Cryoprotective Activity of Different Characterized Fractions Isolated from Enzymatic Hydrolysates of Croceine Croaker (Pseudosciaena crocea)
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Protein Hydrolysate from Croceine Croaker
2.3. Pre-Separation of Enzymatic Hydrolysates of Croceine Croaker by Ultrafiltration (UF)
2.4. Fractionation of Enzymatic Hydrolysates of Croceine Croaker by Solid-Phase Extraction (SPE)
2.5. Sample Pre-Treatment
2.6. Extraction of Turbot Myofibrillar Protein (MFP)
2.7. Determination of Physical Properties
2.7.1. Texture Properties
2.7.2. Moisture Loss
2.7.3. Color
2.7.4. Electronic Nose (E-Nose)
2.8. Determination of MFP Conformation
2.8.1. Fluorescence Spectroscopy
2.8.2. UV Absorption Spectroscopy
2.8.3. Circular Dichroism (CD)
2.9. Scanning Electron Microscope (SEM)
2.10. Determination of Frozen Denaturation and Oxidation of MFP
2.10.1. Particle Size and Zeta Potential
2.10.2. Surface Hydrophobicity
2.10.3. Dityrosine Content
2.10.4. Ca2+-ATPase Activity
2.10.5. Protein Solubility
2.10.6. Total Sulfhydryl Content
2.10.7. Carbonyl Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Texture Properties
3.2. Analysis of Color
3.3. Analyses of Moisture Loss
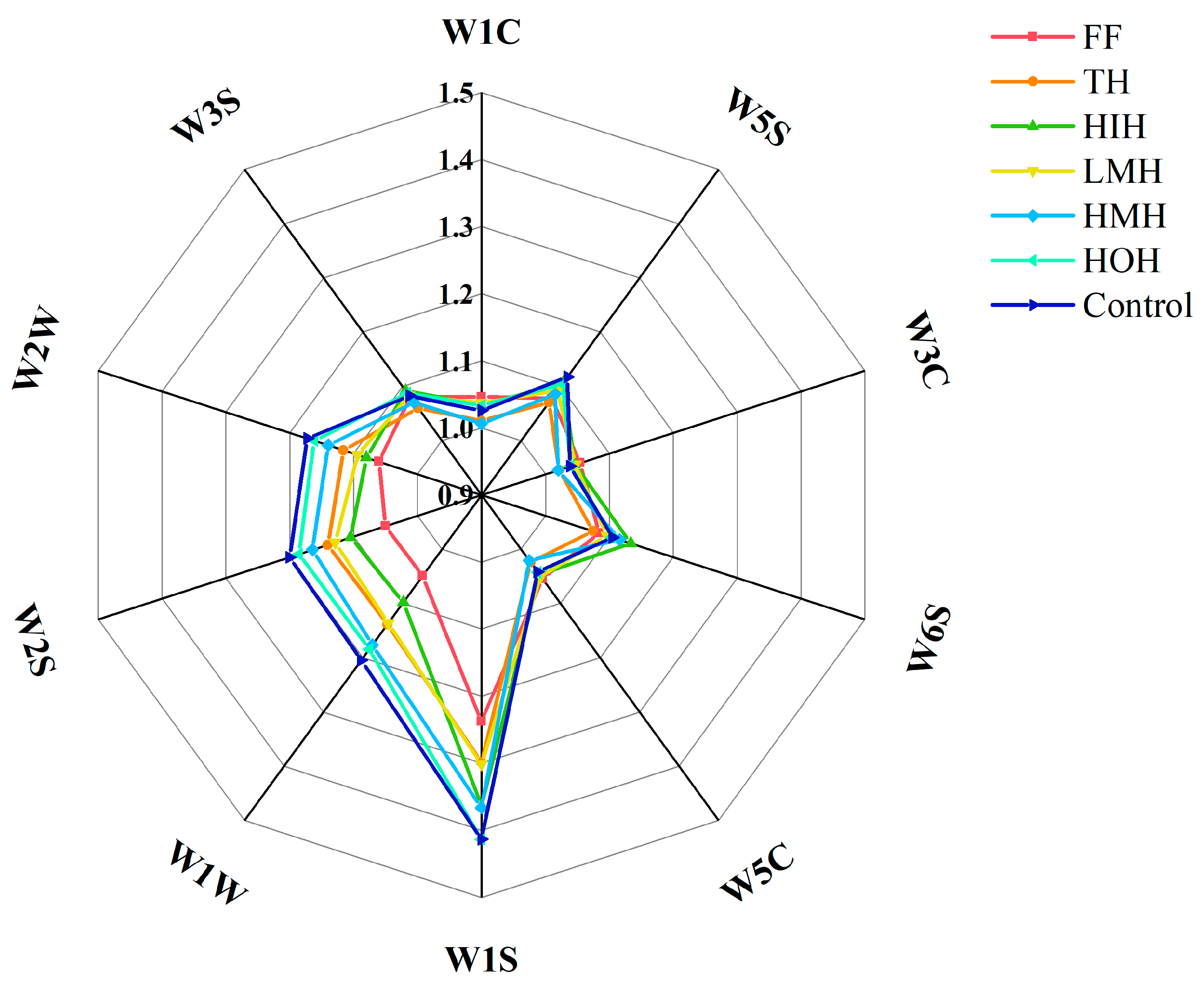
3.4. E-Nose Analysis
3.5. Analysis of MFP Conformational Changes
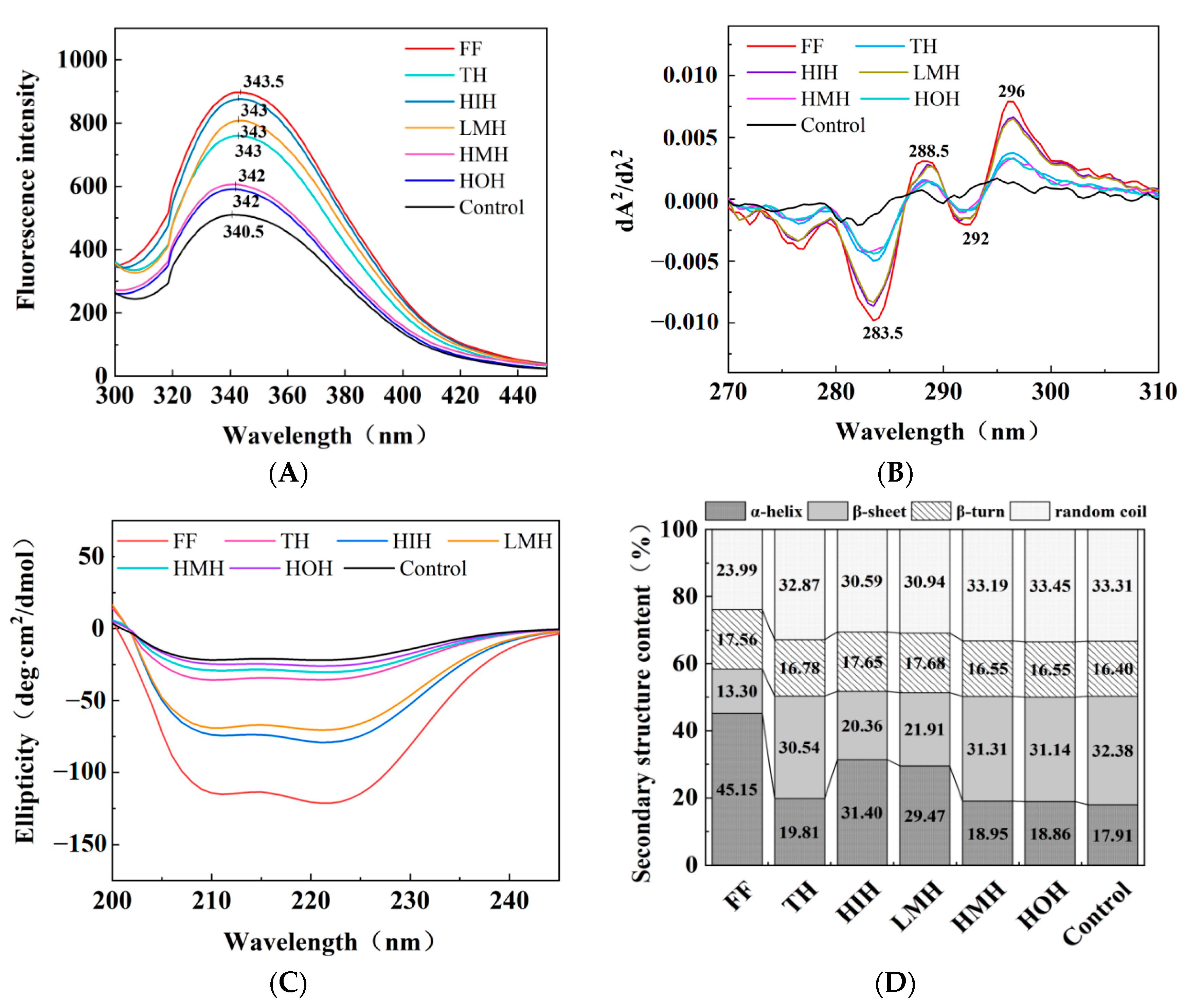
3.5.1. Fluorescence Spectroscopy
3.5.2. UV Absorption Spectroscopy
3.5.3. CD
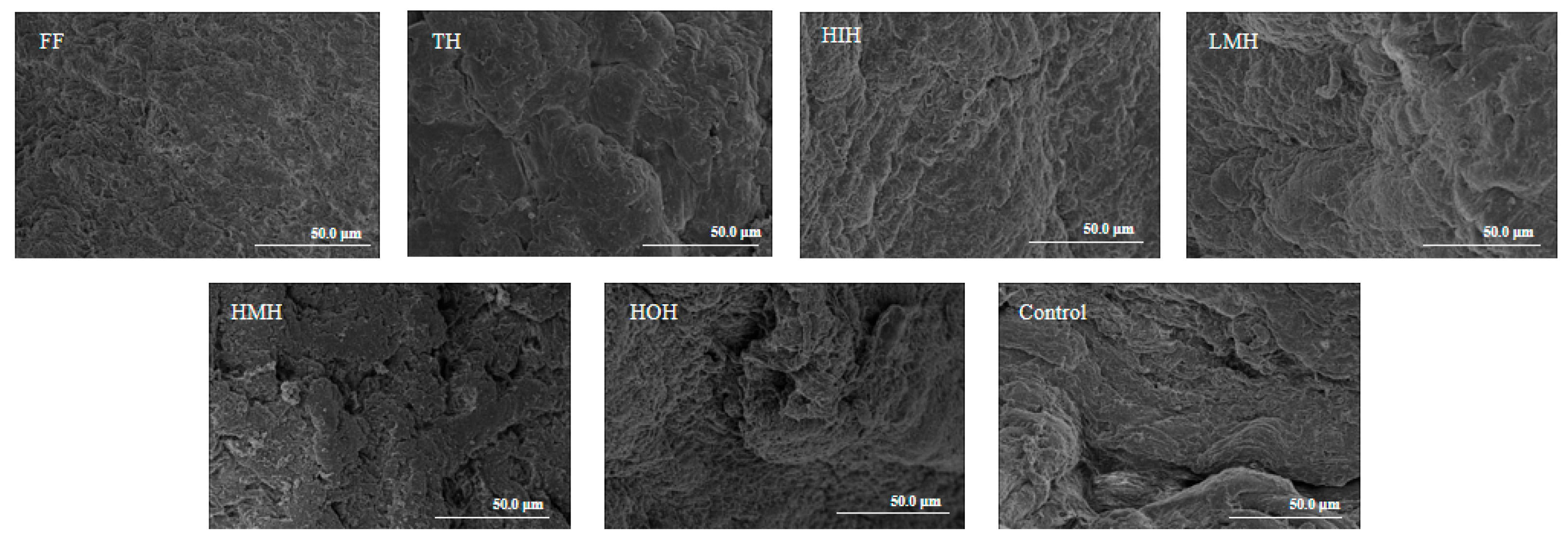
