A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review
Abstract
1. Introduction
2. FFA Analysis Methods
2.1. Classical Titration Methods
2.2. Instrumental Methods
2.2.1. Colorimetric Methods
Copper Soap Method
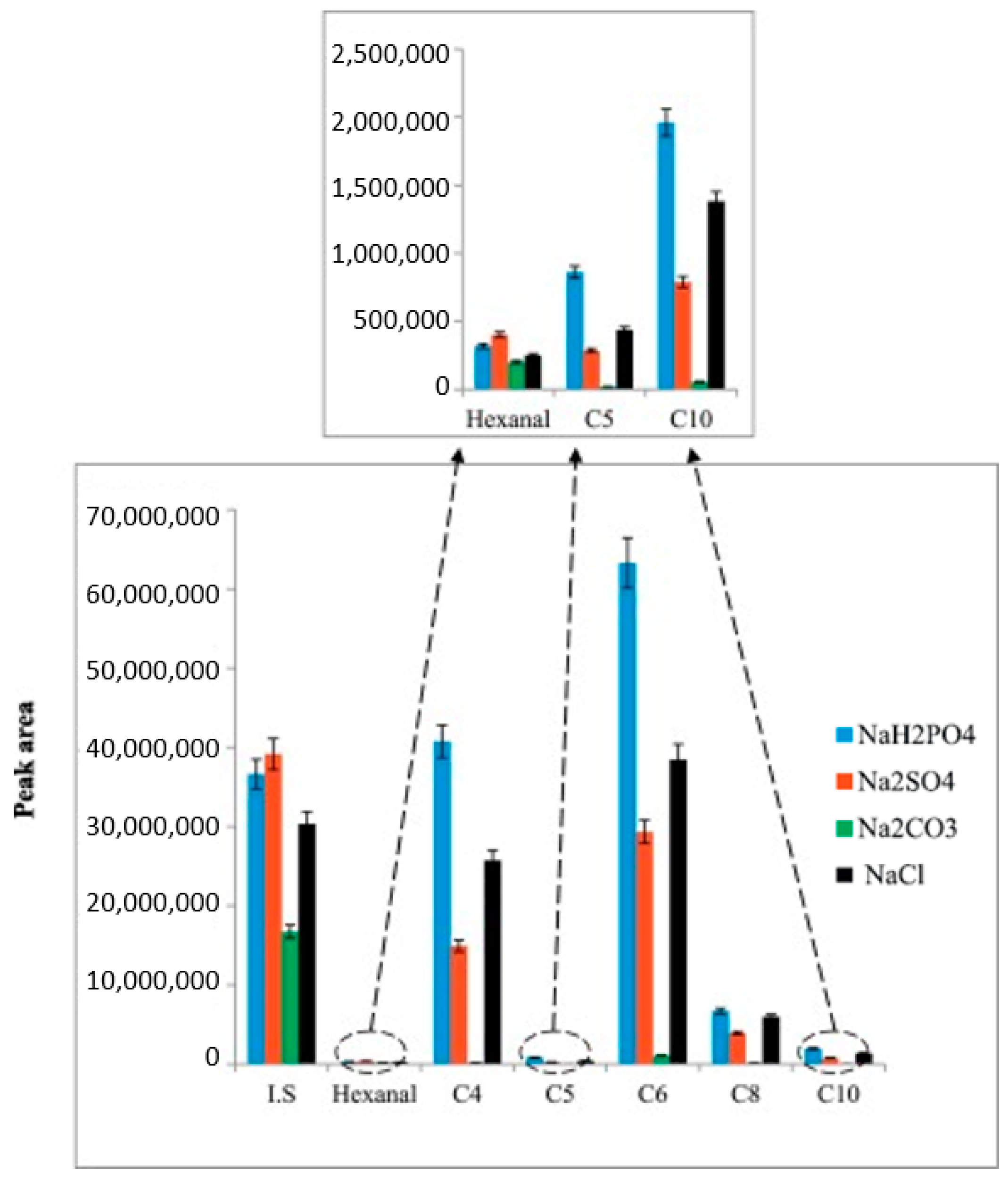
Flow Injection Analysis (FIA) Methods
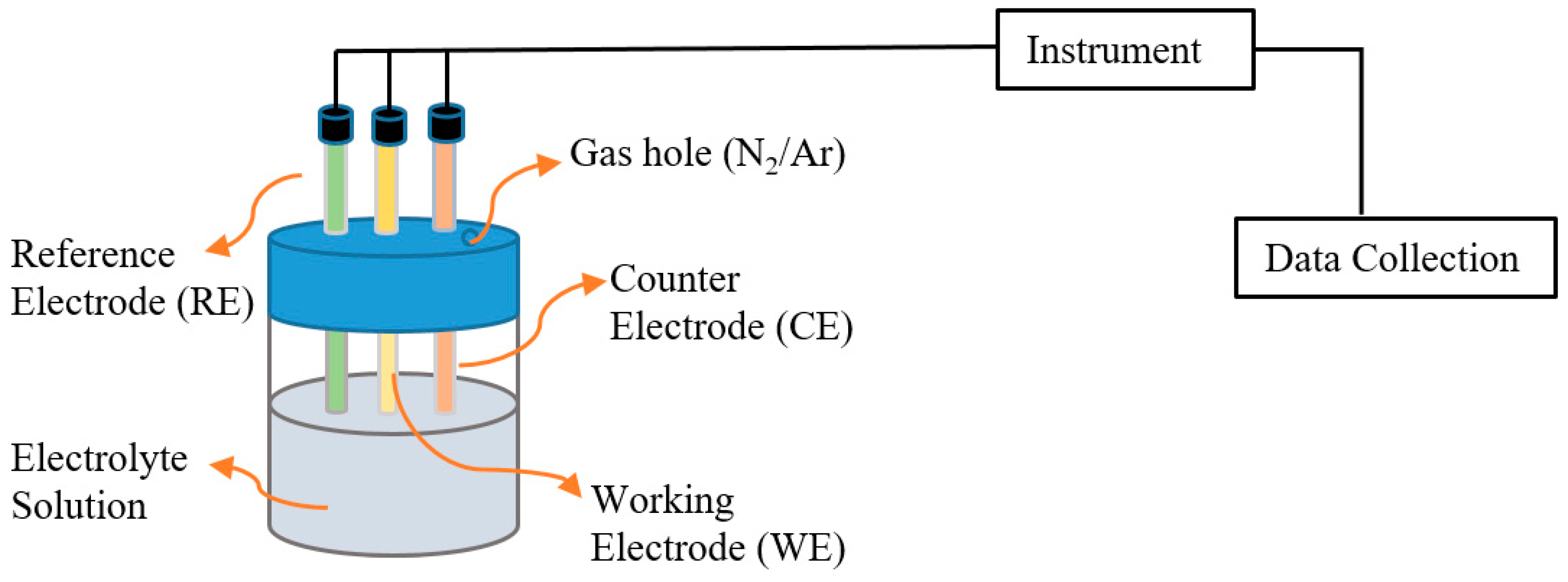
2.2.2. Electrochemical Methods
