmesser, E.; Fallik, E.; Porat, R. Effects of wax coatings and postharvest storage on sensory quality and aroma volatile composition of ‘Mor’ mandarins. J. Sci. Food Agric. 2010, 90, 995–1007. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on ripening and volatile compounds of blueberry fruit. J. Food Process. Preserv. 2020, 44, e14840. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Yang, H.; Zhao, Z. Genetic analysis and QTL mapping of aroma volatile compounds in the apple progeny ‘Fuji’ × ‘Cripps Pink’. Front. Plant Sci. 2023, 14, 1048846. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tieman, D.M.; Knapp, S.J.; Zerbe, P.; Famula, R.; Barbey, C.R.; Folta, K.M.; Amadeu, R.R.; Lee, M.; Oh, Y.; et al. A multi-omics framework reveals strawberry flavor genes and their regulatory elements. New Phytol. 2022, 236, 1089–1107. [Google Scholar] [CrossRef] [PubMed]
| Species | Main Aroma Component | Variety of Aroma Substance | Reference |
|---|---|---|---|
| Apple | N-butyl 2-methylacetate, hexyl acetate | 300 | Qin et al. [16] |
| Kiwifruit | ethyl butyrate, (E)-2-hexene-1-ol, (E)-2-hexenal | 172 | Liang et al. [18] |
| Grape | ethyl butyrate, ethyl hexanoate, octanal, nonanal, limonene | 26 | Zhu et al. [19] |
| Peach | hexanal, (Z)-3-hexene-1-ol, (E)-2-hexenal, 3-mercaptohexanol, nonanal | 100 | Li et al. [20] |
| Banana | 2-methylpropyl butyrate, orthonitrophenyl-beta-d-fucopyranoside | 48 | Pino and Febles [17] |
| Tomato | cis-3-hexenal, trans-2-hexenal, cis-3-hexenol | 400 | Buttery and Takeoka [21] |
| Mango | terpene olefins, 3-pinene, caryophyllene, α-pinene | 60 | Yi et al. [22] |
| Pear | hexyl acetate, alpha-farnesene | 202 | Wang et al. [6] |
| Citrus | limonene, citral, naringene | 162 | Zhang et al. [8] |
| Strawberry | methyl butyrate, ethyl butyrate, butyl acetate, methyl caproate, ethyl hexanoate | 979 | Ulrich et al. [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zhao, H.; Zhong, T.; Chen, D.; Wu, Y.; Xie, Z. Molecular Regulatory Mechanisms Affecting Fruit Aroma. Foods 2024, 13, 1870. https://doi.org/10.3390/foods13121870
Lu H, Zhao H, Zhong T, Chen D, Wu Y, Xie Z. Molecular Regulatory Mechanisms Affecting Fruit Aroma. Foods. 2024; 13(12):1870. https://doi.org/10.3390/foods13121870
Chicago/Turabian StyleLu, Haifei, Hongfei Zhao, Tailin Zhong, Danwei Chen, Yaqiong Wu, and Zhengwan Xie. 2024. "Molecular Regulatory Mechanisms Affecting Fruit Aroma" Foods 13, no. 12: 1870. https://doi.org/10.3390/foods13121870




