Improving Biodegradable Films from Corn Bran Arabinoxylan for Hydrophobic Material and Green Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Milling
2.2. Arabinoxylan Extraction
2.3. Arabinoxylan Polymer Characterization
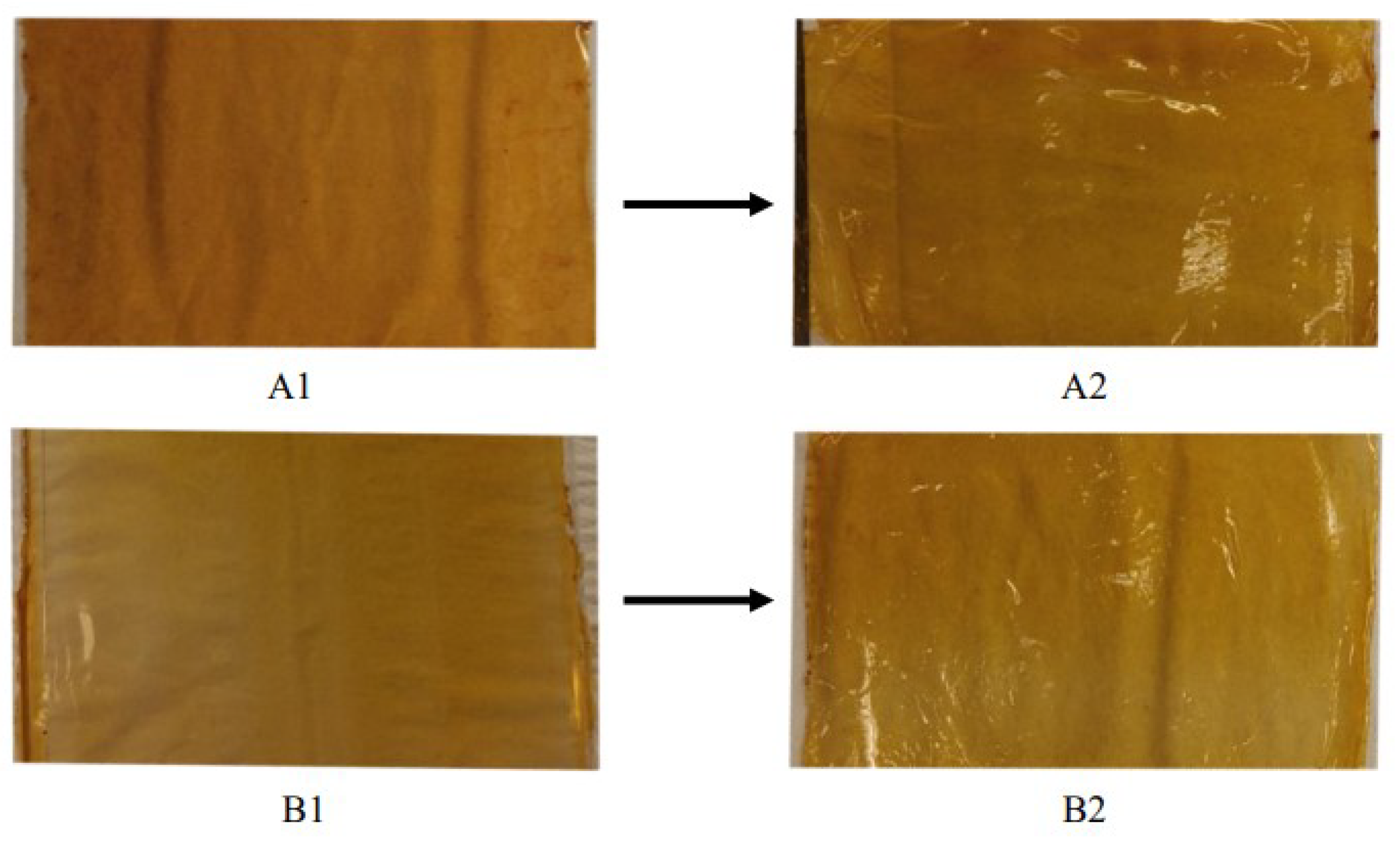
