Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Culture Conditions, and Yeast Suspensions
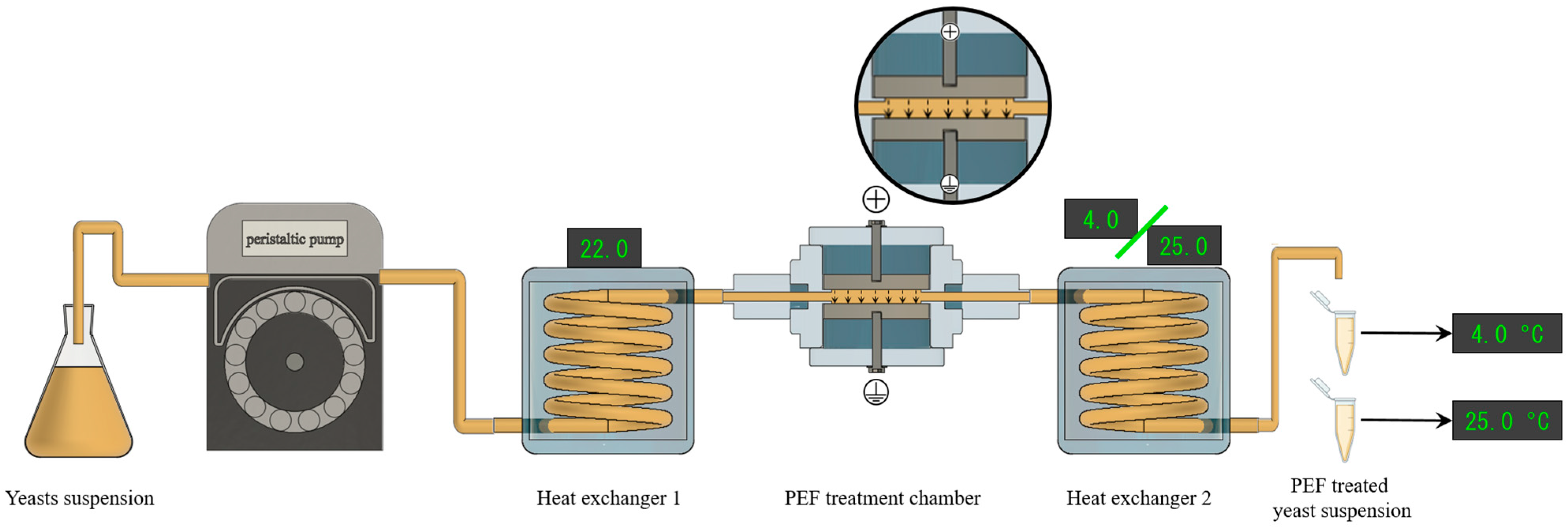
2.2. PEF Processing
Determination of the Effect of PEF on the Electroporation of S. cerevisiae
2.3. Hot Water Treatment
2.4. Bead Milling Treatment
2.5. Analytical Measurements
2.5.1. Reduced Glutathione Concentration
2.5.2. Protein Concentration
2.5.3. Enhancement of the Ratio of Glutathione to Proteins (Δ Ratio Glutathione/Proteins)
2.5.4. Antioxidant Capacity
2.6. Dry Weight Determination
2.7. Electrophoresis (SDS-PAGE)
2.8. Statistical Analysis
3. Results and Discussion
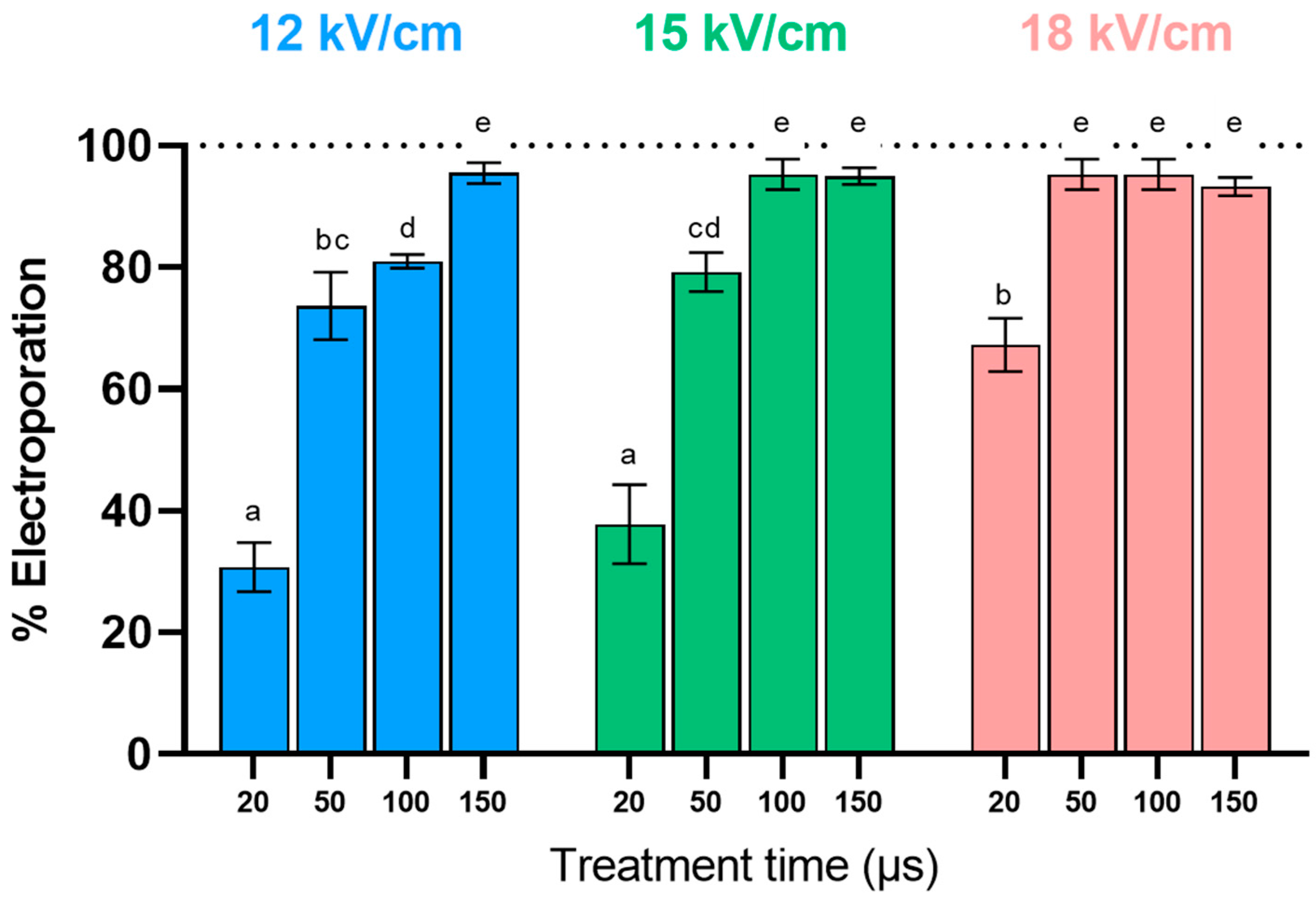
3.1. Selection of PEF Treatment Conditions for Electroporation of Yeast Biomass
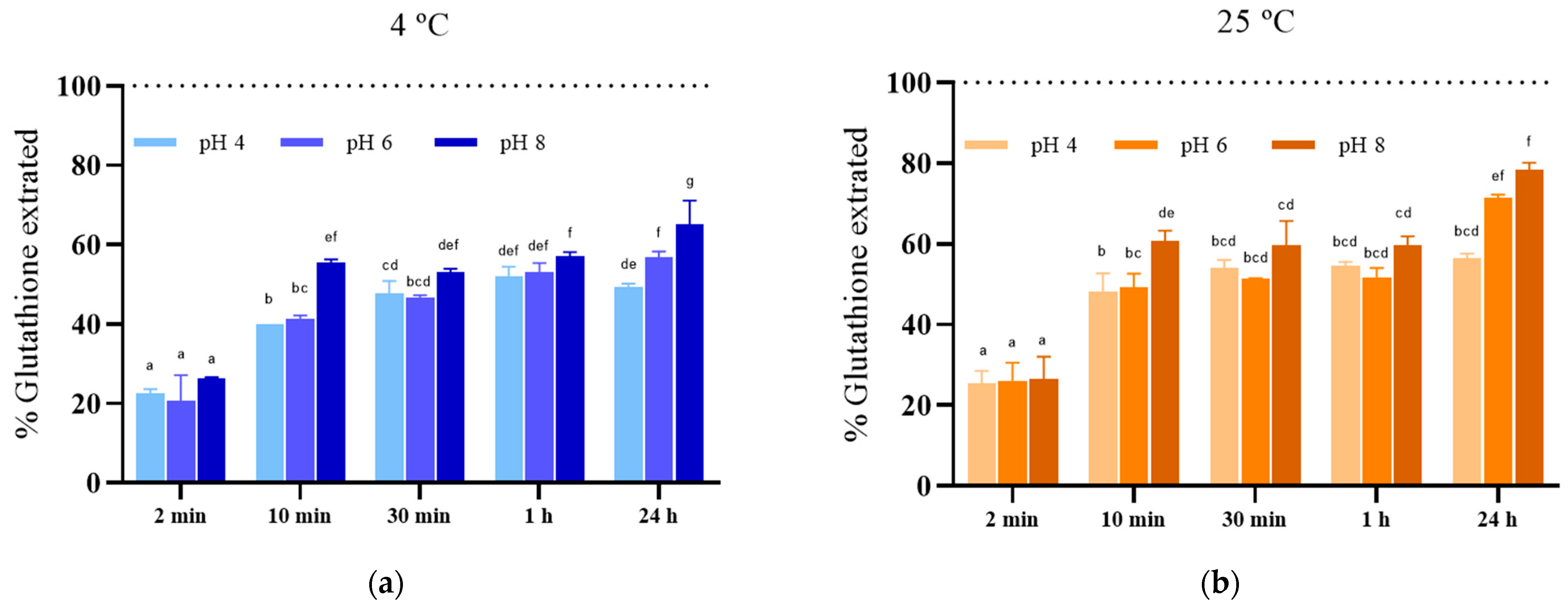
3.2. Effect of the Incubation Conditions after PEF Treatment on the Kinetics of Glutathione Extraction from S. cerevisiae
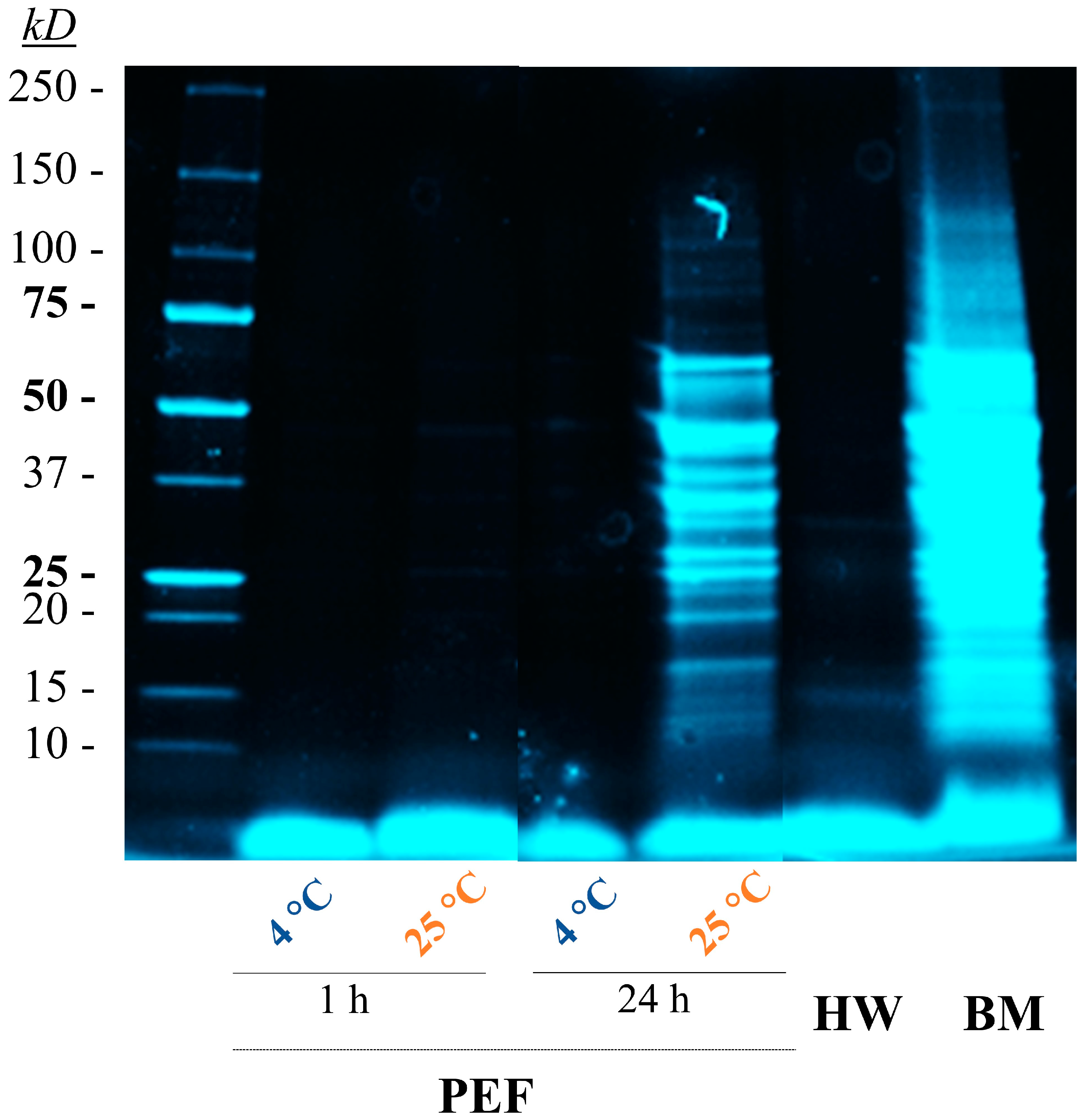
3.3. Release of Proteins from S. cerevisiae after PEF Treatment
3.4. Comparison of PEF with Bead Milling and Hot Water for Extracting Glutathione from S. cerevisiae
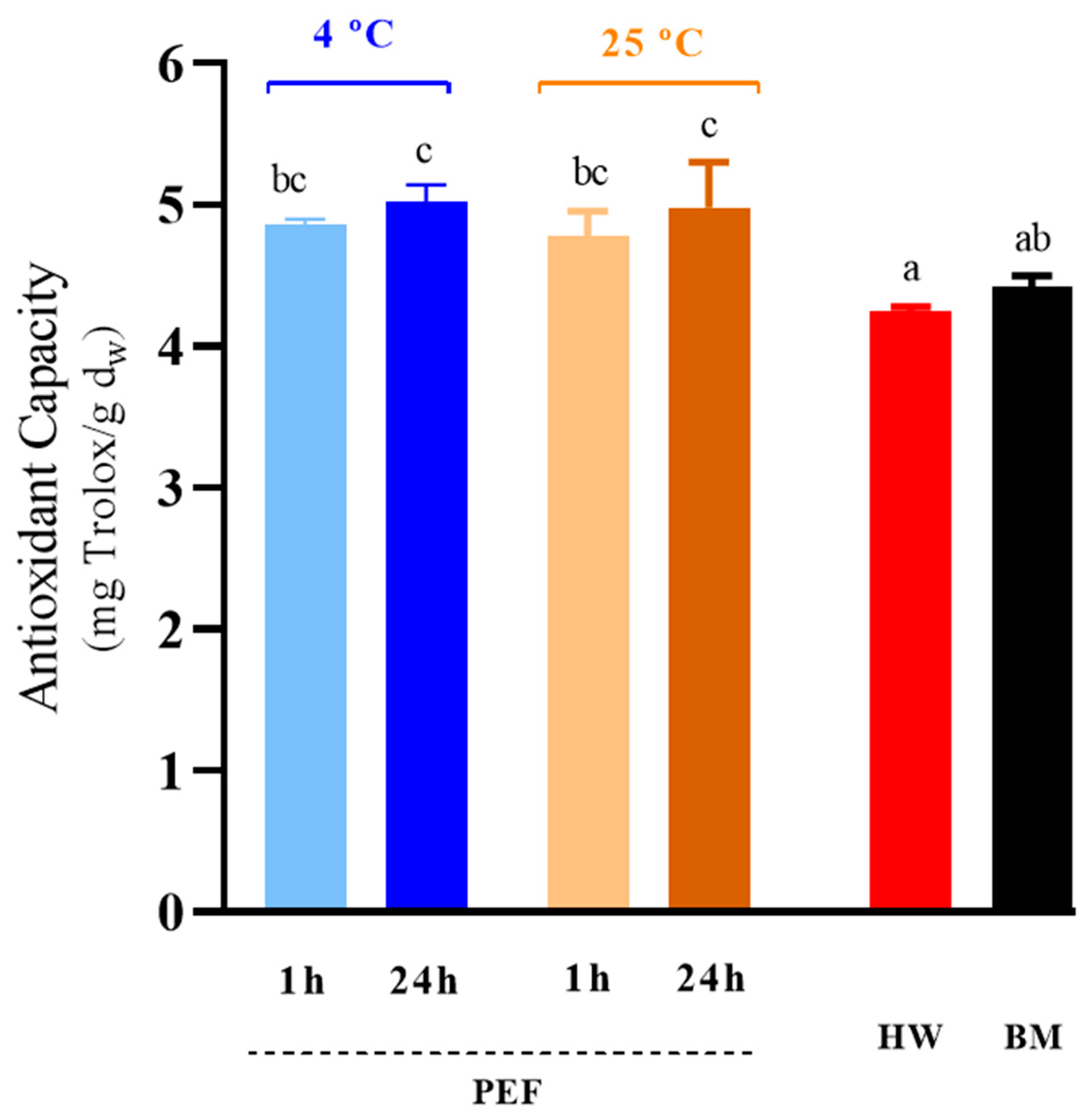
3.5. Antioxidant Capacity of GSH Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wei, G.; Chen, J. Glutathione: A Review on Biotechnological Production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of Glutathione: Implication in Redox and Detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The Glutathione