One-Year Monitoring of Prevalence and Diversity of Dairy Propionic Acid Bacteria in Raw Milk by Means of Culture-Dependent and Culture-Independent Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm Selection and Pre-Screening
2.2. Farm Data and Sampling Information
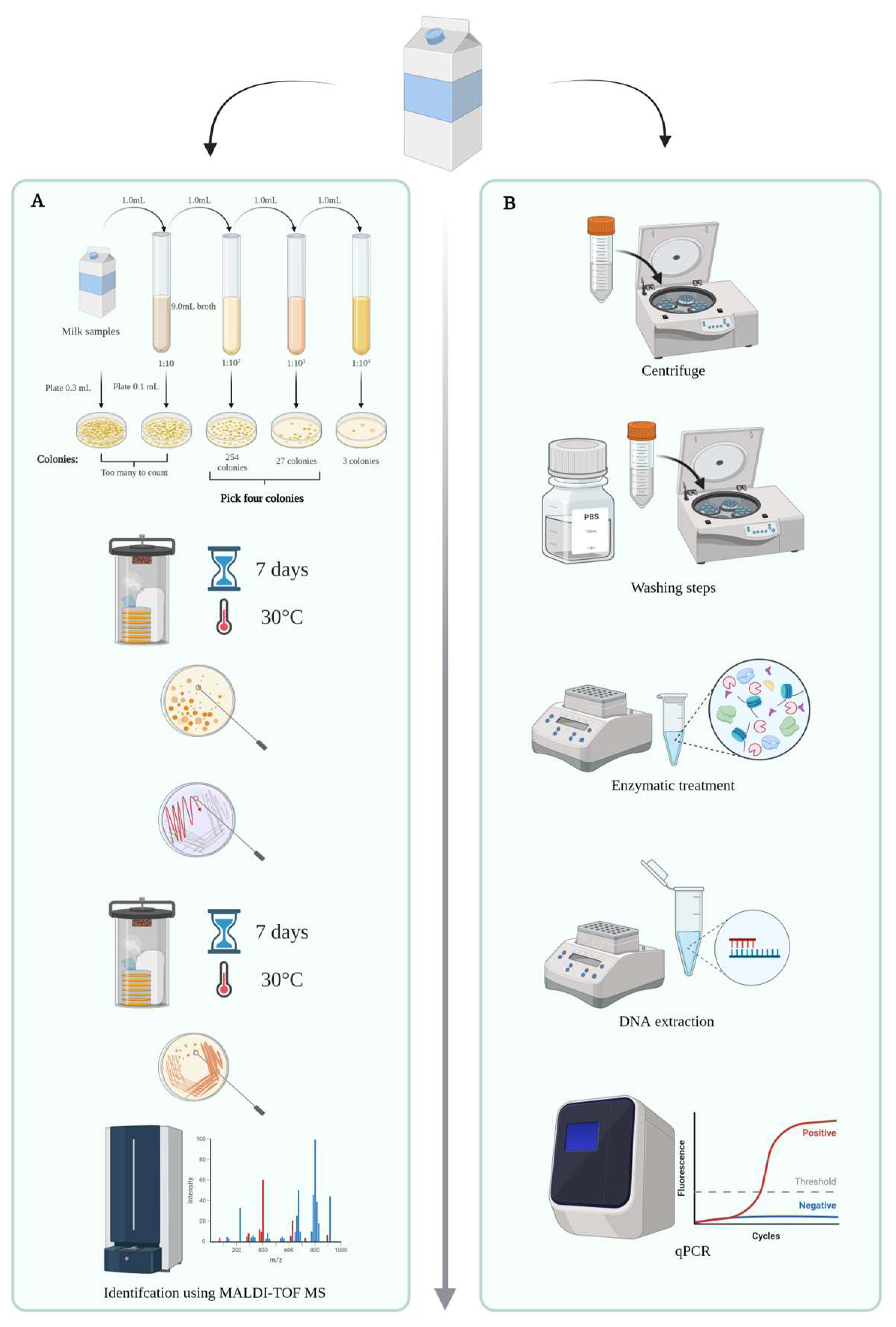
2.3. Milk Screening Using Microbiological Methods
2.4. Milk Screening Using a Culture-Independent Approach
2.5. DNA Extraction
2.6. Creation of a Standard Curve
2.7. Real-Time Quantitative PCR
2.8. Statistical Analysis
3. Results and Discussion
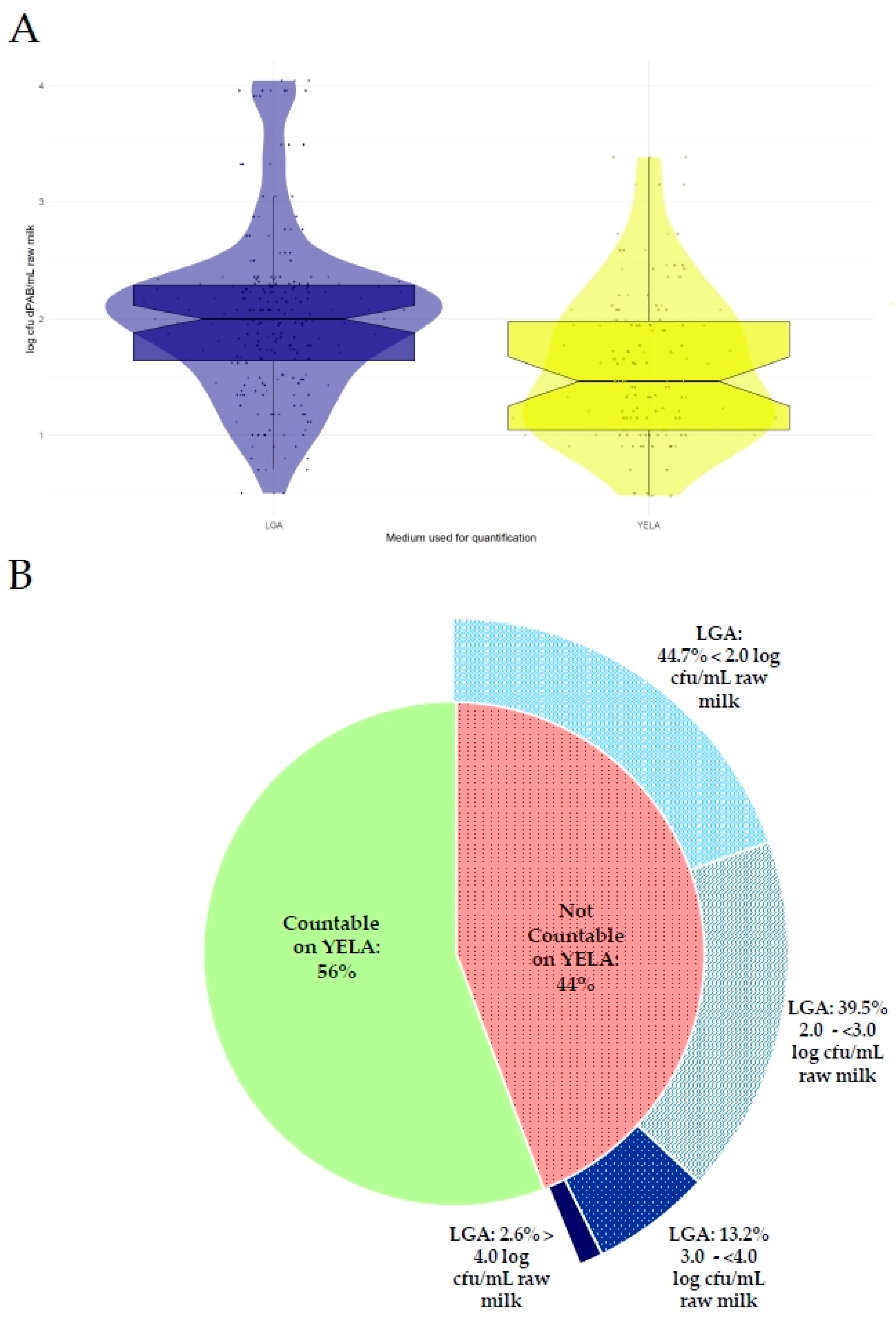
3.1. Comparison of the Culture Media YELA and LGA
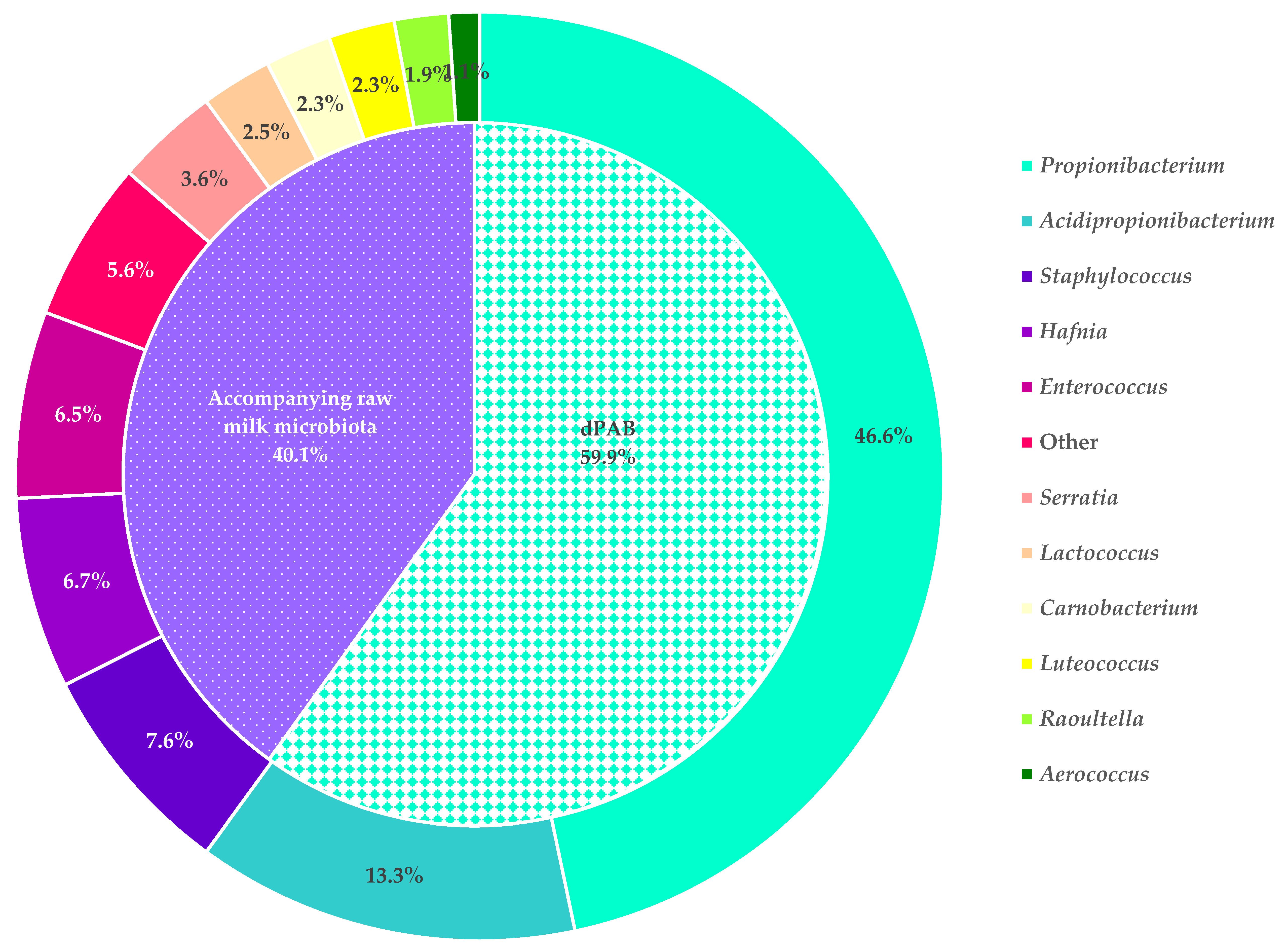
3.2. Comparison of a Cultural and a Culture-Independent Approach for the Long-Term Monitoring of dPAB in Raw Milk
3.3. Relationship between dPAB Levels and Farm Management Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, H.-P.; Fröhlich-Wyder, M.-T.; Jakob, E.; Roth, E.; Wechsler, D.; Beuvier, E.; Buchin, S. Cheese|Raw Milk Cheeses. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 652–660. ISBN 9780123744074. [Google Scholar]