2.4. Preparation of Surface-Modified Arabinoxylan Films
2.5. Hydrophobic and Biodegradable Arabinoxylan Films
2.5.1. Moisture Content
2.5.2. Water Solubility
2.5.3. Water Vapor Transmission Rate
2.5.4. Contact Angles
2.5.5. Biodegradability Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Arabinoxylan Polymer and Molecular Weight
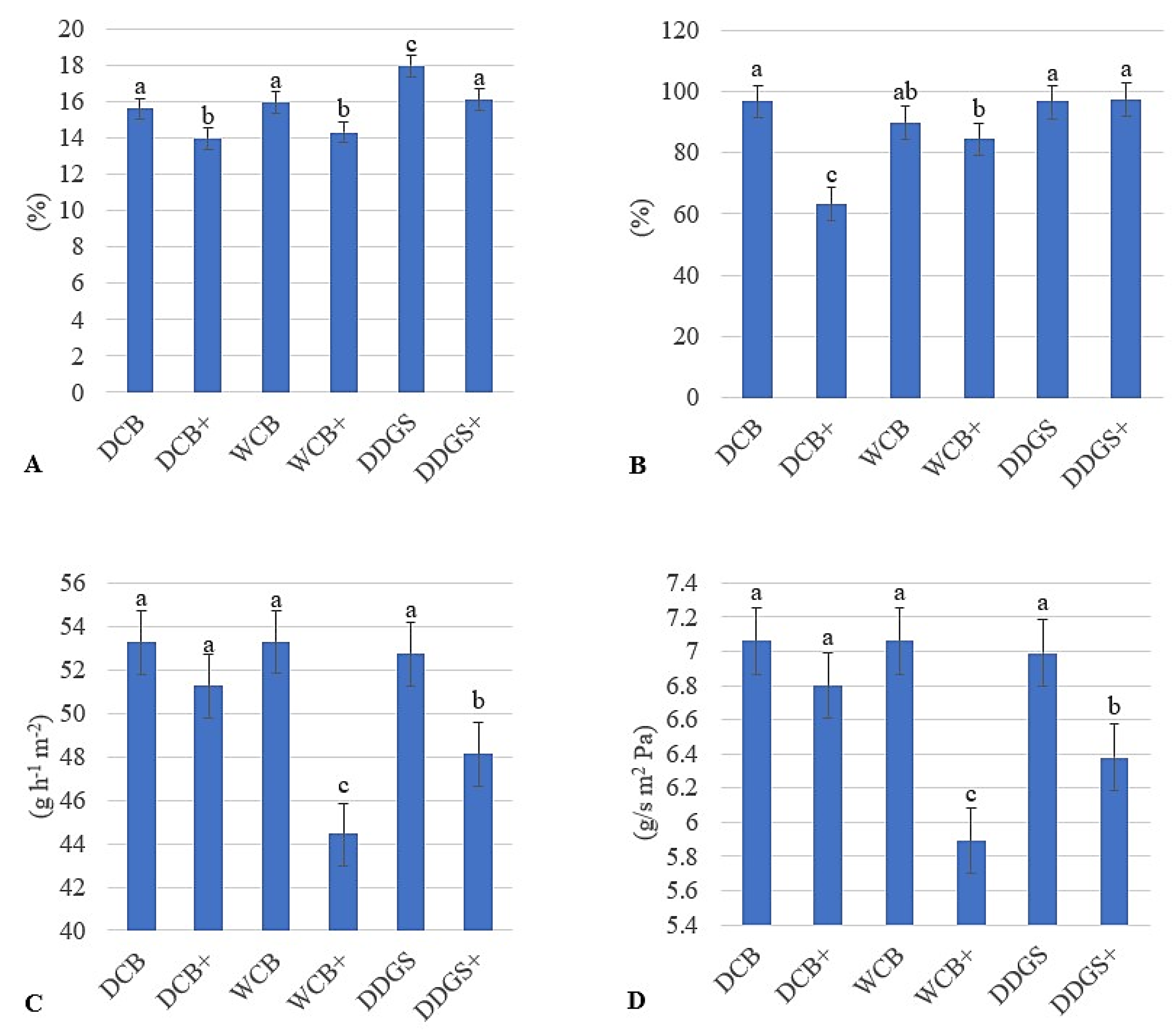
3.2. AX Film Moisture Content
3.3. AX Film Water Solubility
3.4. Water Vapor Transmission Rate
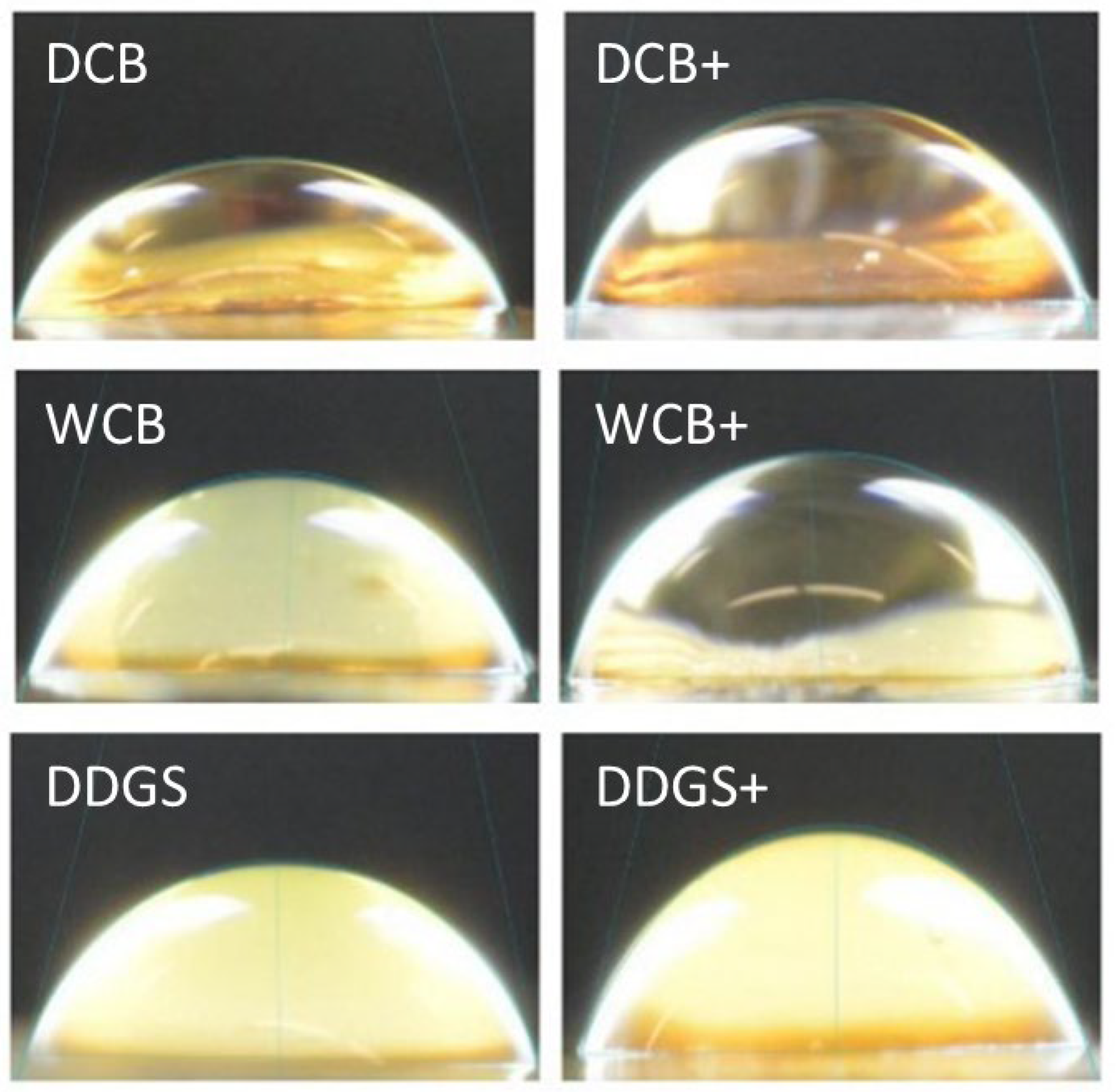
3.5. Contact Angle
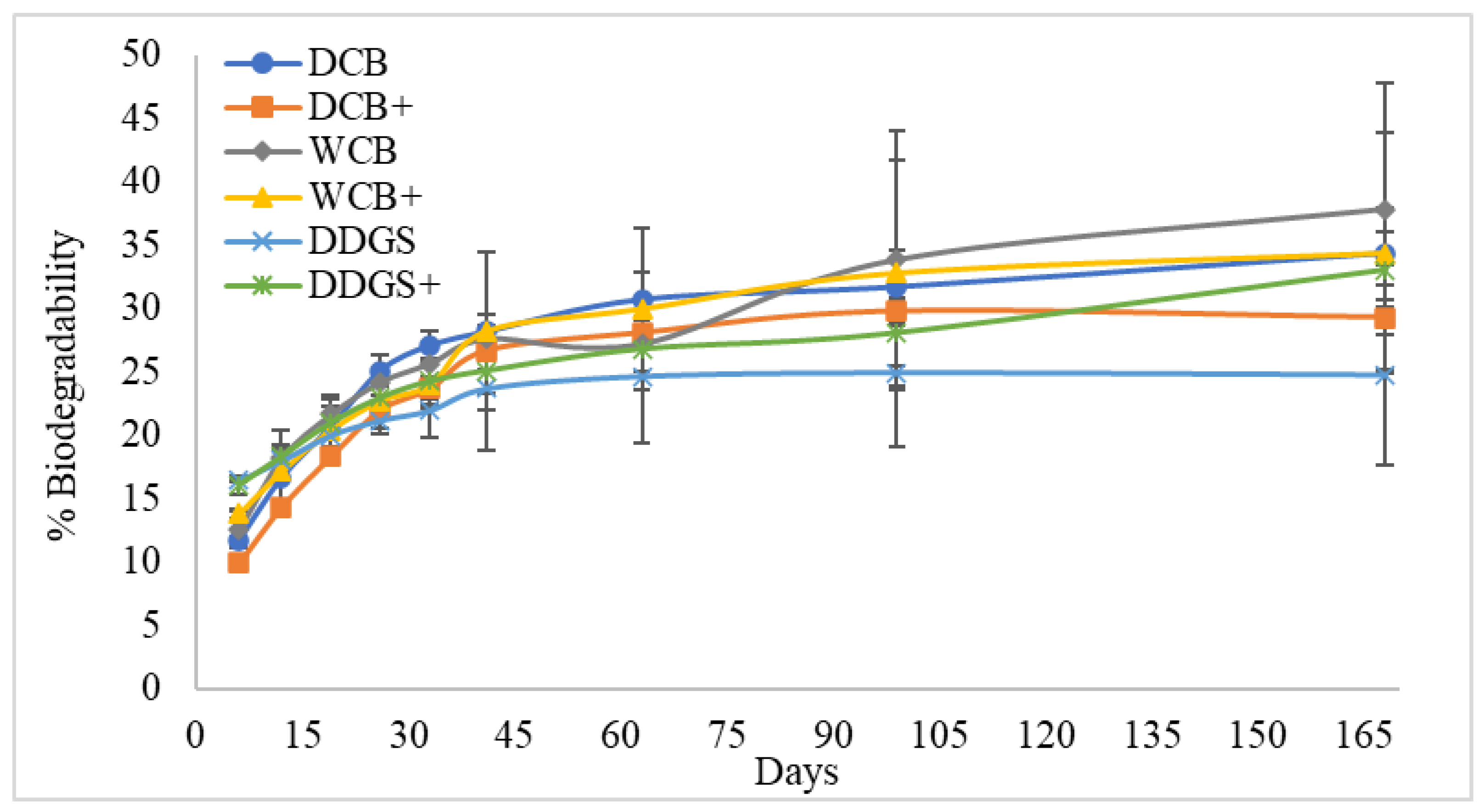
3.6. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Grain Council. Dry-Grind Production of Ethanol, Distillers Corn Oil and Corn Co-Products. In DDGS Handbook; U.S. Grain Council: Washington, DC, USA, 2018; pp. 8–14. Available online: https://grains.org/buying-selling/ddgs/user-handbook/ (accessed on 12 January 2024).