System in Parkinson’s Disease and Its Progression. Neurosci. Biobehav. Rev. 2021, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Ogaya, Y.; Takami, M.; Konishi, T.; Sauchi, Y.; Park, Y.Y.; Wada, S.; Sato, K.; Higashi, A. Glutathione Supplementation Suppresses Muscle Fatigue Induced by Prolonged Exercise via Improved Aerobic Metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 7. [Google Scholar] [CrossRef]
- Grucza, K.; Chołbiński, P.; Kwiatkowska, D.; Szutowski, M. Effects of Supplementation with Glutathione and Its Precursors on Athlete Performance. Biomed. J. Sci. Tech. Res. 2019, 12, 9434–9441. [Google Scholar] [CrossRef]
- Bahut, F.; Romanet, R.; Sieczkowski, N.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Antioxidant Activity from Inactivated Yeast: Expanding Knowledge beyond the Glutathione-Related Oxidative Stability of Wine. Food Chem. 2020, 325, 126941. [Google Scholar] [CrossRef]
- Tang, K.X.; Zhao, C.J.; Gänzle, M.G. Effect of Glutathione on the Taste and Texture of Type I Sourdough Bread. J. Agric. Food Chem. 2017, 65, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Yano, H. Improvements in the Bread-Making Quality of Gluten-Free Rice Batter by Glutathione. J. Agric. Food Chem. 2010, 58, 7949–7954. [Google Scholar] [CrossRef]
- Bustamante, M.; Giménez, P.; Just-Borràs, A.; Solé-Clua, I.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Gil, M.; Pérez-Navarro, J.; Gómez-Alonso, S.; et al. Use of Glutathione, Pure or as a Specific Inactivated Yeast, as an Alternative to Sulphur Dioxide for Protecting White Grape Must from Browning. Foods 2024, 13, 310. [Google Scholar] [CrossRef]