Voltametric Methods
Electrical Conductivity (EC) Methods
pH Metric Methods
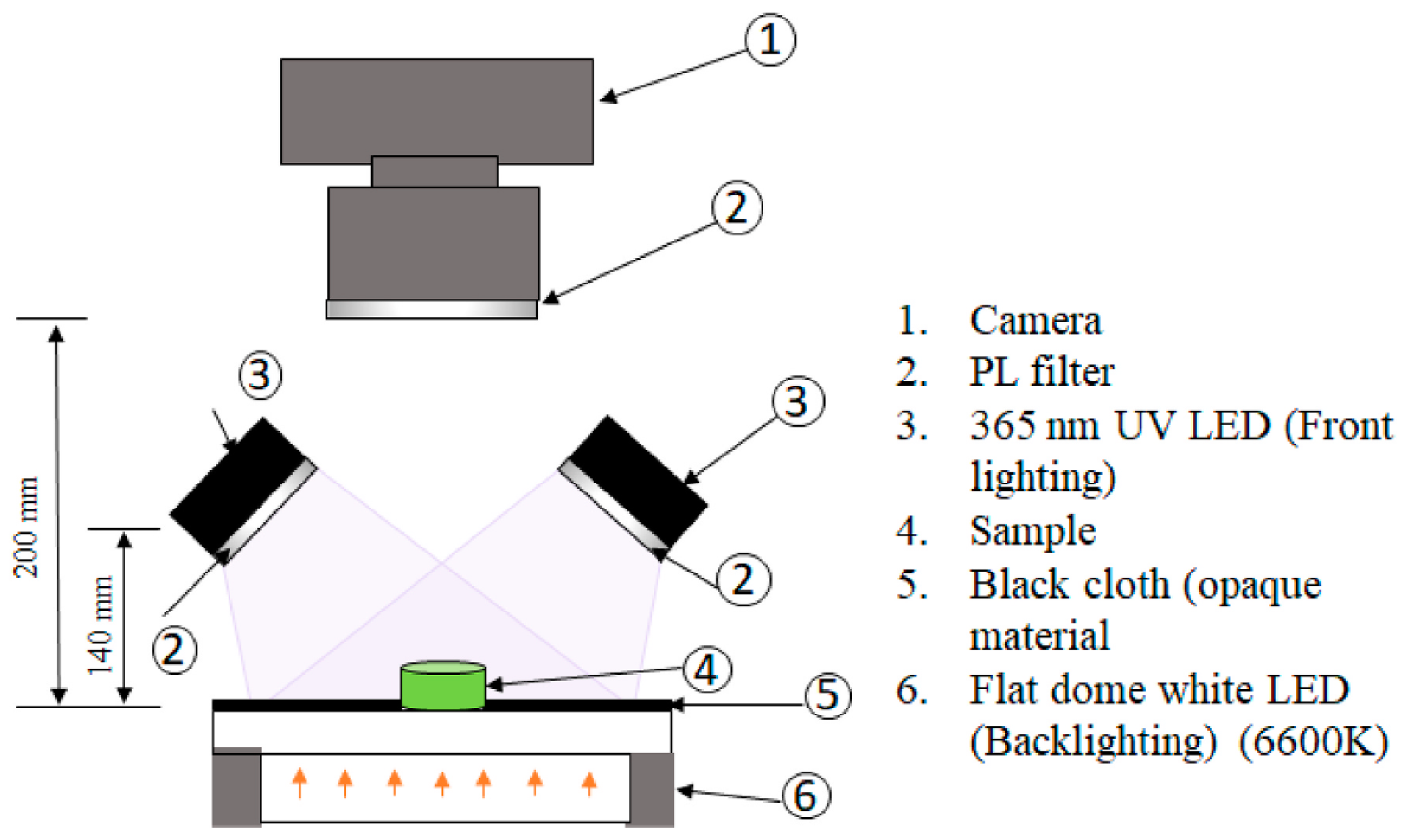
2.2.3. Spectroscopic Methods
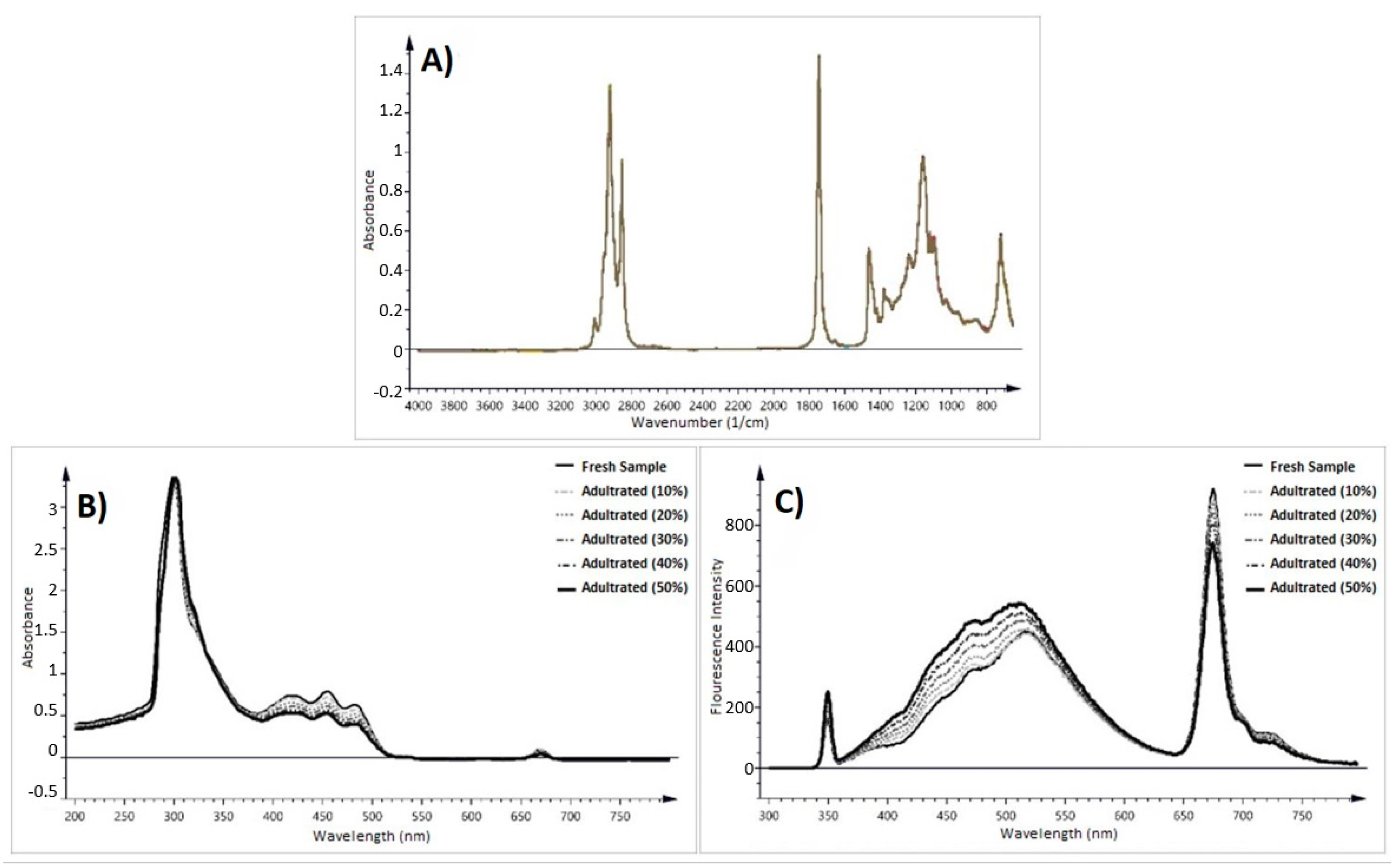
Infrared Methods
- -
- For NIR spectroscopy, the absorption of electromagnetic radiation at wavenumbers ranging from 12,500 cm−1 to 4000 cm−1 (~800–2500 nm wavelength) [73].