- Turgay, M.; Schaeren, W.; Graber, H.U.; Wagner, E.; Amrein, R.; Bütikofer, U.; Wechsler, D. A field study investigating the effectiveness of vat milk controls by qPCR for the prevention of undesired propionic acid fermentation in Sbrinz PDO cheese. Int. Dairy J. 2018, 77, 80–88. [Google Scholar] [CrossRef]
- Bücher, C.; Burtscher, J.; Rudavsky, T.; Zitz, U.; Domig, K.J. Sources of Propionic Acid Bacteria Contamination in the Milking Parlor Environment on Alpine Dairy Farms. J. Dairy Sci. 2024; accepted. [Google Scholar] [CrossRef]
- Turgay, M.; Bachmann, H.-P.; Irmler, S.; von Ah, U.; Fröhlich-Wyder, M.-T.; Falentin, H.; Deutsch, S.-M.; Jan, G.; Thierry, A. Bacteria, Beneficial: Propionibacterium spp. and Acidipropionibacterium spp. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 34–45. ISBN 9780128187678. [Google Scholar]
- Bücher, C.; Burtscher, J.; Domig, K.J. Propionic acid bacteria in the food industry: An update on essential traits and detection methods. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4299–4323. [Google Scholar] [CrossRef]
- Thierry, A.; Madec, M.N. Enumeration of propionibacteria in raw milk using a new selective medium. Lait 1995, 75, 315–323. [Google Scholar] [CrossRef]
- de Freitas, R.; Luiz, L.M.P.; Alves, M.P.; Valence-Bertel, F.; Nero, L.A.; de Carvalho, A.F. Selective enumeration of propionibacteria in Emmental-type cheese using Petrifilm™ aerobic count plates added to lithium glycerol broth. J. Dairy Res. 2013, 80, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Madec, M.N.; Rouault, A.; Maubois, J.