Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tea Polysaccharides Extraction and Purification
2.3. Determination of Monosaccharide Contents of TPs
2.4. Analysis of TP Conformation Using the GPC-RI-MALS Technique
2.5. Animals
2.6. Induction of Type 2 Diabetes Mellitus
2.7. Experimental Design
2.8. Oral Glucose Tolerance Test
2.9. Blood Sampling and Preparation of Tissue Homogenate
2.10. Serum Biochemical Analysis
2.11. ELISA for Serum Insulin
2.12. Protein Extracted from Analysis of the Liver and Western Blot
2.13. Immunofluorescence Analysis of p-Akt in the Liver
2.14. Statistical Analysis
3. Results
3.1. Components of Hydrolyzed Monosaccharides and Molecular Configuration of TPs
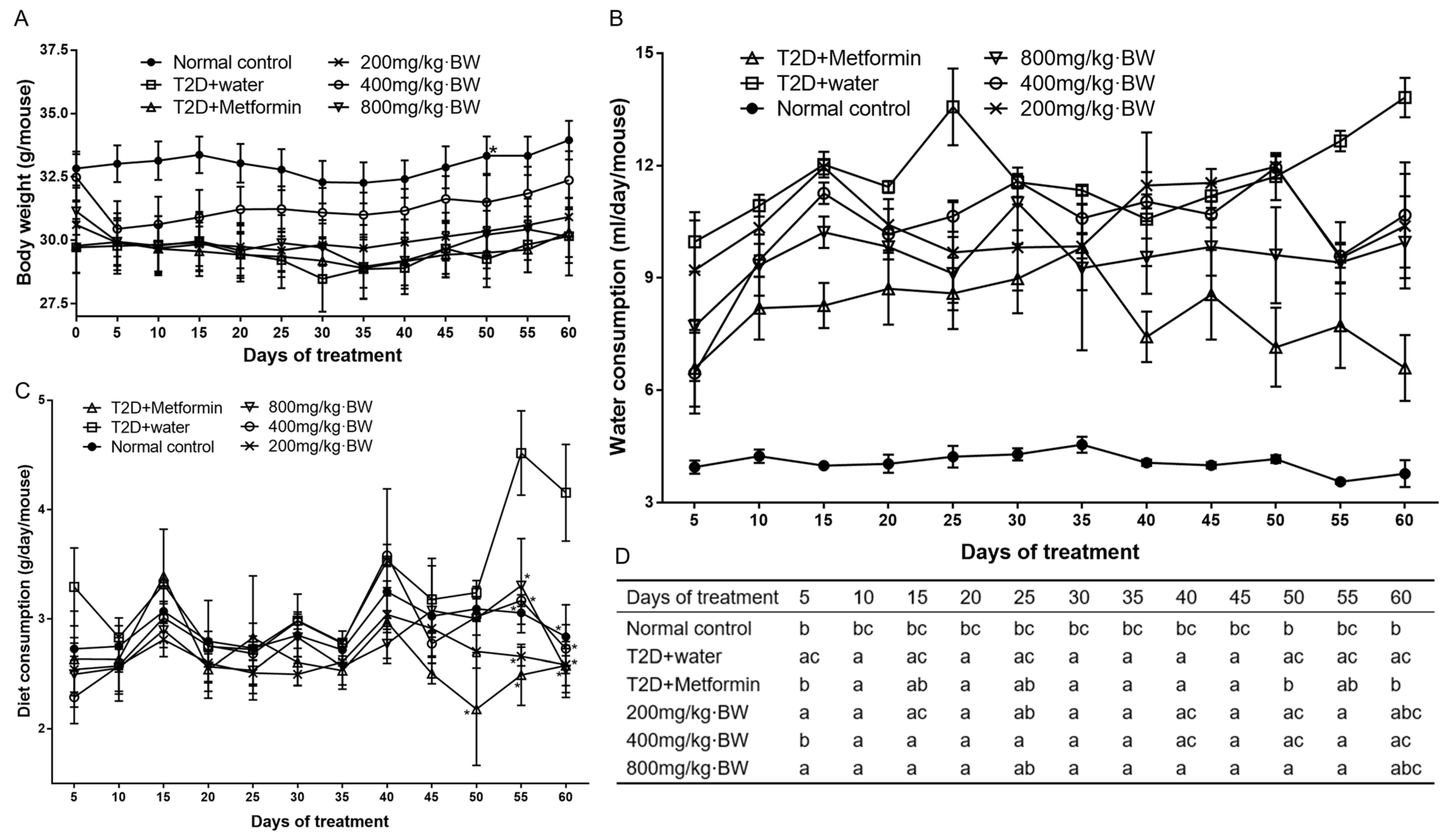
3.2. Effect of TPs on Body Weight, Water, and Diet Consumption
3.3. Effects of TPs on Glucose Tolerance Profiles
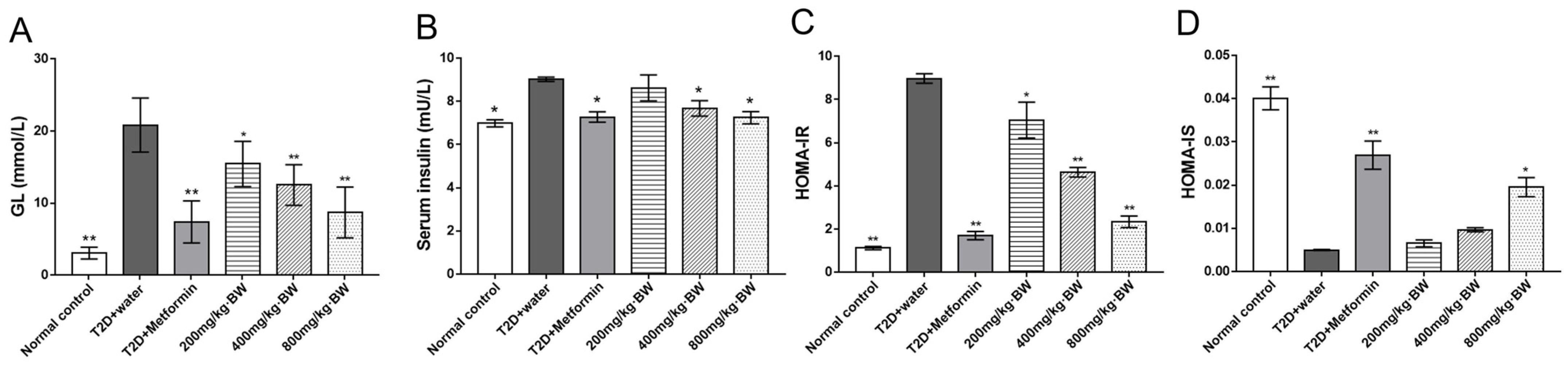
3.4. Effects of TPs on Fasting Blood Glucose and Insulin Tolerance in Serum
3.5. Effects of TPs on Lipid Levels in Serum
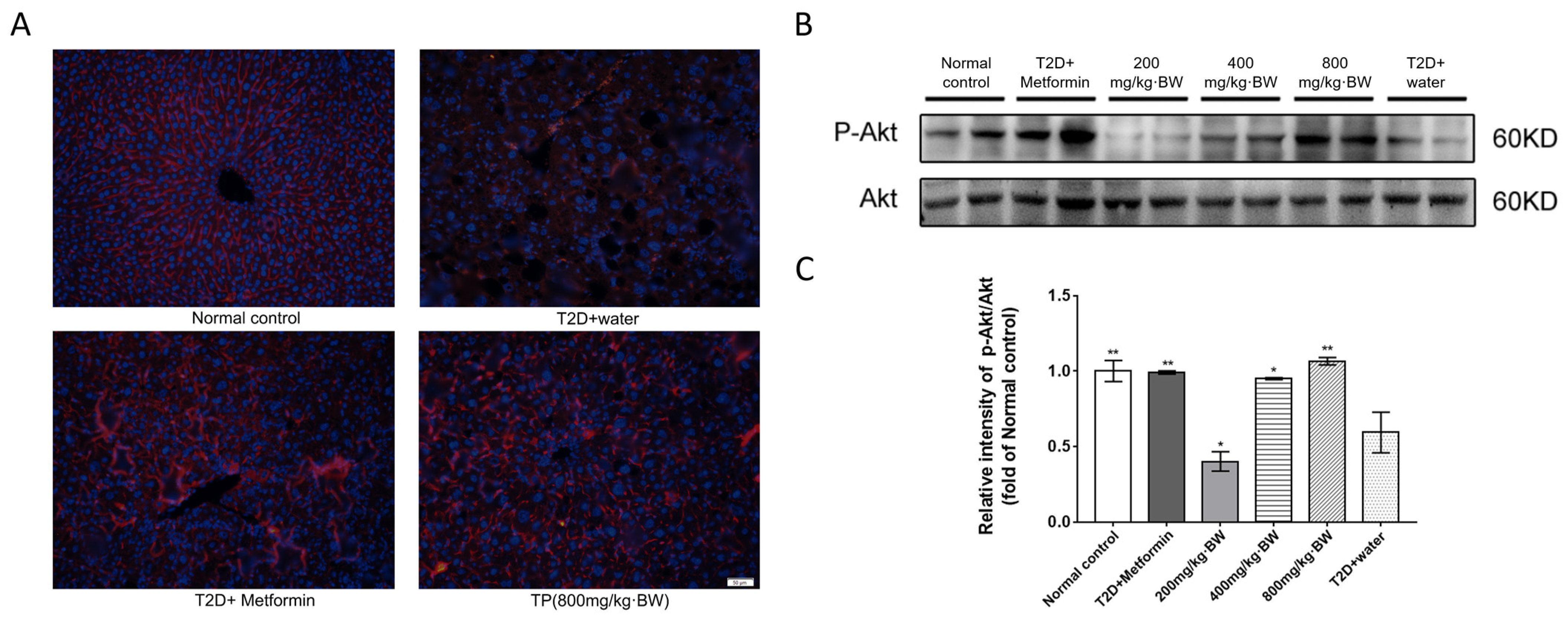
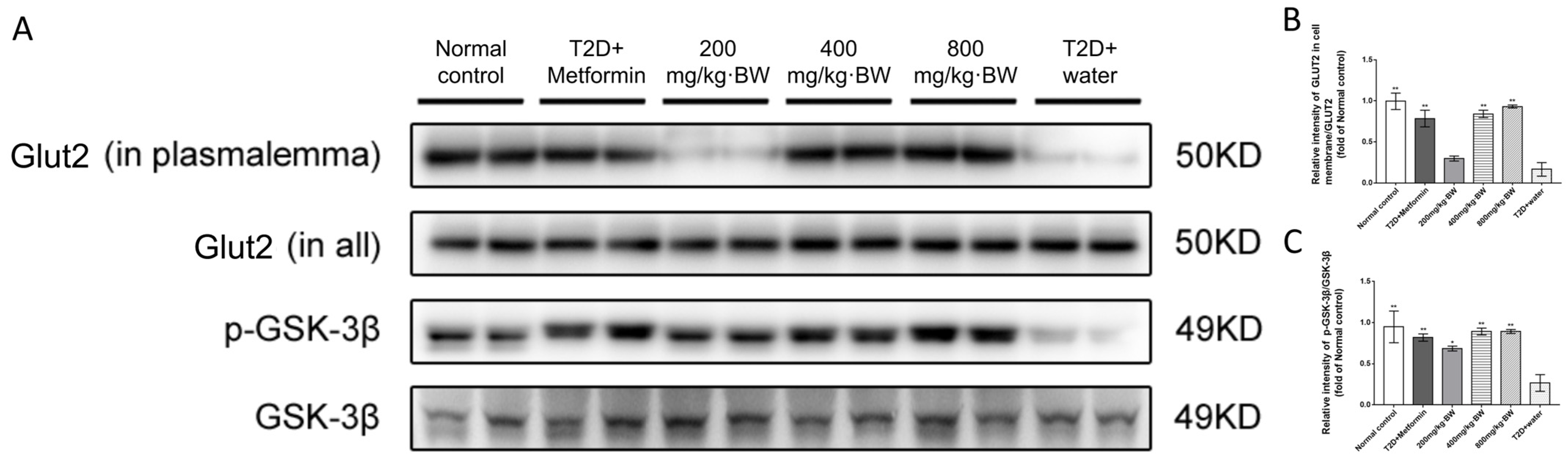
3.6. Effects of TPs on the Akt Signaling Pathway in the Livers of Diabetic Mice
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Chen, J.J.; Ji, X.M.; Hu, X.; Ling, T.J.; Zhang, Z.Z.; Bao, G.H.; Wan, X.C. Changes of major tea polyphenols and production of four new B-ring fission metabolites of catechins from post-fermented Jing-Wei Fu brick tea. Food Chem. 2015, 170, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, Y.; Liu, R.; Wang, K.; Zhang, M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Lombardo Bedran, T.B.; Morin, M.P.; Palomari Spolidorio, D.; Grenier, D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and β-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Lu, X.; Wang, D.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, C.; Zhou, H.; Wei, X.; Wang, Y. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J. Sci. Food Agric. 2021, 101, 6563–6577. [Google Scholar] [CrossRef]