- -
- 4000–2500 cm−1: strong contributions from O-H, N-H, C-H, and S-H stretching vibrations are seen in the X-H stretch region,
- 2500–2000 cm−1: strong contributions from gas-phase CO (2143 cm−1) and linearly adsorbed CO (2000–2200 cm−1) are observed in the triple-bond region,
- 2000–1500 cm−1: bridge-bonded CO as well as carbonyl groups of adsorbed molecules are seen in the double-bond region,
- 1500–500 cm−1: all single bonds between carbon and elements are seen in the fingerprint region, such as nitrogen, oxygen, sulfur, and halogens,
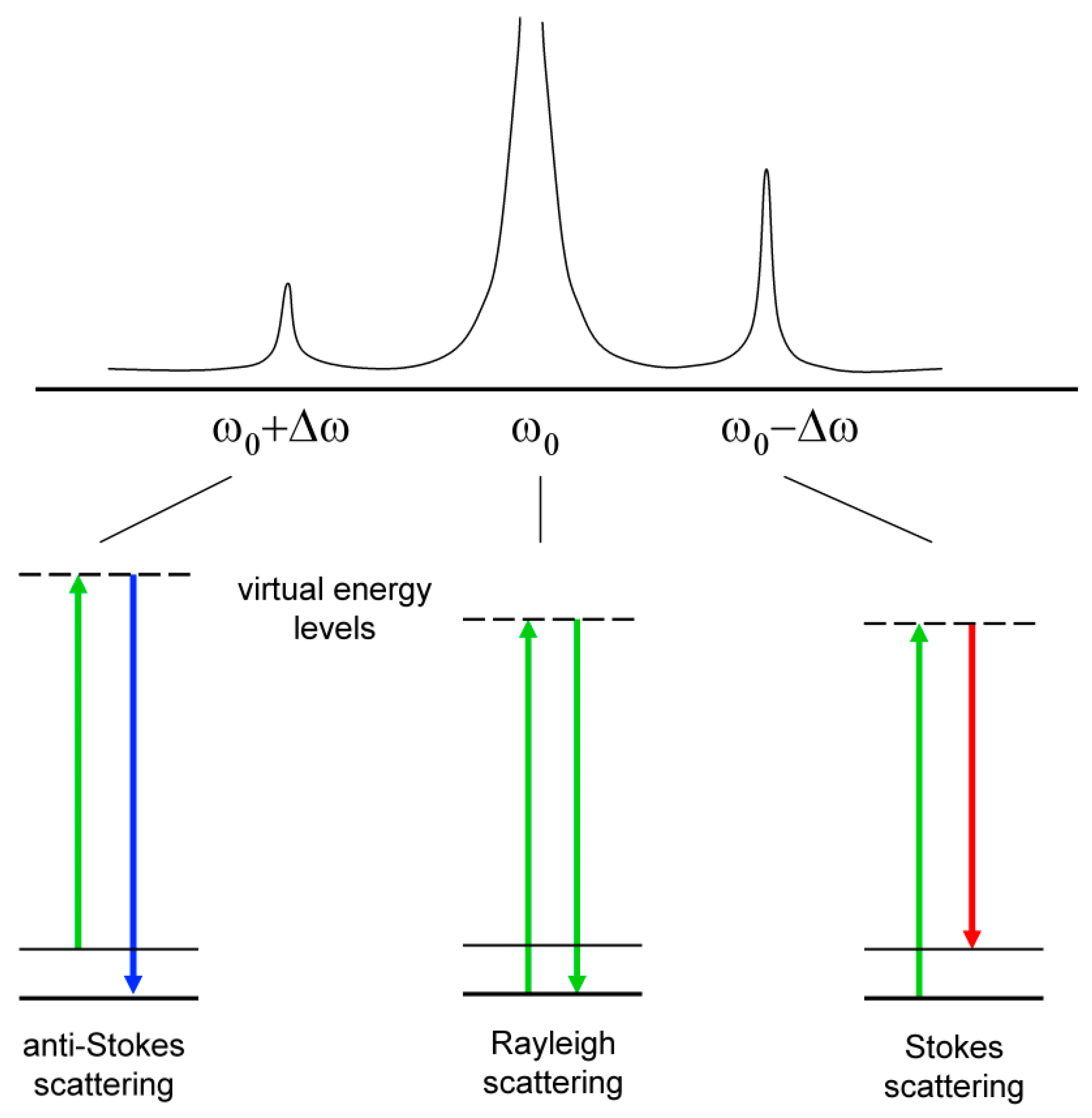
Raman Methods
Nuclear Magnetic Resonance (NMR) Methods
2.2.4. Chromatographic Methods
Gas Chromatography (GC)
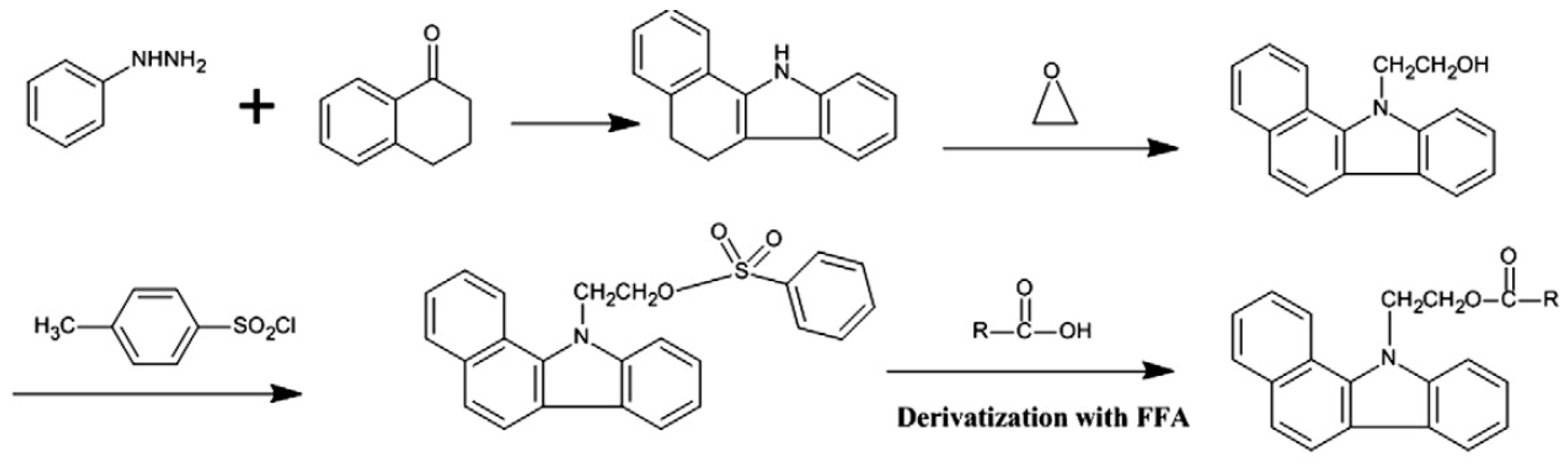
High-Performance Liquid Chromatography (HPLC)
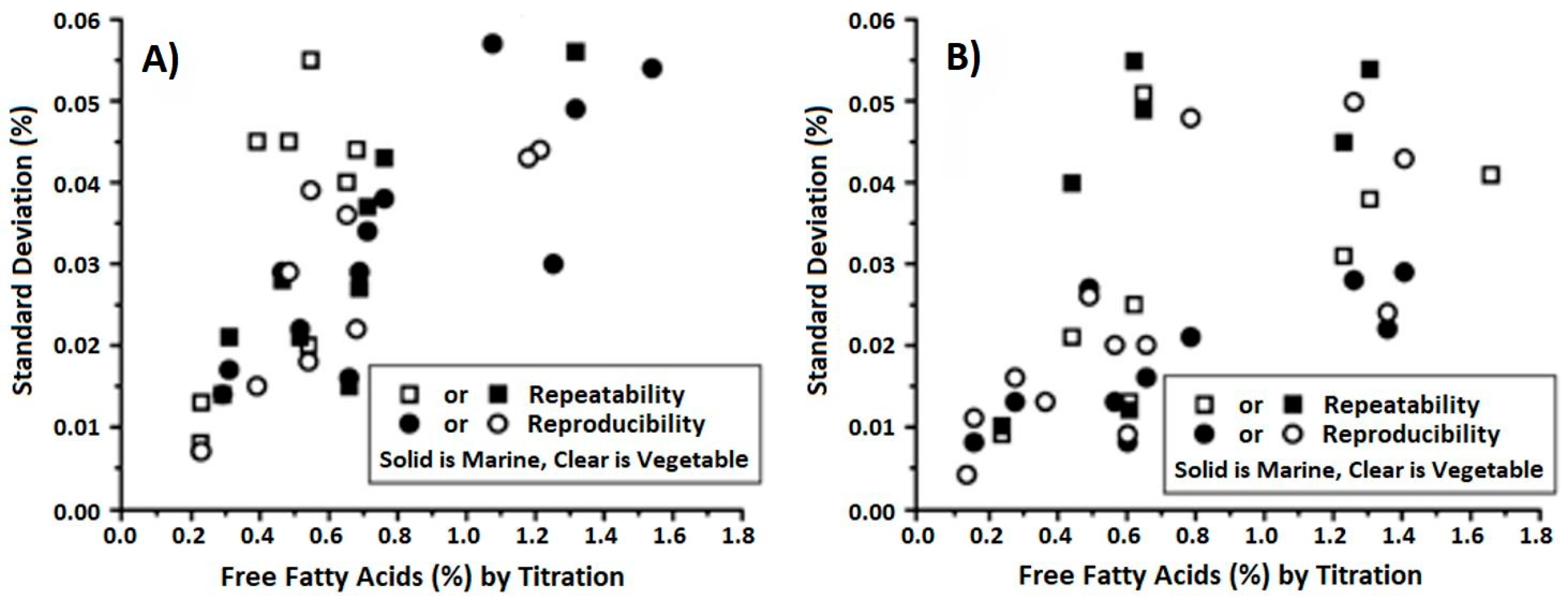
2.2.5. Thermometric Titration Method
3. Alternative Methods for FFA Determination
4. Comparison of Different FFA Analysis Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janampelli, S.; Darbha, S. Selective deoxygenation of fatty acids to fuel-range hydrocarbons over Pt-MOx/ZrO2 (M = Mo and W) catalysts. Catal. Today 2021, 375, 174–180. [Google Scholar] [CrossRef]
- De Paz Carmona, H.; Horáček, J.; Tišler, Z.; Akhmetzyanova, U. Sulfur free supported MoCx and MoNx catalysts for the hydrotreatment of atmospheric gasoil and its blends with rapeseed oil. Fuel 2019, 254, 115582. [Google Scholar] [CrossRef]
- Buffi, M. Initiative Towards sustainable Kerosene for Aviation. In D2.8 Development of UCO Specifications; FP7–308807; ITAKA, 30/04//2016; European Commission: Brussels Belgium, 2016. [Google Scholar]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Madsen, A.T. Catalytic Production of Biodiesel. Centre for Catalysis and Sustainable Chemistry. Ph.D. Thesis, Technical University of Denmark, Department of Chemistry, Kongens Lyngby, Denmark, 2011. [Google Scholar]
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.R.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ. Sci. Technol. 2022, 56, 7512–7521. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; Yu, X.; Chen, X.; Wang, Y. A novel method for determining peroxide value of edible oils using electrical conductivity. Food Control 2014, 39, 198–203. [Google Scholar] [CrossRef]
- Siudem, P.; Zielińska, A.; Paradowska, K. Application of 1H NMR in the study of fatty acids composition of vegetable oils. J. Pharm. Biomed. Anal. 2022, 212, 114658. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, A.; Kumar, N. Hydroprocessed vegetable oil as a fuel for transportation sector: A review. J. Energy Inst. 2019, 92, 1–17. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Porsin, A.A.; Aleksandrov, P.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Co-processing of rapeseed oil–straight run gas oil mixture: Comparative study of sulfide CoMo/Al2O3-SAPO-11 and NiMo/Al2O3-SAPO-11 catalysts. Catal. Today 2021, 378, 119–125. [Google Scholar] [CrossRef]
- Phan, D.-P.; Lee, E.Y. Catalytic Hydroisomerization Upgrading of Vegetable Oil-Based Insulating Oil. Catalysts 2018, 8, 131. [Google Scholar] [CrossRef]
- Andari, F.; Kittel, J.; Fernandes, J.; Godin, N.; Ter-Ovanessian, B.; Ropital, F. High temperature corrosion in various grades of vegetable and waste oils used for bio-fuel production. Corros. Sci. 2022, 206, 110501. [Google Scholar] [CrossRef]
- Palomar, M.; Garcés-Narro, C.; Piquer, O.; Sala, R.; Tres, A.; García-Bautista, J.A.; Soler, M.D. Influence of free fatty acid content and degree of fat saturation on production performance, nutrient digestibility, and intestinal morphology of laying hens. Anim. Nutr. 2023, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Kara, H.; Sherazi, S.T.H. A Novel Approach for Determination of Free Fatty Acids in Vegetable Oils by a Flow Injection System with Manual Injection. Lipids 2011, 46, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Vicentini-Polette, C.; Rodolfo Ramos, P.; Bernardo Gonçalves, C.; Lopes De Oliveira, A. Determination of free fatty acids in crude vegetable oil samples obtained by high-pressure processes. Food Chem. X 2021, 12, 100166. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, E.P.; Georgiou, D.; Romaidi, M.; Exarhopoulos, S.; Petridis, D.; Karastogiannidou, C.; Dimitreli, G.; Karakosta, P. Rapid Methods for Frying Oil Quality Determination: Evaluation with Respect to Legislation Criteria. J. Am. Oil Chem. Soc. 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Wang, H.; Mujumdar, A.S. Monitoring of free fatty acid content in mixed frying oils by means of LF-NMR and NIR combined with BP-ANN. Food Control 2022, 133, 108599. [Google Scholar] [CrossRef]
- Tsoutsos, T.D.; Tournaki, S.; Paraíba, O.; Kaminaris, S.D. The Used Cooking Oil-to-biodiesel chain in Europe assessment of best practices and environmental performance. Renew. Sustain. Energy Rev. 2016, 54, 74–83. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Mei, T.; Xu, M.; Jia, C.; Duan, C.; Dai, H.; Liu, X.; Pi, F. Spectroscopic studies on thermal degradation and quantitative prediction on acid value of edible oil during frying by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122477. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Liu, W.; Zhang, F.; Li, J.; Yang, H.; Bi, Y. Effects of free fatty acids and peroxide on thermal loss of sesamol and formation of its transformation products in soybean oil. LWT 2022, 159, 113236. [Google Scholar] [CrossRef]
- Baldo, M.A.; Oliveri, P.; Simonetti, R.; Daniele, S. A novel electroanalytical approach based on the use of a room temperature ionic liquid for the determination of olive oil acidity. Talanta 2016, 161, 881–887. [Google Scholar] [CrossRef]
- Baldo, M.A.; Oliveri, P.; Fabris, S.; Malegori, C.; Daniele, S. Fast determination of extra-virgin olive oil acidity by voltammetry and Partial Least Squares regression. Anal. Chim. Acta 2019, 1056, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Damiani, T.; Cavanna, D.; Serani, A.; Dall’Asta, C.; Suman, M. GC-IMS and FGC-Enose fingerprint as screening tools for revealing extra virgin olive oil blending with soft-refined olive oils: A feasibility study. Microchem. J. 2020, 159, 105374. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. (Eds.) Chapter 1—Preparatory Information. In Journal of Chromatography Library; Elsevier: Amsterdam, The Netherlands, 2002; pp. 3–111. [Google Scholar]