-L.; Thierry, A. Selective Medium and Method for Enumerating Propionic Bacteria. WO9417201A1, 4 August 1994. Available online: https://worldwide.espacenet.com/patent/search/family/009443411/publication/WO9417201A1?q=pn%3DEP0632838B1 (accessed on 10 November 2023).
- Fessler, D.; Casey, M.G.; Puhan, Z. Propionibacteria flora in Swiss raw milk from lowlands and Alps. Lait 1999, 79, 201–209. [Google Scholar] [CrossRef]
- Rossi, F.; Torriani, S.; Dellaglio, F. Identification and clustering of dairy propionibacteria by RAPD-PCR and CGE-REA methods. J. Appl. Microbiol. 1998, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Turgay, M.; Schaeren, W.; Wechsler, D.; Bütikofer, U.; Graber, H.U. Fast detection and quantification of four dairy propionic acid bacteria in milk samples using real-time quantitative polymerase chain reaction. Int. Dairy J. 2016, 61, 37–43. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2011, 150, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.; Lexer, D.; Palme, R.; Troxler, J.; Waiblinger, S. Milking of Brown Swiss and Austrian Simmental cows in a herringbone parlour or an automatic milking unit. Appl. Anim. Behav. Sci. 2004, 88, 209–225. [Google Scholar] [CrossRef]
- Tse, C.; Barkema, H.W.; DeVries, T.J.; Rushen, J.; Pajor, E.A. Effect of transitioning to automatic milking systems on producers’ perceptions of farm management and cow health in the Canadian dairy industry. J. Dairy Sci. 2017, 100, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) 2016/304 of 2 March 2016 Entering a Name in the Register of Traditional Specialities Guaranteed (Heumilch/Haymilk/Latte fieno/Lait de foin/Leche de heno (TSG)): C/2016/1202. 2016. Available online: https://eur-lex.europa.eu/eli/reg_impl/2016/304/oj (accessed on 10 November 2023).
- ARGE Heumilch. Heumilch. Available online: https://www.heumilch.com/heumilch/arge-heumilch/ (accessed on 10 November 2023).
- Food and Agriculture Organization of the United Nations. Hay Milk in Austria Celebrates Its Recognition as FAO Global Agricultural Heritage. Available online: https://www.fao.org/giahs/news/detail-events/en/c/1678826/ (accessed on 15 March 2024).
- ISO 6887-5:2010; Microbiology of the Food Chain. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 5, Specific Rules for the Preparation of Milk and Milk Products: ISO 6887. ISO International Organization for Standardization: Geneva, Switzerland, 2010.