3.6. Analysis of Microstructure
3.7. Analysis of MFP Oxidative Denaturation
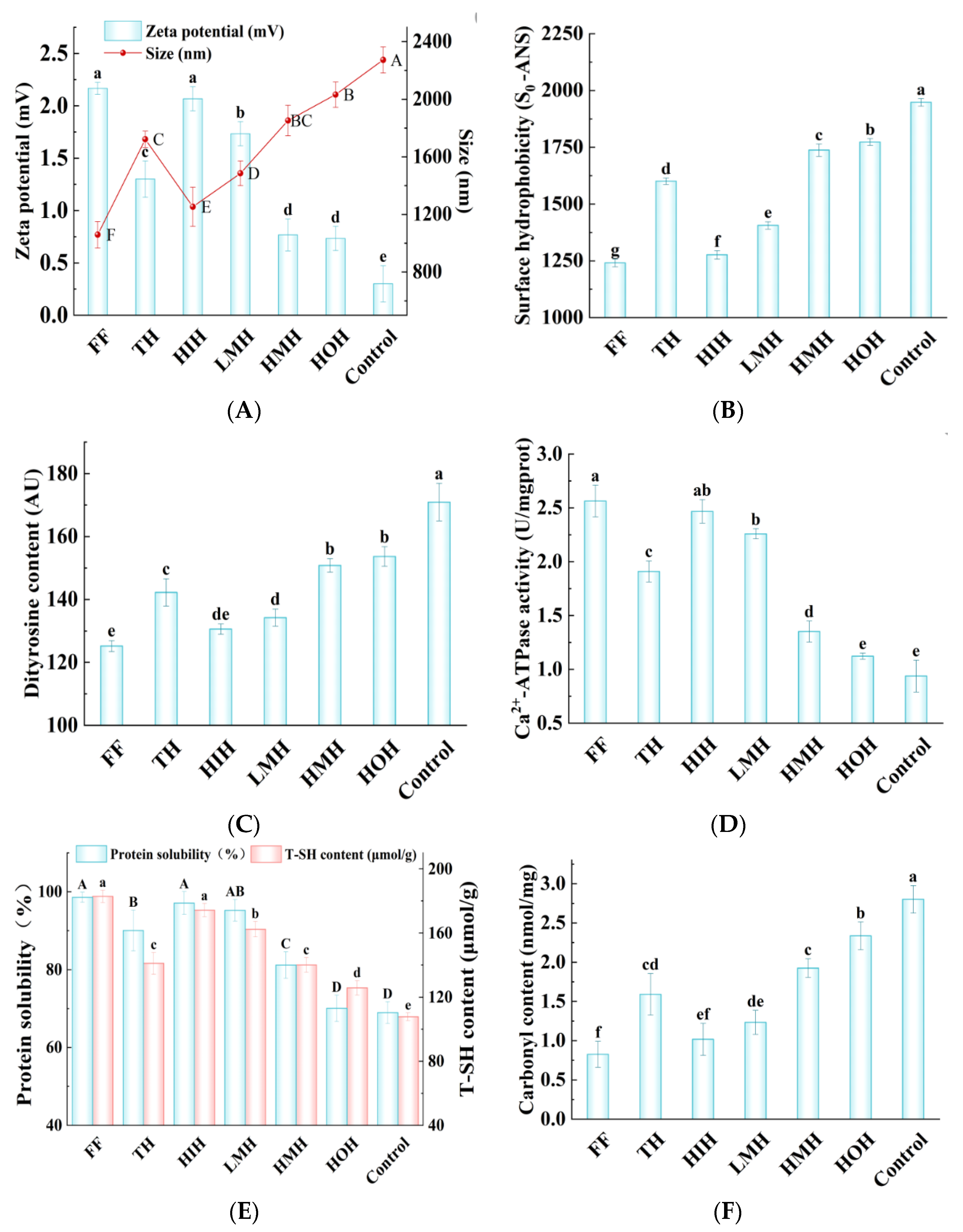
3.7.1. Particle Size and Zeta Potential
3.7.2. So-ANS
3.7.3. Dityrosine Content
3.7.4. Ca2+-ATPase Activity
3.7.5. Protein Solubility
3.7.6. Total Sulfhydryl Content
3.7.7. Carbonyl Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Xue, S.; Han, J.; Yan, J.; Shang, W.; Hong, J.; Wu, H. Simultaneous extraction by acidic and saline solutions and characteristics of the lipids and proteins from large yellow croaker (Pseudosciaena crocea) roes. Food Chem. 2020, 310, 125928. [Google Scholar] [CrossRef]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Kawai, K.; Suzuki, T. Impacts of freezing and molecular size on structure, mechanical properties and recrystallization of freeze-thawed polysaccharide gels. LWT-Food Sci. Technol. 2016, 68, 190–201. [Google Scholar] [CrossRef]
- Hernández, M.; López, M.; Álvarez, A.; Ferrandini, E.; Garcia Garcia, B.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Z.; Sun, D. Naturally sourced biosubstances for regulating freezing points in food researches: Fundamentals, current applications and future trends. Trends Food Sci. Technol. 2019, 95, 131–140. [Google Scholar] [CrossRef]
- Walayat, R.N.; Xiong, H.; Xiong, Z.; Moreno, H.; Nawaz, A.; Niaz, N.; Randhawa, M. Role of cryoprotectants in surimi and factors affecting surimi gel properties: A review. Food Rev. Int. 2020, 38, 1103–1122. [Google Scholar] [CrossRef]