- Barthet, V.J.; Gordon, V.; Daun, J.K. Evaluation of a colorimetric method for measuring the content of FFA in marine and vegetable oils. Food Chem. 2008, 111, 1064–1068. [Google Scholar] [CrossRef]
- Endo, Y. Analytical Methods to Evaluate the Quality of Edible Fats and Oils: The JOCS Standard Methods for Analysis of Fats, Oils and Related Materials (2013) and Advanced Methods. J. Oleo Sci. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Collin, T.; Cunningham, R.; Deb, M.; Villa, R.; MacAdam, J.; Jefferson, B. Energy potential of household fats, oils and grease waste. Water Environ. J. 2022, 36, 132–141. [Google Scholar] [CrossRef]
- Hu, J.Q.; Yang, S.Z.; Zhang, J.J.; Guo, L.; Xin, Y.L. The Determination of Lower Acidity in Several Coloured Oils by Catalyzed Thermometric Titration. Pet. Chem. 2017, 57, 1099–1104. [Google Scholar] [CrossRef]
- Alessio, K.O.; Tischer, B.; Voss, M.; Teixeira, I.D.; Brendler, B.M.; Duarte, F.A.; Helfer, G.A.; Costa, A.B.; Barin, J.S. Open source, low-cost device for thermometric titration with non-contact temperature measurement. Talanta 2020, 216, 120975. [Google Scholar] [CrossRef]
- Micek-Ilnicka, A. Characterization of Acid Catalysts by Thermometric Titration. Catal. Lett. 2002, 81, 233–236. [Google Scholar] [CrossRef]
- Hu, J.Q.; Zhang, J.J.; Yang, S.Z.; Xin, Y.L.; Guo, L.; Yao, T. Thermometric Titration for Rapid Determination of Trace Water in Jet Fuel. J. Spectrosc. 2017, 2017, 8429525. [Google Scholar] [CrossRef]
- Kwon, C.W.; Park, K.-M.; Choi, S.J.; Chang, P.-S. A reliable and reproducible method for the lipase assay in an AOT/isooctane reversed micellar system: Modification of the copper-soap colorimetric method. Food Chem. 2015, 182, 236–241. [Google Scholar] [CrossRef]
- Burguera, J.L.; Burguera, M. Pretreatment of oily samples for analysis by flow injection-spectrometric methods. Talanta 2011, 83, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Suteerapataranon, S.; Jakmunee, J.; Vaneesorn, Y.; Grudpan, K. Exploiting flow injection and sequential injection anodic stripping voltammetric systems for simultaneous determination of some metals. Talanta 2002, 58, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, C.; Du, S.; Gao, J.-m. A New Method for Determining Free Fatty Acid Content in Edible Oils by Using Electrical Conductivity. Food Anal. Methods 2012, 5, 1453–1458. [Google Scholar] [CrossRef]
- Kuselman, I.; Tur’yan, Y.I.; Bourenko, T.; Goldfeld, I.; Anisimov, B. pH-Metric determination of acid values in oilseeds without titration. Talanta 1999, 49, 629–637. [Google Scholar] [CrossRef] [PubMed]
- El-abassy, R.; Donfack, P.; Materny, A. Rapid Determination of Free Fatty Acid in Extra Virgin Olive Oil by Raman Spectroscopy and Multivariate Analysis. J. Am. Oil Chem. Soc. 2009, 86, 507–511. [Google Scholar] [CrossRef]
- Scott, S.L. Spectroscopy in Catalysis: An Introduction, Third, Completely Revised and Enlarged Edition By J. W. Niemantsverdriet (Eindhoven University of Technology, The Netherlands). Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim. 2007, xviii + 326 pp. $135. ISBN 978-3-527-31651-9. J. Am. Chem. Soc. 2008, 130, 383. [Google Scholar]
- Ahmad, M.H.; Shahbaz, Z.; Imran, M.; Khan, M.K.; Muhammad, N.; Iqbal, S.; Ahmed, W.; Ahmad, T. Monitoring of frying process in canola oil blend using fourier transform infrared and chemometrics techniques. Food Sci. Nutr. 2021, 9, 6089–6098. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.A.; van de Voort, F.R.; Simpson, B.K. FTIR determination of free fatty acids in fish oils intended for biodiesel production. Process Biochem. 2009, 44, 401–405. [Google Scholar] [CrossRef]
- Martinez, I.; Aursand, M.; Erikson, U.; Singstad, T.E.; Veliyulin, E.; van der Zwaag, C. Destructive and non-destructive analytical techniques for authentication and composition analyses of foodstuffs. Trends Food Sci. Technol. 2003, 14, 489–498. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Machado, N.; Carvalho, T.; de Almeida, J.M.M.M.; Barros, A.I.R.N.A. Short wavelength Raman spectroscopy applied to the discrimination and characterization of three cultivars of extra virgin olive oils in different maturation stages. Talanta 2015, 132, 829–835. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B. Application of ESI and MALDI-TOF MS for triacylglycerols analysis in edible oils. Eur. J. Lipid Sci. Technol. 2011, 113, 160–167. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Derivatisation-free characterisation and supercritical conversion of free fatty acids into biodiesel from high acid value waste cooking oil. Renew. Energy 2019, 143, 77–90. [Google Scholar] [CrossRef]
- Cert, A.; Moreda, W.; Pérez-Camino, M.C. Chromatographic analysis of minor constituents in vegetable oils. J. Chromatogr. A 2000, 881, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Zambrano, J.M.; Michels, D.; Verkempinck, S.H.E.; Infantes-Garcia, M.R.; Hendrickx, M.E.; Van Loey, A.M.; Grauwet, T. HPLC-CAD method to quantify lipolysis products from plant-based oils rich in unsaturated fatty acids. J. Food Compos. Anal. 2023, 121, 105400. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Pico, Y. 2—Gas chromatography and mass spectroscopy techniques for the detection of chemical contaminants and residues in foods. In Chemical Contaminants and Residues in Food; Schrenk, D., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 17–61. [Google Scholar]
- Li, S.-G.; Zhang, H.; Xue, W.-T. A novel method for the determination of acid value of vegetable oils. Eur. J. Lipid Sci. Technol. 2007, 109, 1088–1094. [Google Scholar] [CrossRef]
- Lam, H.S.; Proctor, A. Rapid Methods for Milled Rice Surface Total Lipid and Free Fatty Acid Determination. Cereal Chem. 2001, 78, 498–499. [Google Scholar] [CrossRef]