- Giménez, P.; Just-Borras, A.; Pons, P.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Canals, J.M.; Zamora, F. Biotechnological Tools for Reducing the Use of Sulfur Dioxide in White Grape Must and Preventing Enzymatic Browning: Glutathione; Inactivated Dry Yeasts Rich in Glutathione; and Bioprotection with Metschnikowia Pulcherrima. Eur. Food Res. Technol. 2023, 249, 1491–1501. [Google Scholar] [CrossRef]
- Martínez, J.; González-Arenzana, L. Use of Glutathione in the Winemaking of White Grape Varieties. In White Wine Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–38. [Google Scholar] [CrossRef]
- Wegmann-Herr, P.; Ullrich, S.; Schmarr, H.-G.; Durner, D. Use of Glutathione during White Wine Production–Impact on S-off-Flavors and Sensory Production. BIO Web Conf. 2016, 7, 02031. [Google Scholar] [CrossRef]
- AL-Temimi, A.A.; Al-Hilifi, S.A.; AL-Mossawi, A.E. bashar An Investigation on Glutathione Derived from Spinach and Red Cabbage Leaves and Their Effects of Adding to Meat Patties. Saudi J. Biol. Sci. 2023, 30, 103632. [Google Scholar] [CrossRef] [PubMed]
- Schmacht, M.; Lorenz, E.; Senz, M. Microbial Production of Glutathione. World J. Microbiol. Biotechnol. 2017, 33, 106. [Google Scholar] [CrossRef]
