Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis
Abstract
:1. Introduction
2. Material and Methods
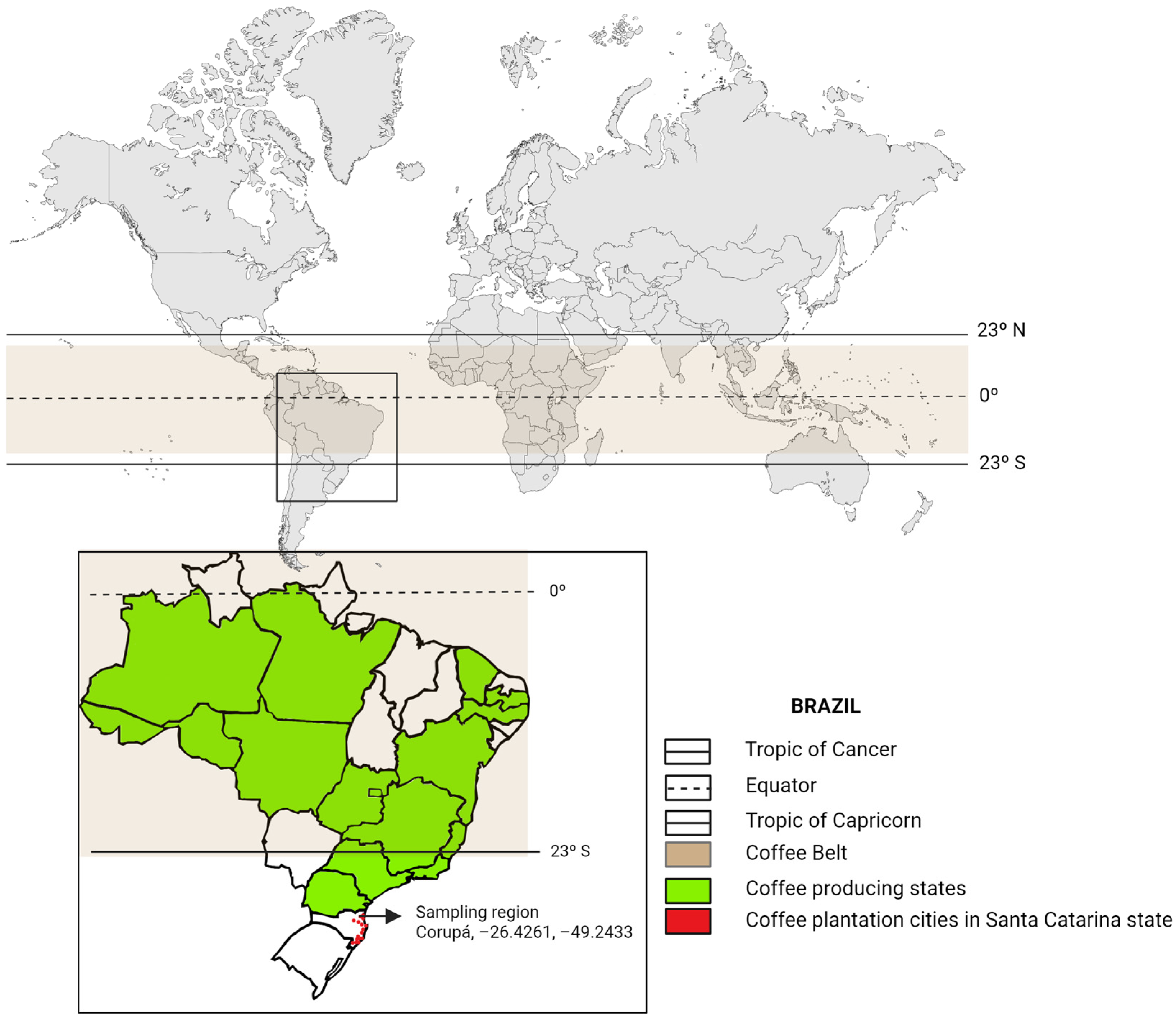
2.1. Area of Study and Sampling Procedure
2.2. Metataxonomic Analysis
2.3. Biochemical Analysis of the Fermentation Liquid Fraction
2.3.1. Analysis of Sugar Consumption and Organic Acid Production by High-Performance Liquid Chromatography
2.3.2. Main Volatile Organic Compounds Identified by Gas Chromatography Coupled to Mass Spectrometry
2.4. Co-Occurrence/Co-Exclusion Analysis
2.5. Sensory Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Coffea Alpha Diversity during the Fermentation Process
3.2. Fermentation Diversity and Dynamics
3.3. Metabolic Profile Changes during Coffee Fermentation
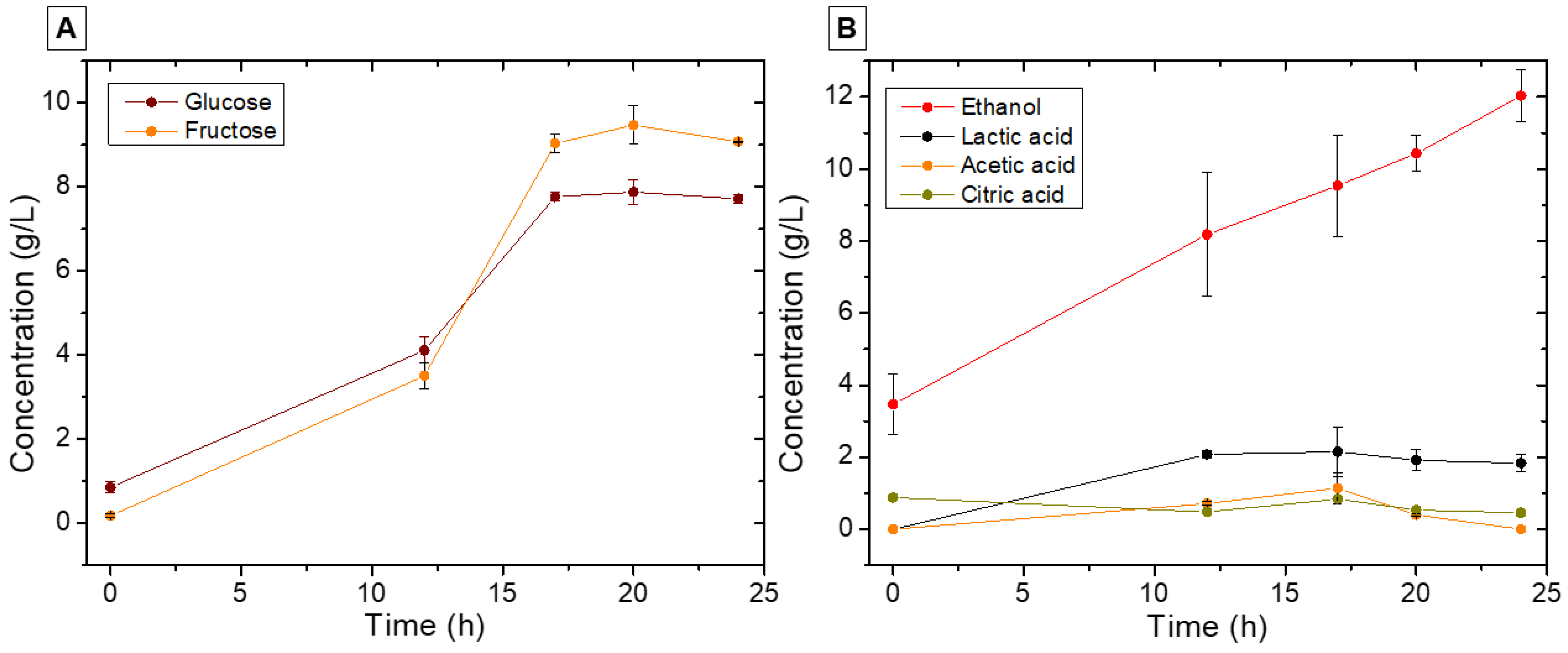
3.3.1. Major Metabolite Changes
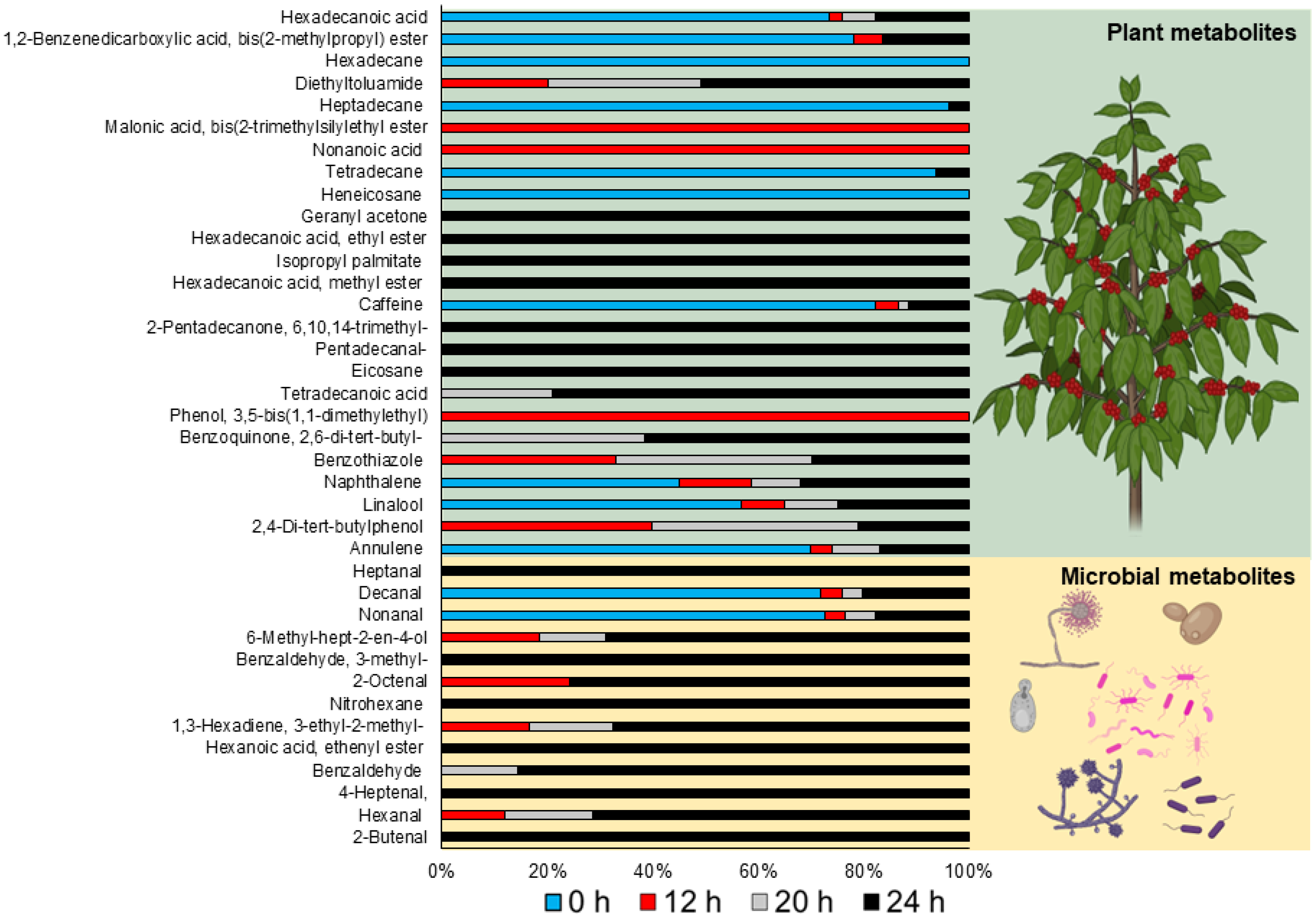
3.3.2. Volatile Compounds
3.4. Co-Occurrence/Co-Exclusion Relationships between Genera and Microbial Metabolites
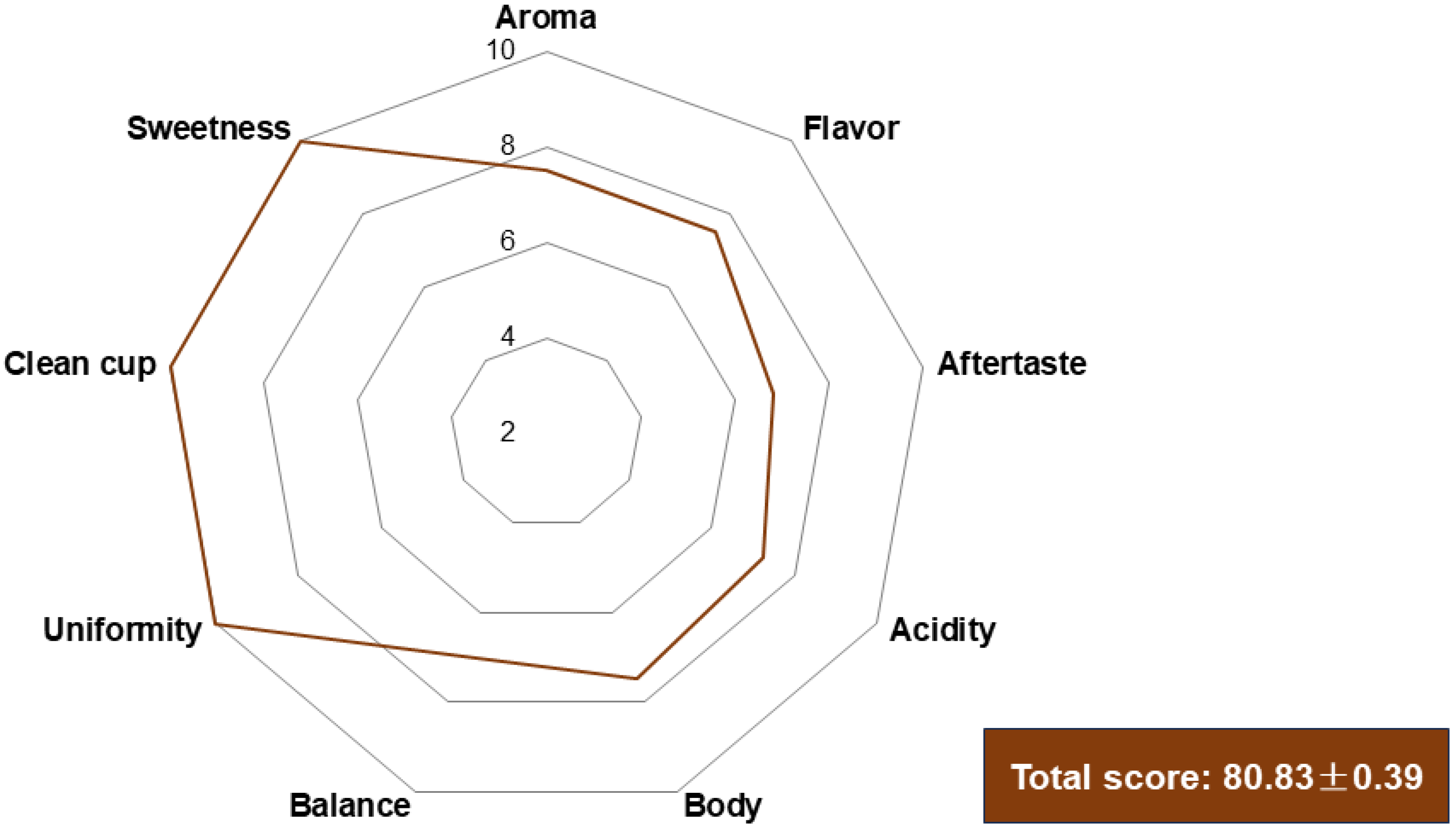
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utrilla-Catalan, R.; Rodríguez-Rivero, R.; Narvaez, V.; Díaz-Barcos, V.; Blanco, M.; Galeano, J. Growing Inequality in the Coffee Global Value Chain: A Complex Network Assessment. Sustainability 2022, 14, 672. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans–A Review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Coffee Fermentation: Expedition from Traditional to Controlled Process and Perspectives for Industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Gomes, L.S.; Oliveira, M.M.; Santos, L.D. Coffee Fermentation Process: A Review. Food Res. Int. 2023, 169, 112793. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junqueira, A.C.; de Pereira, G.V.M.; Coral Medina, J.D.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First Description of Bacterial and Fungal Communities in Colombian Coffee Beans Fermentation Analysed Using Illumina-Based Amplicon Sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee Fermentation and Flavor-An Intricate and Delicate Relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef]