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Minor constituents and phytochemicals of the kernel. In Corn Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Inc.: St. Paul, MN, USA, 2019; pp. 369–403. [Google Scholar] [CrossRef]
- Rose, D.J.; Inglett, G.E.; Liu, S.X. Utilisation of corn (Zea mays) bran and corn fiber in the production of food components. J. Sci. Food Agric. 2010, 90, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Simsek, S. A novel combination of methods for the extraction and purification of arabinoxylan from byproducts of the cereal industry. J. Food Meas. Charact. 2019, 13, 1049–1057. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Yin, J.; Ma, L.; Li, D. Optimisation of double-enzymatic extraction of arabinoxylan from fresh corn fibre. J. Food Sci. Technol. 2020, 57, 4649–4659. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Chuck-Hernandez, C.; Serna-Saldivar, S.O. Development and structure of the corn kernel. In Corn Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Inc.: St. Paul, MN, USA, 2019; pp. 147–163. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Yu, Y.; Wang, S.; Xu, Z.; Huang, H.; Jin, M. Ethanol production from mixtures of Distiller’s Dried Grains with Solubles (DDGS) and corn. Ind. Crops Prod. 2019, 129, 59–66. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M. Fundamental studies on water-washing of the corn ethanol coproduct (DDGS) and its characterization for biocomposite applications. Biomass Bioenergy 2013, 55, 251–259. [Google Scholar] [CrossRef]
- Anderson, C.; Simsek, S. How do arabinoxylan films interact with water and soil? Foods 2019, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Stepan, A.; Anasontzis, G.; Matama, T.; Cavaco-Paulo, A.; Olsson, L.; Gatenholm, P. Lipases efficiently stearate and cutinases acetylate the surface of arabinoxylan films. J. Biotechnol. 2013, 167, 16–23. [Google Scholar] [CrossRef]
- Rumpagaporn, P.; Reuhs, B.L.; Kaur, A.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr. Polym. 2015, 130, 191–197. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Int. Food Res. J. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Agger, J.; Viksø-Nielsen, A.; Meyer, A.S. Enzymatic xylose release from pretreated corn bran arabinoxylan: Differential effects of deacetylation and deferuloylation on insoluble and soluble substrate fractions. J. Agric. Food Chem. 2010, 58, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.S.; Yadav, M.P.; Chau, H.K.; Hotchkiss, A.T., Jr. Molecular and functional properties of a xylanase hydrolysate of corn bran arabinoxylan. Carbohydr. Polym. 2018, 181, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.S.; Pai, D.A.; Hamaker, B.R.; Campanella, O.H. Structure–function relationships for corn bran arabinoxylans. J. Cereal Sci. 2010, 52, 368–372. [Google Scholar] [CrossRef]
- Kale, M.S.; Hamaker, B.R.; Campanella, O.H. Alkaline extraction conditions determine gelling properties of corn bran arabi-noxylans. Food Hydrocoll. 2013, 31, 121–126. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and thermomechanical characterization of tara gum edible films: Effect of polyols as plasticizers. Carbohydr. Polym. 2014, 111, 359–365. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of glycerol and sorbitol on cellulose-based biodegradable films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Bengtsson, M.; Koch, K.; Gatenholm, P. Surface octanoylation of high-amylose potato starch films. Carbohydr. Polym. 2003, 54, 1–11. [Google Scholar] [CrossRef]