- Lee, Y.E.; Yoo, S.H. Hypoglycemic effect of soluble polysaccharide and catechins from green tea on inhibiting intestinal transport of glucose. J. Sci. Food Agric. 2020, 100, 3979–3986. [Google Scholar] [CrossRef]
- Butacnum, A.; Chongsuwat, R.; Bumrungpert, A. Black tea consumption improves postprandial glycemic control in normal and pre-diabetic subjects: A randomized, double-blind, placebo-controlled crossover study. Asia Pac. J. Clin. Nutr. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Pereira, M.A.; Koh, W.P.; Arakawa, K.; Lee, H.P.; Yu, M.C. Coffee, tea, and incident type 2 diabetes: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2008, 88, 979–985. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; Qiu, X.M.; Wang, Y.Q.; Su, Z.Q.; Zhang, B.Y.; Wen, Z.; Yang, Y.F.; Xing, B.F.; Hong, M.; Liao, R. Intervention effect of Potentilla discolor-Euonymus alatus on intestinal flora of type 2 diabetes mellitus rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9062–9071. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.M.; Joo, M.J.; Lee, Y.S.; Kim, M.G. Effects of coffee consumption on insulin resistance and sensitivity: A meta-analysis. Nutrients 2021, 13, 3976. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Guo, C.; Thome, R.; Yang, N.; Zhang, Y.; Li, X.; Cao, X. Hypoglycemic, hypolipidemic and antioxidant effects of iridoid glycosides extracted from Corni fructus: Possible involvement of the PI3K-Akt/PKB signaling pathway. RSC Adv. 2018, 8, 30539–30549. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sanvee, G.M.; Hitzfeld, L.; Bouitbir, J.; Krähenbühl, S. mTORC2 is an important target for simvastatin-associated toxicity in C2C12 cells and mouse skeletal muscle-Roles of Rap1 geranylgeranylation and mitochondrial dysfunction. Biochem. Pharmacol. 2021, 192, 114750. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Schulze, M.B.; Sharp, S.J.; Guevara, M.; Romaguera, D.; Bendinelli, B.; Salamanca-Fernández, E.; Ardanaz, E.; Arriola, L.; Aune, D.; et al. Estimated substitution of tea or coffee for sugar-sweetened beverages was associated with lower type 2 diabetes incidence in case-cohort analysis across 8 European countries in the EPIC-InterAct study. J. Nutr. 2019, 149, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Y.; Chen, Z.; Gao, X.; Wang, C.; Panichayupakaranant, P.; Chen, H. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Krencz, I.; Sebestyen, A.; Papay, J.; Lou, Y.; Lutz, G.F.; Majewicz, T.L.; Khoor, A. Correlation between immunohistochemistry and RICTOR fluorescence in situ hybridization amplification in small cell lung carcinoma. Hum. Pathol. 2019, 93, 74–80. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. CR 2018, 37, 63. [Google Scholar] [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Peng, M.; Xia, T.; Zhong, Y.; Zhao, M.; Yue, Y.; Liang, L.; Zhong, R.; Zhang, H.; Li, C.; Cao, X.; et al. Integrative pharmacology reveals the mechanisms of Erzhi Pill, a traditional Chinese formulation, against diabetic cardiomyopathy. J. Ethnopharmacol. 2022, 296, 115474. [Google Scholar] [CrossRef]
- Dou, Z.; Liu, C.; Feng, X.; Xie, Y.; Yue, H.; Dong, J.; Zhao, Z.; Chen, G.; Yang, J. Camel whey protein (CWP) ameliorates liver injury in type 2 diabetes mellitus rats and insulin resistance (IR) in HepG2 cells via activation of the PI3K/Akt signaling pathway. Food Funct. 2022, 13, 255–269. [Google Scholar] [CrossRef]