- Malik, A.C.; Reinbold, G.W.; Vedamuthu, E.R. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 1968, 14, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.S.; Metzger, D.A.; Higushi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10(4): 506-13 (April 1991). Biotechniques 2013, 54, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Brändle, J.; Fraberger, V.; Schuller, K.; Zitz, U.; Kneifel, W.; Domig, K.J. A critical assessment of four most probable number procedures for routine enumeration of cheese-damaging clostridia in milk. Int. Dairy J. 2017, 73, 109–115. [Google Scholar] [CrossRef]
- Doyle, C.J.; Gleeson, D.; O’Toole, P.W.; Cotter, P.D. High-throughput metataxonomic characterization of the raw milk microbiota identifies changes reflecting lactation stage and storage conditions. Int. J. Food Microbiol. 2017, 255, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Rudavsky, T.; Zitz, U.; Neubauer, V.; Domig, K.J. Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk. Microorganisms 2023, 11, 1337. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Godden, S.M.; Royster, E.; Timmerman, J.; Boyle, M. Postcalving udder health and productivity in cows approaching dry-off with intramammary infections caused by non-aureus Staphylococcus, Aerococcus, Enterococcus, Lactococcus, and Streptococcus species. J. Dairy Sci. 2021, 104, 6061–6079. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, R.N.; Griffiths, H.M.; Munoz, M.A.; Ahlstrom, C.; Bennett, G.J.; Thomas, E.; Schukken, Y.H. Sources of Klebsiella and Raoultella species on dairy farms: Be careful where you walk. J. Dairy Sci. 2011, 94, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Paul Ross, R.; Fitzgerald, G.F.; Cotter, P.D. A comparison of methods used to extract bacterial DNA from raw milk and raw milk cheese. J. Appl. Microbiol. 2012, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Dairawan, M.; Shetty, J.P. The Evolution of DNA Extraction Methods. Am. J. Biomed. Sci. Res. 2020, 8, 39–45. [Google Scholar] [CrossRef]
- Ricchi, M.; Bertasio, C.; Boniotti, M.B.; Vicari, N.; Russo, S.; Tilola, M.; Bellotti, M.A.; Bertasi, B. Comparison among the Quantification of Bacterial Pathogens by qPCR, dPCR, and Cultural Methods. Front. Microbiol. 2017, 8, 1174. [Google Scholar] [CrossRef] [PubMed]
- Pavšič, J.; Žel, J.; Milavec, M. Assessment of the real-time PCR and different digital PCR platforms for DNA quantification. Anal. Bioanal. Chem. 2016, 408, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Dorn-In, S.; Gareis, M.; Schwaiger, K. Differentiation of live and dead Mycobacterium tuberculosis complex in meat samples using PMA qPCR. Food Microbiol. 2019, 84, 103275. [Google Scholar] [CrossRef] [PubMed]
- Wulsten, I.F.; Galeev, A.; Stingl, K. Underestimated Survival of Campylobacter in Raw Milk Highlighted by Viability Real-Time PCR and Growth Recovery. Front. Microbiol. 2020, 11, 1107. [Google Scholar] [CrossRef]
- Kralik, P.; Beran, V.; Pavlik, I. Enumeration of Mycobacterium avium subsp. paratuberculosis by quantitative real-time PCR, culture on solid media and optical densitometry. BMC Res. Notes 2012, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- de Pinho Manzi, M.; Nóbrega, D.B.; Faccioli, P.Y.; Troncarelli, M.Z.; Menozzi, B.D.; Langoni, H. Relationship between teat-end condition, udder cleanliness and bovine subclinical mastitis. Res. Vet. Sci. 2012, 93, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Sant’anna, A.C.; Da Paranhos Costa, M.J.R. The relationship between dairy cow hygiene and somatic cell count in milk. J. Dairy Sci. 2011, 94, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Mendina, G.R.; Damián, J.P.; Meikle, A.; Chilibroste, P.; Bentancur, O.; Adrien, M.d.L. Udder Hygiene and Mastitis Indicators in Contrasting Environmental Conditions during Half-Time Confinement in Pasture-Based Dairy Systems. Animals 2023, 13, 1544. [Google Scholar] [CrossRef]