- Bisso, F.P.; da Rice, W.S.; Zambonim, F.M.; Nachtigall, A.M.; Paiva, C.L.; Schmedler, G.; Oliveira, D. Produção de Cafés Especiais No Estado de Santa Catarina, Brasil: Aspectos Climáticos e Avaliação Qualitativa de Grãos; X Simpósio de Pesquisa dos Cafés do Brasil: Vitória, Brazil, 2019; ISSN 1984-9249. [Google Scholar]
- Salem, F.H.; Lebrun, M.; Mestres, C.; Sieczkowski, N.; Boulanger, R.; Collignan, A. Transfer Kinetics of Labeled Aroma Compounds from Liquid Media into Coffee Beans during Simulated Wet Processing Conditions. Food Chem. 2020, 322, 126779. [Google Scholar] [CrossRef]
- Salem, F.H.; Vasai, F.; Duez, C.; Sieczkowski, N.; Boulanger, R.; Collignan, A. Mass Transfer Kinetics of Nonvolatile Compounds into Coffee Beans during Wet Processing: Study at the Laboratory Scale and in Real Conditions Using Two Yeast Strains. ACS Food Sci. Technol. 2022, 2, 852–861. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; De Vuyst, L.; Björkroth, J. Following Coffee Production from Cherries to Cup: Microbiological and Metabolomic Analysis of Wet Processing of Coffea Arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal Shotgun Metagenomics of an Ecuadorian Coffee Fermentation Process Highlights the Predominance of Lactic Acid Bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological Diversity, Evolution and Metabolism of Microbial Communities in the Wet Fermentation of Australian Coffee Beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef] [PubMed]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial Diversity Associated with Spontaneous Coffee Bean Fermentation Process and Specialty Coffee Production in Northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Braga, A.V.U.; Miranda, M.A.; Aoyama, H.; Schmidt, F.L. Study on Coffee Quality Improvement by Self-Induced Anaerobic Fermentation: Microbial Diversity and Enzymatic Activity. Food Res. Int. 2023, 165, 112528. [Google Scholar] [CrossRef] [PubMed]