- Liao, J.; Yang, Z.; Yao, Y.; Yang, X.; Shen, J.; Zhao, P. Andrographolide promotes uptake of glucose and GLUT4 transport through the PKC pathway in L6 cells. Pharmaceuticals 2022, 15, 1346. [Google Scholar] [CrossRef]
- Saqier; Bao, S.; Han, S.; Ao, W. Effects of Agriophyllum squarrosum extracts on glucose metabolism in KKAy mice and the associated underlying mechanisms. J. Ethnopharmacol. 2019, 241, 112009. [Google Scholar] [CrossRef]
- Nwabueze, O.P.; Sharma, M.; Balachandran, A.; Gaurav, A.; Abdul Rani, A.N.; Małgorzata, J.; Beata, M.M.; Lavilla, C.A., Jr.; Billacura, M.P. Comparative studies of palmatine with metformin and glimepiride on the modulation of insulin dependent signaling pathway in vitro, in vivo & ex vivo. Pharmaceuticals 2022, 15, 1317. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, Y.; Wu, G.; Shi, Y.; Ge, Y.; Li, B.; Cheng, X.; Tang, X.; Zhu, J.; Le, G. Dietary methionine restriction improves gastrocnemius muscle glucose metabolism through improved insulin secretion and H19/IRS-1/Akt pathway in middle-aged mice. J. Agric. Food Chem. 2023, 71, 5655–5666. [Google Scholar] [CrossRef]
- Tang, P.; Tang, Y.; Liu, Y.; He, B.; Shen, X.; Zhang, Z.J.; Qin, D.L.; Tian, J. Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyranoside isolated from Eucommia ulmoides leaf relieves insulin resistance in HepG2 cells via the IRS-1/PI3K/Akt/GSK-3β pathway. Biol. Pharm. Bull. 2023, 46, 219–229. [Google Scholar] [CrossRef]
- Jeon, Y.G.; Kim, Y.Y.; Lee, G.; Kim, J.B. Physiological and pathological roles of lipogenesis. Nat. Metab. 2023, 5, 735–759. [Google Scholar] [CrossRef]