- Anconi, A.C.S.A.; de Jesus Fonseca, J.L.; Nunes, C.A. A digital image-based colorimetric method for measuring free acidity in edible vegetable oils. Food Chem. 2024, 443, 138555. [Google Scholar]
- Baker, D. A colorimetric method for determining free fatty acids in vegetable oils. J. Am. Oil Chem. Soc. 1964, 41, 21–22. [Google Scholar] [CrossRef]
- Russo, S.; Brambilla, L.; Thomas, J.B.; Joseph, E. But aren’t all soaps metal soaps? A review of applications, physico-chemical properties of metal soaps and their occurrence in cultural heritage studies. Herit. Sci. 2023, 11, 172. [Google Scholar] [CrossRef]
- Saisuburamaniyan, N.; Krithika, L.; Dileena, K.P.; Sivasubramanian, S.; Puvanakrishnan, R. Lipase assay in soils by copper soap colorimetry. Anal. Biochem. 2004, 330, 70–73. [Google Scholar] [CrossRef]
- Mehrotra, K.N.; Mehta, V.P.; Nagar, T.N. Studies on Colorimetry, Solubility and Thermodynamic Properties of Copper Soap Solutions. J. Prakt. Chem. 1971, 313, 607–613. [Google Scholar] [CrossRef]
- Bhutra, R.; Sharma, R.; Sharma, A.K.; Biotechnol, S.A.J. Fungicidal Activities of Cu (II) Soaps Derived from Various Oils Treated at High Temperature for Biomedical Use. SAJ Biotechnol. 2017, 5, 1–6. [Google Scholar]
- Mahesar, S.A.; Sherazi, S.T.H.; Khaskheli, A.R.; Kandhro, A.A.; uddin, S. Analytical approaches for the assessment of free fatty acids in oils and fats. Anal. Methods 2014, 6, 4956–4963. [Google Scholar] [CrossRef]
- Makahleh, A.; Saad, B. Flow injection determination of free fatty acids in vegetable oils using capacitively coupled contactless conductivity detection. Anal. Chim. Acta 2011, 694, 90–94. [Google Scholar] [CrossRef]
- Saad, B.; Ling, C.W.; Jab, M.S.; Lim, B.P.; Mohamad Ali, A.S.; Wai, W.T.; Saleh, M.I. Determination of free fatty acids in palm oil samples using non-aqueous flow injection titrimetric method. Food Chem. 2007, 102, 1407–1414. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Kara, H. A Highly Efficient Automated Flow Injection Method for Rapid Determination of Free Fatty Acid Content in Corn Oils. J. Am. Oil Chem. Soc. 2014, 91, 549–558. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E. Flow injection analysis: From beaker to microfluidics. Anal. Chem. 2000, 72, 212A–217A. [Google Scholar] [CrossRef][Green Version]
- Parisi, S.; Escarpa, A.; González, M.C.; López, M.Á. Agricultural and food electroanalysis. Anal. Bioanal. Chem. 2016, 408. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kotani, A.; Kusu, F.; Takamura, K. New electrochemical detection method in high-performance liquid chromatography for determining free fatty acids. Anal. Chim. Acta 2002, 465, 199–206. [Google Scholar] [CrossRef]
- Ghorbani, M.A.; Deo, R.C.; Kashani, M.H.; Shahabi, M.; Ghorbani, S. Artificial intelligence-based fast and efficient hybrid approach for spatial modelling of soil electrical conductivity. Soil Tillage Res. 2019, 186, 152–164. [Google Scholar] [CrossRef]
- Tur’yan, Y.I.; Kardash, E.; Garibyan, I. pH-Metric Determination of Free Fatty Acids in Oils and Fats During Frying in the Absence of a Chemical Laboratory. J. Am. Oil Chem. Soc. 2008, 85, 91–92. [Google Scholar] [CrossRef]
- Gerasimenko, E.O.; Tur’yan, Y.I. Automated flow pH-method for the determination of total free fatty acids content in edible oils. Food Chem. 2012, 132, 1562–1565. [Google Scholar] [CrossRef]
- Meng, H.; Li, S.; Xiao, L.; Li, C. Functionalized assembly of solid membranes for chiral separation using polyelectrolytes and chiral ionic liquid. AIChE J. 2013, 59, 4772–4779. [Google Scholar] [CrossRef]
- Du, R.; Lai, K.; Xiao, Z.; Shen, Y.; Wang, X.; Huang, Y. Evaluation of the Quality of Deep Frying Oils with Fourier Transform Near-infrared and Mid-infrared Spectroscop. J. Food Sci. 2012, 77, C261–C266. [Google Scholar] [CrossRef]
- Al-Alawi, A.; van de Voort, F.R.; Sedman, J. New FTIR method for the determination of FFA in oils. J. Am. Oil Chem. Soc. 2004, 81, 441–446. [Google Scholar] [CrossRef]
- van Wyngaard, E.; Blancquaert, E.; Nieuwoudt, H.; Aleixandre-Tudo, J.L. Infrared Spectroscopy and Chemometric Applications for the Qualitative and Quantitative Investigation of Grapevine Organs. Front. Plant Sci. 2021, 12, 723247. [Google Scholar] [CrossRef]
- Adewale, P.; Mba, O.; Dumont, M.-J.; Ngadi, M.; Cocciardi, R. Determination of the iodine value and the free fatty acid content of waste animal fat blends using FT-NIR. Vib. Spectrosc. 2014, 72, 72–78. [Google Scholar] [CrossRef]
- Gertz, C.; Behmer, D. Application of FT-NIR spectroscopy in assessment of used frying fats and oils. Eur. J. Lipid Sci. Technol. 2014, 116, 756–762. [Google Scholar] [CrossRef]
- Kaufmann, K.C.; Favero, F.; Vasconcelos, M.; Godoy, H.; Sampaio, K.; Barbin, D. Portable NIR Spectrometer for Prediction of Palm Oil Acidity. J. Food Sci. 2019, 84, 406–411. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Mele, M.; Secchiari, P.; Bittante, G. Effectiveness of mid-infrared spectroscopy to predict fatty acid composition of Brown Swiss bovine milk. Animal 2011, 5, 1653–1658. [Google Scholar] [CrossRef]
- Tarhan, I.; Ismail, A.; Kara, H. Quantitative determination of free fatty acids in extra virgin olive oils by multivariate methods and Fourier transform infrared spectroscopy considering different absorption modes. Int. J. Food Prop. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Nascimento, T.A.D.; Lopes, T.I.B.; Nazario, C.E.D.; Oliveira, S.L.; Alcantara, G.B. Vegetable oils: Are they true? A point of view from ATR-FTIR, 1H NMR, and regiospecific analysis by 13C NMR. Food Res. Int. 2021, 144, 110362. [Google Scholar] [CrossRef]
- Touffet, M.; Patsioura, A.; Ziaiifar, A.M.; Eveleigh, L.; Vitrac, O. Online reconstruction of oil oxidation kinetics and reaction schemes during deep-frying by deconvolution of ATR-FTIR spectra. J. Food Eng. 2018, 224, 1–16. [Google Scholar] [CrossRef]
- Rozali, N.L.; Azizan, K.A.; Singh, R.; Syed Jaafar, S.N.; Othman, A.; Weckwerth, W.; Ramli, U.S. Fourier transform infrared (FTIR) spectroscopy approach combined with discriminant analysis and prediction model for crude palm oil authentication of different geographical and temporal origins. Food Control 2023, 146, 109509. [Google Scholar] [CrossRef]