- Vacheyrou, M.; Normand, A.-C.; Guyot, P.; Cassagne, C.; Piarroux, R.; Bouton, Y. Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int. J. Food Microbiol. 2011, 146, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hogenboom, J.A.; Pellegrino, L.; Sandrucci, A.; Rosi, V.; D’Incecco, P. Invited review: Hygienic quality, composition, and technological performance of raw milk obtained by robotic milking of cows. J. Dairy Sci. 2019, 102, 7640–7654. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Pereira, J.M.; Amiama, C.; Barrasa, M. Long-term variability of bulk milk somatic cell and bacterial counts associated with dairy farms moving from conventional to automatic milking systems. Ital. J. Anim. Sci. 2018, 17, 218–225. [Google Scholar] [CrossRef]
- Klungel, G.H.; Slaghuis, B.A.; Hogeveen, H. The effect of the introduction of automatic milking systems on milk quality. J. Dairy Sci. 2000, 83, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, D.; Smistad, M.; Bombelli, A.; Abdelghani, A.; Jørgensen, H.J.; Skeie, S.B. Longitudinal Study of the Bulk Tank Milk Microbiota Reveals Major Temporal Shifts in Composition. Front. Microbiol. 2021, 12, 616429. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, P.; Malacarne, M.; Bortolazzo, E.; Coloretti, F.; Formaggioni, P.; Garavaldi, A.; Musi, V.; Summer, A. Automatic Milking Systems in the Production of Parmigiano Reggiano Cheese: Effects on the Milk Quality and on Cheese Characteristics. Agriculture 2022, 12, 104. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.-source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Falentin, H.; Deutsch, S.-M.; Jan, G.; Loux, V.; Thierry, A.; Parayre, S.; Maillard, M.-B.; Dherbécourt, J.; Cousin, F.J.; Jardin, J.; et al. The complete genome of Propionibacterium freudenreichii CIRM-BIA1, a hardy actinobacterium with food and probiotic applications. PLoS ONE 2010, 5, e11748. [Google Scholar] [CrossRef] [PubMed]
- Jakob, E.; Amrein, R.; Turgay, M.; Winkler, H. Propionsäurebakterien in der Rohmilch und deren Bedeutung für die Qualität von Hart- und Halbhartkäse. No. 133. 2016. Available online: https://ira.agroscope.ch/de-CH/publication/36077 (accessed on 16 March 2024).
- Vissers, M.M.M.; Driehuis, F.; Te Giffel, M.C.; de Jong, P.; Lankveld, J.M.G. Minimizing the level of butyric acid bacteria spores in farm tank milk. J. Dairy Sci. 2007, 90, 3278–3285. [Google Scholar] [CrossRef] [PubMed]
- Paiva, N.M.L.; Ribeiro, S.C.; Rosa, H.J.D.; Silva, C.C.G. Comparative study of the bacterial community of organic and conventional cow’s milk. Food Microbiol. 2024, 120, 104488. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited review: Organic and conventionally produced milk-an evaluation of factors influencing milk composition. J. Dairy Sci. 2015, 98, 721–746. [Google Scholar] [CrossRef] [PubMed]
- van den Oever, S.P.; Haselmann, A.; Schreiner, M.; Fuerst-Waltl, B.; Zebeli, Q.; Mayer, H.K.; Knaus, W. Hay versus silage: Does hay feeding positively affect milk composition? Int. Dairy J. 2021, 118, 105024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bücher, C.; Burtscher, J.; Zitz, U.; Domig, K.J. One-Year Monitoring of Prevalence and Diversity of Dairy Propionic Acid Bacteria in Raw Milk by Means of Culture-Dependent and Culture-Independent Methods. Foods 2024, 13, 1921. https://doi.org/10.3390/foods13121921
Bücher C, Burtscher J, Zitz U, Domig KJ. One-Year Monitoring of Prevalence and Diversity of Dairy Propionic Acid Bacteria in Raw Milk by Means of Culture-Dependent and Culture-Independent Methods. Foods. 2024; 13(12):1921. https://doi.org/10.3390/foods13121921
Chicago/Turabian StyleBücher, Carola, Johanna Burtscher, Ulrike Zitz, and Konrad J. Domig. 2024. "One-Year Monitoring of Prevalence and Diversity of Dairy Propionic Acid Bacteria in Raw Milk by Means of Culture-Dependent and Culture-Independent Methods" Foods 13, no. 12: 1921. https://doi.org/10.3390/foods13121921