- Oliveira Santos, L.; Garcia Pereira Silva, P.; Fernandes Lemos Junior, W.J.; Sales de Oliveira, V.; Anschau, A. Glutathione Production by Saccharomyces cerevisiae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Guo, M.J.; Guo, Y.X.; Chu, J.; Zhuang, Y.P.; Zhang, S.L. Efficient Extraction of Intracellular Reduced Glutathione from Fermentation Broth of Saccharomyces cerevisiae by Ethanol. Bioresour. Technol. 2009, 100, 1011–1014. [Google Scholar] [CrossRef]
- Wu, X.; Tang, L.; Du, Y.; Xu, Z. Improving Glutathione Extraction from Crude Yeast Extracts by Optimizing Aqueous Two-Phase System Composition and Operation Conditions. Korean J. Chem. Eng. 2010, 27, 1829–1835. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction of Compounds of Interest from Yeast Biomass Assisted by Pulsed Electric Fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Miklavčič, D. The Phenomenon of Electroporation. In Pulsed Electric Fields Technology for the Food Industry: Fundamentals and Applications; Javier, R., Heinz, V., Alvarez, I., Toepfl, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 107–141. ISBN 978-3-030-70586-2. [Google Scholar]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of Cell Membrane Electropermeabilization: A Minireview of Our Present (Lack of?) Knowledge. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1724, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Ladner, C.L.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible Fluorescent Detection of Proteins in Polyacrylamide Gels without Staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, K.; Rönner, U.; Borch, E. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in Relation to Membrane Permeabilization and Subsequent Leakage of Intracellular Compounds Due to Pulsed Electric Field Processing. Int. J. Food Microbiol. 2005, 99, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cserhalmi, Z.; Vidács, I.; Beczner, J.; Czukor, B. Inactivation of Saccharomyces cerevisiae and Bacillus cereus by Pulsed Electric Fields Technology. Innov. Food Sci. Emerg. Technol. 2002, 3, 41–45. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field and PH Assisted Selective Extraction of Intracellular Components from Microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Aguilar-Machado, D.; Delso, C.; Martinez, J.M.; Morales-Oyervides, L.; Montañez, J.; Raso, J. Enzymatic Processes Triggered by PEF for Astaxanthin Extraction from Xanthophyllomyces dendrorhous. Front. Bioeng. Biotechnol. 2020, 8, 857. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhang, Z.; Feng, X. Extraction and Concentration of Glutathione from Yeast by Membranes. Can. J. Chem. Eng. 2021, 100, S195–S204. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of Pulsed Electric Fields on the Production of Yeast Extract by Autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of Cell Membranes: The Fundamental Effects of Pulsed Electric Fields in Food Processing. Food Eng. Rev. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Yang, G.; Wang, R.; Gao, J.R.; Niu, D.; Li, J.; Wen, Q.H.; Zeng, X.A. The Effect of Moderate Pulsed Electric Fields on Autolysis of Saccharomyces Cerevisiae and the Amino Acid Content in Autolysates. Int. J. Food Sci. Technol. 2021, 56, 441–451. [Google Scholar] [CrossRef]
- Rollini, M.; Musatti, A.; Manzoni, M. Production of Glutathione in Extracellular Form by Saccharomyces cerevisiae. Process Biochem. 2010, 45, 441–445. [Google Scholar] [CrossRef]
- Hara, K.Y.; Kim, S.; Yoshida, H.; Kiriyama, K.; Kondo, T.; Okai, N.; Ogino, C.; Fukuda, H.; Kondo, A. Development of a Glutathione Production Process from Proteinaceous Biomass Resources Using Protease-Displaying Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2012, 93, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of Food Grade Yeasts. Food Technol. Biotechnol. 2006, 44, 407–415. [Google Scholar]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of Different Extraction Techniques for Isolation of Antioxidants from Sweet Grass (Hierochloë odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B. Electropulsation as an Alternative Method for Protein Extraction from Yeast. FEMS Microbiol. Lett. 1999, 174, 279–284. [Google Scholar] [CrossRef][Green Version]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar]
- Santiago, L.A.; Mori, A. Antioxidant Defenses of Baker′s Yeast against Free Radicals and Lipid Peroxides in Rat Brain. Arch. Biochem. Biophys. 1993, 306, 16–21. [Google Scholar] [CrossRef]