- Triyasmono, L.; Schollmayer, C.; Holzgrabe, U. Chemometric analysis applied to 1H NMR and FTIR data for a quality parameter distinction of red fruit (Pandanus conoideus, lam.) oil products. Phytochem. Anal. 2022, 34, 788–799. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and Applications in Nanoscale Infrared Spectroscopy and Chemical Imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Mulla, M.; Ahmed, J.; Alagarsamy, S.; Farvin, S. Utilization of novel and rapid techniques for characterization of neem Azadirachta indica seed oil and palm oil blends. Int. J. Food Eng. 2020, 16, 20200047. [Google Scholar] [CrossRef]
- Valasi, L.; Kokotou, M.G.; Pappas, C.S. GC-MS, FTIR and Raman spectroscopic analysis of fatty acids of Pistacia vera (Greek variety “Aegina”) oils from two consecutive harvest periods and chemometric differentiation of oils quality. Food Res. Int. 2021, 148, 110590. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- García Martín, J.F.; López Barrera, M.D.; Torres García, M.; Zhang, Q.-A.; Álvarez Mateos, P. Determination of the Acidity of Waste Cooking Oils by Near Infrared Spectroscopy. Processes 2019, 7, 304. [Google Scholar] [CrossRef]
- Mishchik, K. Ultrafast Laser-Induced Modification of Optical Glasses: A Spectroscopy Insight into the Microscopic Mechanisms. Ph.D. Thesis, Université Jean Monnet-Saint-Etienne, Saint-Étienne, France, 2012. [Google Scholar]
- Colarusso, P.; Kidder, L.H.; Levin, I.W.; Lewis, E.N. Raman and Infrared Microspectroscopy. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 844–852. [Google Scholar]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. TrAC Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.-Y.; Yang, I.-S.; Chen, X.-B. Raman Spectroscopy Analysis of Free Fatty Acid in Olive Oil. Appl. Sci. 2019, 9, 4510. [Google Scholar] [CrossRef]
- Gu, H.; Huang, X.; Sun, Y.; Chen, Q.; Wei, Z.; Lv, R. Intelligent evaluation of total polar compounds (TPC) content of frying oil based on fluorescence spectroscopy and low-field NMR. Food Chem. 2021, 342, 128242. [Google Scholar] [CrossRef]
- Lankhorst, P.P.; Chang, A.-N. The Application of NMR in Compositional and Quantitative Analysis of Oils and Lipids. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1743–1764. [Google Scholar]
- Bao, R.; Tang, F.; Rich, C.; Hatzakis, E. A comparative evaluation of low-field and high-field NMR untargeted analysis: Authentication of virgin coconut oil adulterated with refined coconut oil as a case study. Anal. Chim. Acta 2023, 1273, 341537. [Google Scholar] [CrossRef]
- Monakhova, Y.; Hafer, E.; Diehl, B. Quality Control of Krill Oil by Nuclear Magnetic Resonance (NMR) Spectroscopy: Composition and Detection of Foreign Species. Anal. Lett. 2018, 51, 2549–2560. [Google Scholar] [CrossRef]
- Zailer, E.; Holzgrabe, U.; Diehl, B.W.K. Interlaboratory Comparison Test as an Evaluation of Applicability of an Alternative Edible Oil Analysis by 1H NMR Spectroscopy. J. AOAC Int. 2017, 100, 1819–1830. [Google Scholar] [CrossRef]
- Triyasmono, L.; Holzgrabe, U. Combination quantitative 1H NMR and chemometric approaches for the assessment of quality control in commercially available products of red fruit (Pandanus conoidues, Lam.) oil. J. Pharm. Biomed. Anal. Open 2023, 1, 100010. [Google Scholar] [CrossRef]
- Hafer, E.; Holzgrabe, U.; Wiedemann, S.; Adams, K.M.; Diehl, B. NMR Spectroscopy: Determination of Peroxide Value in Vegetable and Krill Oil by Using Triphenylphosphine as Tagging Reagent. Eur. J. Lipid Sci. Technol. 2020, 122, 1900442. [Google Scholar] [CrossRef]
- Schripsema, J. Comprehensive Analysis of Polar and Apolar Constituents of Butter and Margarine by Nuclear Magnetic Resonance, Reflecting Quality and Production Processes. J. Agric. Food Chem. 2008, 56, 2547–2552. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Monakhova, Y.; Diehl, B. Fingerprinting Krill Oil by 31P, 1H and 13C NMR Spectroscopies. J. Am. Oil Chem. Soc. 2016, 93, 1037–1049. [Google Scholar] [CrossRef]
- Spyros, A.; Dais, P. 31P NMR spectroscopy in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 54, 195–207. [Google Scholar] [CrossRef]
- Monakhova, Y.; Diehl, B. Quantitative Analysis of Sunflower Lecithin Adulteration with Soy Species by NMR Spectroscopy and PLS Regression. J. Am. Oil Chem. Soc. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Comparison and validation of 2 analytical methods for the determination of free fatty acids in dairy products by gas chromatography with flame ionization detection. J. Dairy Sci. 2016, 99, 5047–5063. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Wu, H. Recent progress in the analysis of unsaturated fatty acids in biological samples by chemical derivatization-based chromatography-mass spectrometry methods. J. Chromatogr. B 2023, 1215, 123572. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Poulsen, M.E. Clean-up of cereal extracts for gas chromatography–tandem quadrupole mass spectrometry pesticide residues analysis using primary secondary amine and C18. J. Chromatogr. A 2015, 1423, 47–53. [Google Scholar] [CrossRef]
- Beneito-Cambra, M.; Moreno-González, D.; García-Reyes, J.F.; Bouza, M.; Gilbert-López, B.; Molina-Díaz, A. Direct analysis of olive oil and other vegetable oils by mass spectrometry: A review. TrAC Trends Anal. Chem. 2020, 132, 116046. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Caprioli, G.; Fiorini, D.; Torregiani, E.; Vittori, S.; Sagratini, G. HS-SPME-GC-MS technique for FFA and hexanal analysis in different cheese packaging in the course of long term storage. Food Res. Int. 2019, 121, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kangani, C.O.; Kelley, D.E.; DeLany, J.P. New method for GC/FID and GC–C-IRMS analysis of plasma free fatty acid concentration and isotopic enrichment. J. Chromatogr. B 2008, 873, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kalo, P.J.; Ollilainen, V.; Rocha, J.M.; Malcata, F.X. Identification of molecular species of simple lipids by normal phase liquid chromatography–positive electrospray tandem mass spectrometry, and application of developed methods in comprehensive analysis of low erucic acid rapeseed oil lipids. Int. J. Mass Spectrom. 2006, 254, 106–121. [Google Scholar] [CrossRef]