- Piraino, P.; Zotta, T.; Ricciardi, A.; Parente, E. Discrimination of Commercial Caciocavallo Cheeses on the Basis of the Diversity of Lactic Microflora and Primary Proteolysis. Int. Dairy J. 2005, 15, 1138–1149. [Google Scholar] [CrossRef]
- da Vale, A.S.; Balla, G.; Rodrigues, L.R.S.; de Carvalho Neto, D.P.; Soccol, C.R.; de Melo Pereira, G.V. Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies. Foods 2022, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Garcia-Armisen, T.; Papalexandratou, Z.; Hendryckx, H.; Camu, N.; Vrancken, G.; De Vuyst, L.; Cornelis, P. Diversity of the Total Bacterial Community Associated with Ghanaian and Brazilian Cocoa Bean Fermentation Samples as Revealed by a 16 S RRNA Gene Clone Library. Appl. Microbiol. Biotechnol. 2010, 87, 2281–2292. [Google Scholar] [CrossRef]
- Hugouvieux-Cotte-Pattat, N.; Jacot-des-Combes, C.; Briolay, J. Genomic Characterization of a Pectinolytic Isolate of Serratia Oryzae Isolated from Lake Water. J. Genom. 2019, 7, 64–72. [Google Scholar] [CrossRef]
- Pregolini, V.B.; de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Soccol, C.R. Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation. Fermentation 2021, 7, 278. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of Bacterial and Fungal Communities during Natural Coffee (Coffea arabica) Fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- do Rosário, D.K.A.; da Silva Mutz, Y.; Vieira, K.M.; Schwan, R.F.; Bernardes, P.C. Effect of Self-Induced Anaerobiosis Fermentation (SIAF) in the Volatile Compounds and Sensory Quality of Coffee. Eur. Food Res. Technol. 2024, 250, 667–675. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Velasquez-Emiliani, A.V.; Angel, C.A. Microbial Diversity Using a Metataxonomic Approach, Associated with Coffee Fermentation Processes in the Department of Quindío, Colombia. Fermentation 2023, 9, 343. [Google Scholar] [CrossRef]