| Extraction Method | Glutathione Concentration (mg/g dw) | Protein Concentration (mg/g dw) | Δ Ratio Glutathione/Proteins | ||
|---|---|---|---|---|---|
| Bead mill (BM) | 10.7 ± 0.3 d | 637.9 ± 25.7 d | 1.0 a | ||
| PEF (12 kV/cm, 150 µs) | 4 °C | 1 h | 6.3 ± 0.2 b | 77.4 ± 18.3 a | 5.2 ± 1.4 d |
| 24 h | 6.9 ± 0.5 b | 152.2 ± 7.5 b | 2.7 ± 0.3 bc | ||
| 25 °C | 1 h | 6.4 ± 0.2 b | 101.1 ± 10.9 a | 3.8 ± 0.5 cd | |
| 24 h | 8.4 ± 0.1 c | 304.4 ± 24.6 c | 1.6 ± 0.2 ab | ||
| Hot water (HW) (95 °C, 3 min) | 4.9 ± 0.1 a | 78.5 ± 4.9 a | 3.7 ± 0.3 c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzosa, A.; Marín-Sánchez, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces cerevisiae. Foods 2024, 13, 1916. https://doi.org/10.3390/foods13121916
Berzosa A, Marín-Sánchez J, Álvarez I, Sánchez-Gimeno C, Raso J. Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces cerevisiae. Foods. 2024; 13(12):1916. https://doi.org/10.3390/foods13121916
Chicago/Turabian StyleBerzosa, Alejandro, Javier Marín-Sánchez, Ignacio Álvarez, Cristina Sánchez-Gimeno, and Javier Raso. 2024. "Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces cerevisiae" Foods 13, no. 12: 1916. https://doi.org/10.3390/foods13121916
APA StyleBerzosa, A., Marín-Sánchez, J., Álvarez, I., Sánchez-Gimeno, C., & Raso, J. (2024). Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces cerevisiae. Foods, 13(12), 1916. https://doi.org/10.3390/foods13121916