- Zhao, G.-H.; Hu, Y.-Y.; Zeng, X.; Zhang, M.; Zhou, Z.; Qin, L.; Yin, F.-W.; Zhou, D.-Y.; Shahidi, F. A direct and facile simultaneous quantification of non-polar and polar lipids in different species of marine samples using normal-phase HPLC–CAD. J. Food Compos. Anal. 2022, 114, 104813. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.E.; Lee, J.C.; Maharjan, R.; Oh, H.; Lee, K.; Kim, N.A.; Jeong, S.H. Optimization of HPLCCAD method for simultaneous analysis of different lipids in lipid nanoparticles with analytical QbD. J. Chromatogr. A 2023, 1709, 464375. [Google Scholar] [CrossRef]
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Zhao, X.; Shi, J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011, 125, 1365–1372. [Google Scholar] [CrossRef]
- Zhao, X.E.; Wang, H.; Ding, C.; Suo, Y.; Sun, J.; Chen, G.; Sun, X.; You, J. Determination of Free Fatty Acids from Soil and Bryophyte by HPLC with Fluorescence Detection and Identification with Mass Spectrometry. Chin. J. Anal. Chem. 2006, 34, 150–155. [Google Scholar] [CrossRef]
- Farid, N.F.; Abdelwahab, N.S. A new HPLC methodology for the analysis of metronidazole and dexibuprofen: Application to pharmacokinetic study and comparative greenness assessment. Microchem. J. 2022, 183, 108048. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.; Choi, H.; Lee, J. Development of methods for determining free fatty acid contents in red colored oils. Food Sci. Biotechnol. 2021, 30, 1435–1443. [Google Scholar] [CrossRef]
- Obisesan, K.A.; Jiménez-Carvelo, A.M.; Cuadros-Rodriguez, L.; Ruisánchez, I.; Callao, M.P. HPLC-UV and HPLC-CAD chromatographic data fusion for the authentication of the geographical origin of palm oil. Talanta 2017, 170, 413–418. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Chen, J.; Zhang, J.; Chen, L.; Wang, B.; Wu, C.; Yuan, Y. Identification and direct determination of fatty acids profile in oleic acid by HPLC-CAD and MS-IT-TOF. J. Pharm. Biomed. Anal. 2021, 204, 114238. [Google Scholar] [CrossRef] [PubMed]
- Aksoylu, Ö.; Günç Ergönül, P. Determination of Physicochemical Properties, Fatty Acid, Tocopherol, Sterol, and Phenolic Profiles of Expeller–Pressed Poppy Seed Oils from Turkey. J. Am. Oil Chem. Soc. 2020, 97, 591–602. [Google Scholar] [CrossRef]
- Lyashenko, S.; Yunusova, S.; López-Ruiz, R.; Vasfilova, E.; Kiseleva, O.; Chimitov, D.; Bahanova, M.; Bojko, N.; Guil-Guerrero, J.L. Lipid Fractions, Fatty Acid Profiles, and Bioactive Compounds of Lithospermum officinale L. Seeds. J. Am. Oil Chem. Soc. 2021, 98, 425–437. [Google Scholar] [CrossRef]
- Yuenyong, J.; Pokkanta, P.; Phuangsaijai, N.; Kittiwachana, S.; Mahatheeranont, S.; Sookwong, P. GC-MS and HPLC-DAD analysis of fatty acid profile and functional phytochemicals in fifty cold-pressed plant oils in Thailand. Heliyon 2021, 7, e06304. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, E.; Dunshea, F.R.; Muralitharan, M.; Cross, R.F. Comparison of silver-ion high-performance liquid chromatographic quantification of free and methylated conjugated linoleic acids. Lipids 2000, 35, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, M.; Kowalczyk, J.; Wa̧sowska, I.; Niedźwiedzka, K.M. Determination of conjugated linoleic acid isomers by liquid chromatography and photodiode array detection. J. Anim. Feed. Sci. 2003, 12, 369–382. [Google Scholar] [CrossRef]
- Luna, P.; Juárez, M.; de la Fuente, M.A. Gas chromatography and silver-ion high-performance liquid chromatography analysis of conjugated linoleic acid isomers in free fatty acid form using sulphuric acid in methanol as catalyst. J. Chromatogr. A 2008, 1204, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Yurawecz, M.P.; Morehouse, K.M. Silver-ion HPLC of conjugated linoleic acid isomers. Eur. J. Lipid Sci. Technol. 2001, 103, 609–613. [Google Scholar] [CrossRef]
- Białek, M.; Czauderna, M.; Białek, A. Conjugated linolenic acid (CLnA) isomers as new bioactive lipid compounds in ruminant-derived food products. A review. J. Anim. Feed. Sci. 2017, 26, 3–17. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Korniluk, K.; Wa̧sowska, I. Improved saponification then mild base and acid-catalyzed methylation is a useful method for quantifying fatty acids, with special emphasis on conjugated dienes. Acta Chromatogr. 2007, 18, 59–71. [Google Scholar]
- Salimon, J.; Abdullah, B.M.; Salih, N. Hydrolysis optimization and characterization study of preparing fatty acids from Jatropha curcasseed oil. Chem. Cent. J. 2011, 5, 67. [Google Scholar] [CrossRef]
- Dong, Z.; Jin, J.; Wei, W.; Wang, X.; Wu, G.; Wang, X.; Jin, Q. Fabrication of immobilized lipases from Candida rugosa on hierarchical mesoporous silica for enzymatic enrichment of ω-3 polyunsaturated fatty acids by selective hydrolysis. Food Chem. X 2024, 22, 101434. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Marounek, M.; Mlchalski, J.P.; Rozbicka-Wieczorek, A.J.; Krajewska, K.A. A new internal standard for HPLC assay of conjugated linoleic acid in animal tissues and milk. Czech J. Anim. Sci. 2011, 56, 23–29. [Google Scholar] [CrossRef]
- Smith, T.K. Analysis of FFA in edible oils by catalyzed end-point thermometric titrimetry (CETT). J. Am. Oil Chem. Soc. 2003, 80, 21–24. [Google Scholar] [CrossRef]
- Metrohm. Determination of Free Fatty Acid (FFA) in Edible Oils with 859 Titrotherm; Application Bulletin; 315 e; Metrohm: Herisau, Switzerland, 2014. [Google Scholar]
- Tahmasbian, I.; Wallace, H.M.; Gama, T.; Hosseini Bai, S. An automated non-destructive prediction of peroxide value and free fatty acid level in mixed nut samples. LWT 2021, 143, 110893. [Google Scholar] [CrossRef]
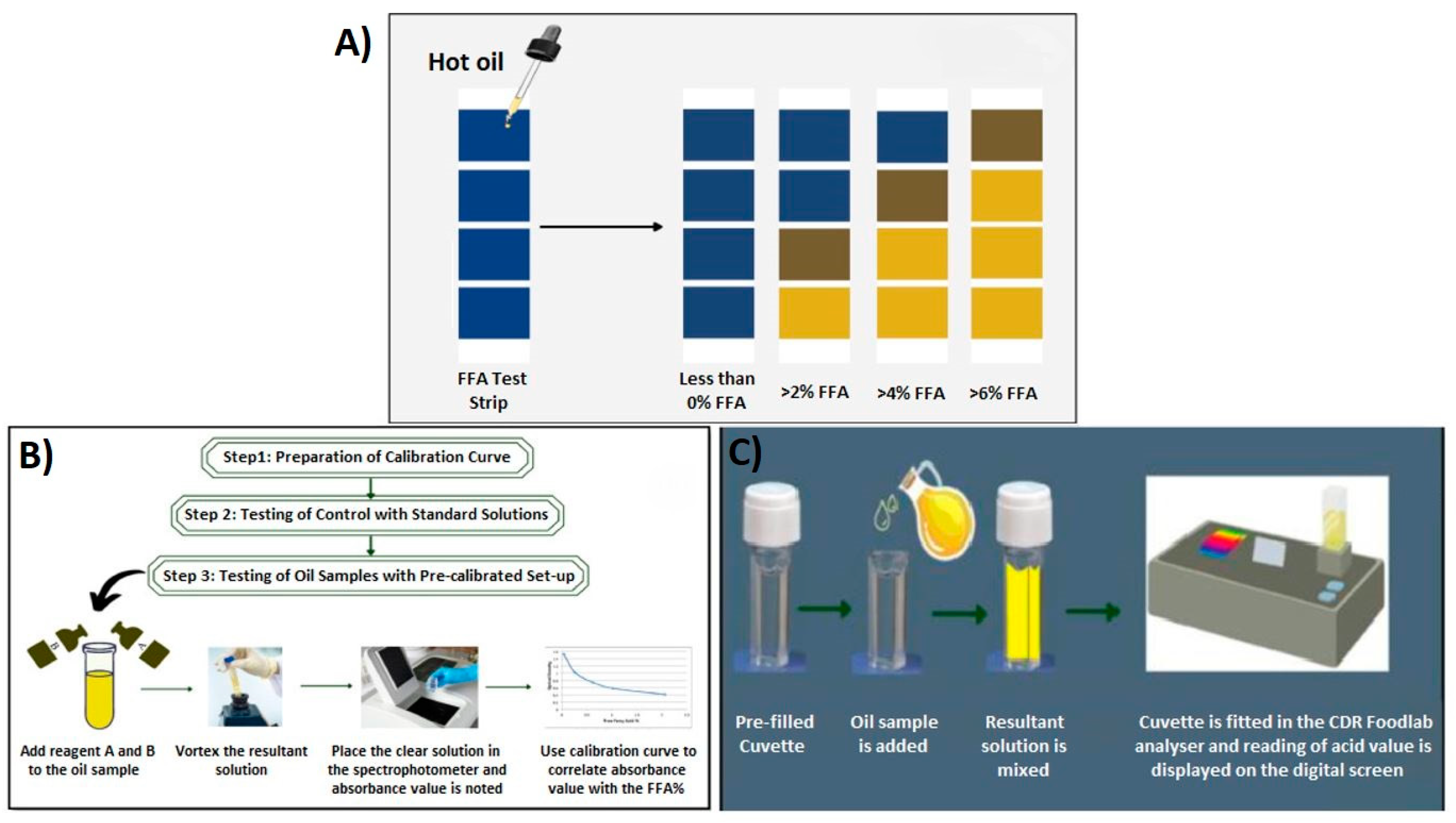
- Fatima, S.; Kumar, V.; Bhadauria, G.; Verma, H. Quality indicators based rapid test kits for detection of frying oil quality: A review. Food Chem. Adv. 2023, 2, 100305. [Google Scholar] [CrossRef]