- da Vale, A.S.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Sorto, R.D.; Goés-Neto, A.; Kato, R.; Soccol, C.R. Facility-Specific ‘House’ Microbiome Ensures the Maintenance of Functional Microbial Communities into Coffee Beans Fermentation: Implications for Source Tracking. Environ. Microbiol. Rep. 2021, 13, 470–481. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Stephanus, N. Venter Pantoea Ananatis: An Unconventional Plant Pathogen. Mol. Plant Pathol. 2009, 10, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Luo, J.; Ahmed, T.; Zaki, H.E.M.; Tian, Y.; Shahid, M.S.; Chen, J.; Li, B. Beneficial Effect and Potential Risk of Pantoea on Rice Production. Plants 2022, 11, 2608. [Google Scholar] [CrossRef] [PubMed]
- Walterson, A.M.; Stavrinides, J. Pantoea: Insights into a Highly Versatile and Diverse Genus within the Enterobacteriaceae. FEMS Microbiol. Rev. 2015, 39, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, P.R.; Patil, S.M.; Jadhav, S.L.; Jeon, B.-H.; Govindwar, S.P. Utilization of Agricultural Waste Biomass by Cellulolytic Isolate Enterobacter Sp. SUK-Bio. Agric. Nat. Resour. 2018, 52, 399–406. [Google Scholar] [CrossRef]
- Campos, E.; Negro Alvarez, M.J.; Sabarís di Lorenzo, G.; Gonzalez, S.; Rorig, M.; Talia, P.; Grasso, D.H.; Sáez, F.; Manzanares Secades, P.; Ballesteros Perdices, M.; et al. Purification and Characterization of a GH43 β-Xylosidase from Enterobacter sp. Identified and Cloned from Forest Soil Bacteria. Microbiol. Res. 2014, 169, 213–220. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium Oxysporum: Genomics, Diversity and Plant–Host Interaction. In Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 159–199. [Google Scholar]
- de Wit, P.J.G.M.; van der Burgt, A.; Ökmen, B.; Stergiopoulos, I.; Abd-Elsalam, K.A.; Aerts, A.L.; Bahkali, A.H.; Beenen, H.G.; Chettri, P.; Cox, M.P.; et al. The Genomes of the Fungal Plant Pathogens Cladosporium Fulvum and Dothistroma Septosporum Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry. PLoS Genet. 2012, 8, e1003088. [Google Scholar] [CrossRef] [PubMed]