- Kontkanen, H.; Tenkanen, M.; Fagerström, R.; Reinikainen, T. Characterisation of steryl esterase activities in commercial lipase preparations. J. Biotechnol. 2004, 108, 51–59. [Google Scholar] [CrossRef]
- Sakai, S.; Antoku, K.; Yamaguchi, T.; Kawakami, K. Transesterification by lipase entrapped in electrospun poly (vinyl alcohol) fibers and its application to a flow-through reactor. J. Biosci. Bioeng. 2008, 105, 687–689. [Google Scholar] [CrossRef]
- Utsugi, A.; Kanda, A.; Hara, S. Lipase specificity in the transacylation of triacylglycerin. J. Oleo Sci. 2009, 58, 123–132. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Effect of clay nanofillers on the mechanical and water vapor permeability properties of xylan–alginate films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef] [PubMed]
- Salvada, J.; Alke, B.; Brazinha, C.; Alves, V.D.; Coelhoso, I.M. Development and characterisation of arabinoxylan-based com-posite films. Coatings 2022, 12, 813. [Google Scholar] [CrossRef]
- Eissenberger, K.; Ballesteros, A.; De Bisschop, R.; Bugnicourt, E.; Cinelli, P.; Defoin, M.; Demeyer, E.; Fürtauer, S.; Gioia, C.; Gómez, L. Approaches in Sustainable, Biobased Multilayer Packaging Solutions. Polymers 2023, 15, 1184. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. CRFSFS 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Lindh, H.; Olsson, A.; Williams, H. Consumer perceptions of food packaging: Contributing to or counteracting environmentally sustainable development? Packag. Technol. Sci. 2016, 29, 3–23. [Google Scholar] [CrossRef]
- Anderson, C.; Simsek, S. Mechanical profiles and topographical properties of films made from alkaline extracted arabinoxylans from wheat bran, maize bran, or dried distillers grain. Food Hydrocoll. 2019, 86, 78–86. [Google Scholar] [CrossRef]
- Alahmed, A.; Simsek, S. Enhancing Mechanical Properties of Corn Bran Arabinoxylan Films for Sustainable Food Packaging. Foods 2024, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Watson, J.; Tobimatsu, Y.; Runge, T. Film-forming polymers from distillers’ grains: Structural and material properties. Ind. Crops Prod. 2014, 59, 282–289. [Google Scholar] [CrossRef]
- Nagel, A.; Sirisakulwat, S.; Carle, R.; Neidhart, S. An acetate–hydroxide gradient for the quantitation of the neutral sugar and uronic acid profile of pectins by HPAEC-PAD without postcolumn pH adjustment. J. Agric. Food Chem. 2014, 62, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, Y.; Whitney, K.; Nakamura, K.; Hayakawa, K.; Simsek, S. Analysis of molecular weight and structural changes in water-extractable arabinoxylans during the breadmaking process. Food Chem. 2022, 386, 132772. [Google Scholar] [CrossRef] [PubMed]
- García, M.a.A.; Pinotti, A.; Martino, M.N.; Zaritzky, N.E. Characterization of composite hydrocolloid films. Carbohydr. Polym. 2004, 56, 339–345. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- ASTM Standard E96/E96M-22aε1; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM Standard D7490-13(2022); Standard Test Method for Measurement of the Surface Tension of Solid Coatings, Substrates and Pigments Using Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- AASTM Standard D5988-18; Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials under High-Solids Anaerobic-Digestion Conditions. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- SAS Institute Inc. SAS Software, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Anderson, B.; Almeida, H. Corn dry milling: Processes, products, and applications. In Corn Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Inc.: St. Paul, MN, USA, 2019; pp. 405–433. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Li, L.; Zhang, M.; Tian, S.; Wang, D.; Liu, K.; Liu, H.; Zhu, W.; Wang, X. Comprehensive utilization of corn starch processing by-products: A review. Grain Oil Sci. Technol. 2021, 4, 89–107. [Google Scholar] [CrossRef]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as functional food ingredients: A review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Characterization of edible emulsified films with low affinity to water based on kefiran and oleic acid. Int. J. Biol. Macromol. 2011, 49, 378–384. [Google Scholar] [CrossRef]
- Heikkinen, S.L.; Mikkonen, K.S.; Pirkkalainen, K.; Serimaa, R.; Joly, C.; Tenkanen, M. Specific enzymatic tailoring of wheat arabinoxylan reveals the role of substitution on xylan film properties. Carbohydr. Polym. 2013, 92, 733–740. [Google Scholar] [CrossRef]
- Weng, V.; Brazinha, C.; Coelhoso, I.M.; Alves, V.D. Decolorization of a corn fiber arabinoxylan extract and formulation of biodegradable films for food packaging. Membranes 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, barrier, mechanical, and biodegradability properties of modified starch films with nut by-products extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, T.; Lim, J.; Gu, F.; Fang, F.; Cheng, L.; Campanella, O.H.; Hamaker, B.R. Acid gelation of soluble laccase-crosslinked corn bran arabinoxylan and possible gel formation mechanism. Food Hydrocoll. 2019, 92, 1–9. [Google Scholar] [CrossRef]
- ASTM Standard D3985-17; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
| Yield (%, DWB) | AX Content (%, DWB) | A/X | Mw | PI | |
|---|---|---|---|---|---|
| DCB | 23.30 b | 76.60 b | 1.02 c | 4,063,667 a | 3.05 a |
| WCB | 25.39 a | 77.64 a | 1.20 b | 1,876,333 b | 2.37 b |
| DDGS | 23.01 b | 66.68 c | 1.32 a | 879,133 c | 1.80 c |
| Factors | AX Films | Moisture Content (%) | Water Solubility (%) | WVTR g h−1 m−2 | WVP g/s m2 Pa |
|---|---|---|---|---|---|
| Sources | DCB | 14.77 b | 79.92 c | 52.24 a | 6.92 a |
| WCB | 15.13 b | 87.08 b | 48.85 b | 6.47 b | |
| DDGS | 17.03 a | 96.94 a | 50.42 b | 6.68 b | |
| Modification | Unmodified | 16.50 a | 94.35 a | 53.08 a | 7.03 a |
| Modified | 14.79 b | 81.61 b | 47.93 b | 6.35 b |
| Factors | AX Films | Contact Angle (°) | C-CO2 (mg) | Biodegradability (%) |
|---|---|---|---|---|
| Sources | DCB | 106.11 a | 9.84 a | 31.82 ab |
| WCB | 109.46 a | 12.43 a | 36.09 a | |
| DDGS | 108.87 a | 11.73 a | 28.91 b | |
| Modification | Unmodified | 109.86 a | 11.45 a | 32.31 a |
| Modified | 106.43 a | 11.22 a | 32.24 a | |
| Interactions | DCB | 107.08 a | 12.27 a | 34.35 ab |
| DCB+ | 105.15 a | 7.41 a | 29.30 bc | |
| WCB | 115.70 a | 14.25 a | 37.82 a | |
| WCB+ | 103.23 a | 10.62 a | 34.36 ab | |
| DDGS | 106.81 a | 7.83 a | 24.75 c | |
| DDGS+ | 110.92 a | 15.63 a | 33.06 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahmed, A.; Simsek, S. Improving Biodegradable Films from Corn Bran Arabinoxylan for Hydrophobic Material and Green Food Packaging. Foods 2024, 13, 1914. https://doi.org/10.3390/foods13121914
Alahmed A, Simsek S. Improving Biodegradable Films from Corn Bran Arabinoxylan for Hydrophobic Material and Green Food Packaging. Foods. 2024; 13(12):1914. https://doi.org/10.3390/foods13121914
Chicago/Turabian StyleAlahmed, Abdulrahman, and Senay Simsek. 2024. "Improving Biodegradable Films from Corn Bran Arabinoxylan for Hydrophobic Material and Green Food Packaging" Foods 13, no. 12: 1914. https://doi.org/10.3390/foods13121914