- McSavage, J.; Trevisan, S. The use and abuse of frying oil. Food Serv. Technol. 2001, 1, 85–92. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, S.; Feng, L. Rapid and direct determination of fatty acids and glycerides profiles in Schisandra chinensis oil by using UPLC-Q/TOF-MSE. J. Chromatogr. B 2019, 1104, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Uncu, O.; Ozen, B. A comparative study of mid-infrared, UV–Visible and fluorescence spectroscopy in combination with chemometrics for the detection of adulteration of fresh olive oils with old olive oils. Food Control 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Abamba Omwange, K.; Al Riza, D.F.; Saito, Y.; Suzuki, T.; Ogawa, Y.; Shiraga, K.; Giametta, F.; Kondo, N. Potential of front face fluorescence spectroscopy and fluorescence imaging in discriminating adulterated extra-virgin olive oil with virgin olive oil. Food Control 2021, 124, 107906. [Google Scholar] [CrossRef]
- Scano, P.; Cagliani, L.R.; Consonni, R. 1H NMR characterisation of the lipid fraction and the metabolite profiles of Fossa (pit) cheese. Int. Dairy J. 2019, 90, 39–44. [Google Scholar] [CrossRef]
- Martín, I.; Vivar-Quintana, A.; Revilla, I.; Salvador-Esteban, J. The determination of fatty acids in cheeses of variable composition (cow, ewe’s, and goat) by means of near infrared spectroscopy. Microchem. J. 2020, 156, 104854. [Google Scholar] [CrossRef]
- Siciliano, C.; Belsito, E.; De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A. Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy. Food Chem. 2013, 136, 546–554. [Google Scholar] [CrossRef] [PubMed]






| LF-NMR | Infrared |
|---|---|
| Easy to apply. | Trained personnel needed to apply. |
| Non-destructive method and sample preparation is not necessary. | Non-destructive method and sample preparation is required. |
| NMR method is surface-independent. | Since the surface of the samples is utilized for analysis, it is a surface-dependent method. |
| Color-independent. | Color of the sample can affect the IR spectra, so different calibration curves are necessary regarding sample color and size. |
| NMR calibration curves require 3–5 reference samples. | Minimum of 10 reference samples are necessary for creating a calibration curve. |
| For calibration, a simple linear regression fit can be easily applied. | For calibration, different methodologies can be applied, such as derivatization methods, the partial least squares (PLS) regression method, etc. |
| High reproducibility of analysis. | Reproducibility of analysis is good. |
| The quantitative results show that the integral of a signal corresponds to the number of nuclei by utilizing the whole sample. | The surface of the sample is utilized for the analysis. |
| Test Kits | Producers | Limitations | Ref. |
|---|---|---|---|
| 3M TM Oil Quality Test | 3M Commercial Solutions, St. Paul, MN, USA | This test gives an idea about the range of FFA, instead of presenting detailed compound information. | [132] |
| Strips | Divisions, Newport, KY, USA | This test gives an idea about the range of FFA, instead of presenting detailed compound information. | [132] |
| FASafe | MP Biomedicals, Irvine, CA, USA | Laboratory environment, chemical usage, and trained personnel needed. | [17,26] |
| CDR FoodLabR | CDR Mediared Co., Ltd., Firenze, Italy | Validity and reliability of the results have not been proven. | [132] |
| Instrumental Methods | Solvent Requirement | Data Variability | Strengths | Weaknesses | |
|---|---|---|---|---|---|
| Colorimetric Methods | Copper Soap Method | Relatively High | Overall value of FFAs | Simplicity, low cost, reproducibility | Limited determination on dark-colored samples Inadequate determination of FFA |
| FIA Method | Moderated | Sensitivity, accuracy | Stability Problem Inadequate determination of FFA | ||
| Thermometric Titration Method | Moderated | Overall value of FFAs | Simplicity, low cost, reproducibility | Inadequate determination of FFA | |
| Electrochemical Methods | Voltametric Method | Moderated | Overall value of FFAs | Low cost | Overlapping and reproducibility problems Not applicable for viscous sample Inadequate determination of FFA |
| EC Method | Low cost | Accuracy and reproducibility of the method mainly depend on the conditions of the electrodes Inadequate determination of FFA | |||
| pH Metric Method | Low cost, accuracy, repeatability, reproducibility, sensitivity | Inadequate determination of FFA | |||
| Spectroscopic Methods | Infrared Method | Low | In-depth understanding of FFAs | Non-destructive, accuracy, repeatability, reproducibility, sensitivity | Sample preparation is required Calibration could be required in colored samples |
| Raman Method | Overlapping Limitation for detecting low-intensity peaks | ||||
| NMR Method | Expensive Trained person needed | ||||
| Chromatographic Methods | GC | Low | In-depth understanding of FFAs | Feasible, accuracy, repeatability, reproducibility sensitivity | Sample preparation is required Heat application might affect the component composition |
| HPLC | Sample preparation is required Coupling with other methods could be required for sensitivity and accuracy | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uçar, B.; Gholami, Z.; Svobodová, K.; Hradecká, I.; Hönig, V. A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review. Foods 2024, 13, 1891. https://doi.org/10.3390/foods13121891
Uçar B, Gholami Z, Svobodová K, Hradecká I, Hönig V. A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review. Foods. 2024; 13(12):1891. https://doi.org/10.3390/foods13121891
Chicago/Turabian StyleUçar, Beyza, Zahra Gholami, Kateřina Svobodová, Ivana Hradecká, and Vladimír Hönig. 2024. "A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review" Foods 13, no. 12: 1891. https://doi.org/10.3390/foods13121891
APA StyleUçar, B., Gholami, Z., Svobodová, K., Hradecká, I., & Hönig, V. (2024). A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review. Foods, 13(12), 1891. https://doi.org/10.3390/foods13121891