- Prasoulas, G.; Gentikis, A.; Konti, A.; Kalantzi, S.; Kekos, D.; Mamma, D. Bioethanol Production from Food Waste Applying the Multienzyme System Produced On-Site by Fusarium Oxysporum F3 and Mixed Microbial Cultures. Fermentation 2020, 6, 39. [Google Scholar] [CrossRef]
- dos Santos Gomes, W.; Pereira, L.L.; Rodrigues da Luz, J.M.; de Soares da Silva, M.C.; Reis Veloso, T.G.; Partelli, F.L. Exploring the Microbiome of Coffee Plants: Implications for Coffee Quality and Production. Food Res. Int. 2024, 179, 113972. [Google Scholar] [CrossRef] [PubMed]
- da Vale, A.S.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with Pichia Fermentans and Pediococcus Acidilactici on Metabolite Produced during Fermentation and Volatile Composition of Coffee Beans. Fermentation 2019, 5, 67. [Google Scholar] [CrossRef]
- Góngora, C.E.; Holguín-Sterling, L.; Pedraza-Claros, B.; Pérez-Salinas, R.; Ortiz, A.; Navarro-Escalante, L. Metataxonomic Identification of Microorganisms during the Coffee Fermentation Process in Colombian Farms (Cesar Department). Foods 2024, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- de Sampaio, V.M.; Wiele, N.; da Silva Vale, A.; Woiciechowski, A.L.; Karp, S.G.; Soccol, C.R.; de Melo Pereira, G.V. Modulation of Aroma and Chemical Composition of Coffee Beans through Simultaneous and Sequential Inoculation of Pichia Fermentans and Pediococcus pentosaceus during Wet Fermentation. Syst. Microbiol. Biomanuf. 2024. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Evangelista, S.R.; Dias, D.R.; Schwan, R.F. Characteristics of Fermented Coffee Inoculated with Yeast Starter Cultures Using Different Inoculation Methods. LWT-Food Sci. Technol. 2018, 92, 212–219. [Google Scholar] [CrossRef]