| Monosaccharide Name | Content of Monosaccharide (%) | Monosaccharide Name | Content of Monosaccharide (%) |
|---|---|---|---|
| Arabinose | 0.07 ± 0.03 | Mannose | 0.30 ± 0.03 |
| Fucose | 0.05 ± 0.01 | Ribose | 0.20 ± 0.02 |
| Fructose | 0.08 ± 0.01 | Xylose | 0.53 ± 0.07 |
| Galactose | 1.04 ± 0.28 | Galacturonic acid | 0.31 ± 0.03 |
| Glucose | 1.92 ± 0.74 | Alduronic acid | 77.80 ± 1.89 |
| Component | Weight-Average Molecular Weight/g·mol−1 | Number-Average Molecular Weight/g·mol−1 | Polydispersity (aMw/bMn) | RMS Radius of Gyration/nm |
|---|---|---|---|---|
| TP-A | 7.09 × 104 (±3.33%) | 1.01 × 104 (±23.21%) | 7.00(±23.44%) | 25.50 (±48.20%) |
| TP-B | 6.75 × 104 (±1.84%) | 1.10 × 104 (±8.77%) | 6.17 (±8.97%) | 28.00 (±19.80%) |
| TP-C | 6.71 × 104 (±1.00%) | 1.42 × 104 (±3.00%) | 4.72 (±3.16%) | 27.90 (±8.20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Deng, X.; Chen, R.; Li, Q.; Sun, L.; Cao, J.; Lai, Z.; Lai, X.; Wang, Z.; Sun, S.; et al. Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway. Foods 2024, 13, 1908. https://doi.org/10.3390/foods13121908
Zhang Z, Deng X, Chen R, Li Q, Sun L, Cao J, Lai Z, Lai X, Wang Z, Sun S, et al. Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway. Foods. 2024; 13(12):1908. https://doi.org/10.3390/foods13121908
Chicago/Turabian StyleZhang, Zhenbiao, Xuming Deng, Ruohong Chen, Qiuhua Li, Lingli Sun, Junxi Cao, Zhaoxiang Lai, Xingfei Lai, Zaihua Wang, Shili Sun, and et al. 2024. "Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway" Foods 13, no. 12: 1908. https://doi.org/10.3390/foods13121908
APA StyleZhang, Z., Deng, X., Chen, R., Li, Q., Sun, L., Cao, J., Lai, Z., Lai, X., Wang, Z., Sun, S., & Zhang, L. (2024). Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway. Foods, 13(12), 1908. https://doi.org/10.3390/foods13121908