- Lin, Z.; Wei, J.; Hu, Y.; Pi, D.; Jiang, M.; Lang, T. Caffeine Synthesis and Its Mechanism and Application by Microbial Degradation, A Review. Foods 2023, 12, 2721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Long, Y.; Ma, Y.; Chen, Y.; Yu, Q.; Xie, J.; Li, B.; Tian, J. Comparison of Chemical and Fatty Acid Composition of Green Coffee Bean (Coffea arabica L.) from Different Geographical Origins. LWT 2021, 140, 110802. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Cárdenas-Vargas, S.; Saavedra-Barrera, R.; Durán, D.C. GC–MS Study of Compounds Isolated from Coffea Arabica Flowers by Different Extraction Techniques. J. Sep. Sci. 2013, 36, 2901–2914. [Google Scholar] [CrossRef]
- Hafsah, H.; Iriawati, I.; Syamsudin, T.S. Dataset of Volatile Compounds from Flowers and Secondary Metabolites from the Skin Pulp, Green Beans, and Peaberry Green Beans of Robusta Coffee. Data Brief. 2020, 29, 105219. [Google Scholar] [CrossRef]
- D’Amelio, N.; De Angelis, E.; Navarini, L.; Schievano, E.; Mammi, S. Green Coffee Oil Analysis by High-Resolution Nuclear Magnetic Resonance Spectroscopy. Talanta 2013, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Smits, T.H.M.; Rezzonico, F.; Kamber, T.; Goesmann, A.; Ishimaru, C.A.; Stockwell, V.O.; Frey, J.E.; Duffy, B. Genome Sequence of the Biocontrol Agent Pantoea Vagans Strain C9-1. J. Bacteriol. 2010, 192, 6486–6487. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Wang, X.; He, J.; Li, W.; Lu, M.; Sun, X.; Yin, Y. Revealing Core Functional Microorganisms in the Fermentation Process of Qicaipaojiao (Capsicum annuum L.) Based on Microbial Metabolic Network. Food Res. Int. 2024, 187, 114315. [Google Scholar] [CrossRef]
- de Cassimiro, D.M.J.; Batista, N.N.; Fonseca, H.C.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Coinoculation of Lactic Acid Bacteria and Yeasts Increases the Quality of Wet Fermented Arabica Coffee. Int. J. Food Microbiol. 2022, 369, 109627. [Google Scholar] [CrossRef]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The Lipid Fraction of the Coffee Bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Isquierdo, E.P.; Borém, F.M.; de Oliveira, P.D.; Siqueira, V.C.; Alves, G.E. Quality of Natural Coffee Subjected to Different Rest Periods during the Drying Process. Cienc. Agrotecnologia 2012, 36, 439–445. [Google Scholar] [CrossRef]
- Tang, V.C.Y.; Sun, J.; Cornuz, M.; Yu, B.; Lassabliere, B. Effect of Solid-State Fungal Fermentation on the Non-Volatiles Content and Volatiles Composition of Coffea Canephora (Robusta) Coffee Beans. Food Chem. 2021, 337, 128023. [Google Scholar] [CrossRef]
- Silva, C.F.; Vilela, D.M.; de Souza Cordeiro, C.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a Potential Starter Culture for Enhance Quality of Coffee Fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Silva, C.F.; da Miguel, M.G.P.C.; de Cordeiro, C.S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of Coffee Beverage Quality by Using Selected Yeasts Strains during the Fermentation in Dry Process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, A.d.S.; Pereira, C.M.T.; De Dea Lindner, J.; Rodrigues, L.R.S.; Kadri, N.K.E.; Pagnoncelli, M.G.B.; Kaur Brar, S.; Soccol, C.R.; Pereira, G.V.d.M. Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis. Foods 2024, 13, 1871. https://doi.org/10.3390/foods13121871
Vale AdS, Pereira CMT, De Dea Lindner J, Rodrigues LRS, Kadri NKE, Pagnoncelli MGB, Kaur Brar S, Soccol CR, Pereira GVdM. Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis. Foods. 2024; 13(12):1871. https://doi.org/10.3390/foods13121871
Chicago/Turabian StyleVale, Alexander da Silva, Cecília Marques Tenório Pereira, Juliano De Dea Lindner, Luiz Roberto Saldanha Rodrigues, Nájua Kêmil El Kadri, Maria Giovana Binder Pagnoncelli, Satinder Kaur Brar, Carlos Ricardo Soccol, and Gilberto Vinícius de Melo Pereira. 2024. "Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis" Foods 13, no. 12: 1871. https://doi.org/10.3390/foods13121871
APA StyleVale, A. d. S., Pereira, C. M. T., De Dea Lindner, J., Rodrigues, L. R. S., Kadri, N. K. E., Pagnoncelli, M. G. B., Kaur Brar, S., Soccol, C. R., & Pereira, G. V. d. M. (2024). Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis. Foods, 13(12), 1871. https://doi.org/10.3390/foods13121871