- Yang, F.; Chen, X.; Huang, M.; Yang, Q.; Cai, X.; Chen, X.; Du, M.; Huang, J.; Wang, S. Molecular characteristics and structure–activity relationships of food-derived bioactive peptides. J. Integr. Agric. 2021, 20, 2313–2332. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, J.; Chen, X.; Zhang, Q.; Cai, X.; Ding, Y.; Zhou, X.; Wang, S. Dual cryoprotective strategies for ice-binding and stabilizing of frozen seafood: A review. Trends Food Sci. Technol. 2021, 111, 223–232. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, W.; Luo, W.; Wu, J.; Liu, Y.; Wang, F.; Li, X.; Yu, J.; Wang, S. Component stabilizing mechanism of membrane-separated hydrolysates on frozen surimi. Food Chem. 2024, 431, 137114. [Google Scholar] [CrossRef]
- Wu, J.; Rong, Y.; Wang, Z.; Zhou, Y.; Wang, S.; Zhao, B. Isolation and characterisation of sericin antifreeze peptides and molecular dynamics modelling of their ice-binding interaction. Food Chem. 2015, 174, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhao, Y.; Zhu, Y.; Xu, F.; Yu, J.; Yuan, M. Antifreeze and cryoprotective activities of ice-binding collagen peptides from pig skin. Food Chem. 2016, 194, 1245–1253. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Chen, L.; Zhou, Y.; Wu, J. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT-Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Z.; Tu, M.; Chang, J.; Han, S.; Han, L.; Chen, H.; Tan, Z.; Du, M.; Li, T. Characterizations and the mechanism underlying cryoprotective activity of peptides from enzymatic hydrolysates of Pseudosciaena crocea. Foods 2023, 12, 875. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, S.; Zhu, Z.; Hu, B.; Chen, H.; Tu, M.; Tan, Z.; Du, M.; Li, T. Characterization and the mechanism underlying the cryoprotective activity of a peptide from large yellow croaker (Pseudosciaena crocea). Food Chem. 2024, 435, 137512. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Zhao, G.; Zhang, Y.; Sha, L.; Li, J. Effect of herring antifreeze protein combined with chitosan magnetic nanoparticles on quality attributes in red sea bream (Pagrosomus major). Food Bioprocess Technol. 2018, 12, 409–421. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, F.; Wu, J.; Huang, D.; Huang, J.; Wang, S. Effects and mechanism of antifreeze peptides from silver carp scales on the freeze-thaw stability of frozen surimi. Food Chem. 2022, 396, 133717. [Google Scholar] [CrossRef]
- Du, X.; Chang, P.; Tian, J.; Kong, B.; Sun, F.; Xia, X. Effect of ice structuring protein on the pquality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT-Food Sci. Technol. 2020, 124, 109140. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Jiang, Q. Aggregation and structural changes of silver carp actomyosin as affected by mild acidification with D-gluconic acid delta-lactone. Food Chem. 2012, 134, 1005–1010. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Cao, A.; Regenstein, J.; Li, J.; Guan, R. Ultrasound or microwave vacuum thawing of red seabream (Pagrus major) fillets. Ultrason. Sonochem. 2018, 47, 122–132. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Cao, A.; Zhang, Y.; Li, X. Effect of carboxymethyl chitosan magnetic nanoparticles plus herring antifreeze protein on conformation and oxidation of myofibrillar protein from red sea bream (Pagrosomus major) after freeze-thaw treatment. Food Bioprocess Technol. 2019, 13, 355–366. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation, oxidizing system. Food Chem. 2016, 223, 96–103. [Google Scholar] [CrossRef]
- Davies, K.; Delsignore, M.; Lin, S. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on the effect of the freeze-thawing process on the physicochemical properties and microstructures of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) muscle. Food Chem. 2008, 104, 113–121. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohyd. Polym. 2016, 156, 294–302. [Google Scholar] [CrossRef]
- Deng, Y.; Rosenvold, K.; Karlsson, A.H.; Horn, P.; Hedegaard, J.; Steffensen, C.L.; Andersen, H.J. Relationship between thermal denaturation of porcine muscle proteins and water-holding capacity. J. Food Sci. 2002, 67, 1642–1647. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Chung, H.Y.; Yung, I.K.S.; Ma, W.C.J.; Kim, J.S. Analysis of volatile components in frozen and dried scallops (Patinopecten yessoensis) by gas chromatography-mass spectrometry. Food Res. Int. 2002, 35, 43–53. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, J.; Li, X.; Wang, J.; Yi, S.; Zhu, W.; Xu, Y.; Li, J. Prediction of the freshness of horse mackerel (Trachurus japonicus) using E-nose, E-tongue, and colorimeter based on biochemical indexes analyzed during frozen storage of whole fish. Food Chem. 2023, 402, 134325. [Google Scholar] [CrossRef]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, R.; Zhao, Y.; Zhang, S.; Lin, S. Immunomodulatory sctivity improvement of pine nut peptides by pulsed electric field and their structure activity relationships. J. Agric. Food Chem. 2019, 67, 3796–3810. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R. The effect of Pulsed Electric Fields on the inactivation and structure of lysozyme. Food Chem. 2008, 110, 334–343. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020, 325, 126918. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. The effect of protein concentration and heat treatment temperature on micellar casein–soy protein mixtures. Food Hydrocoll. 2011, 25, 1448–1460. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Zhao, X.; Wan, W.; Du, X.; Kong, B.; Xia, X. Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT-Food Sci. Technol. 2021, 137, 110403. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; Augustin, M.A. Deamidated wheat protein–dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocoll. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Liu, J.; Fang, C.; Luo, Y.; Ding, Y.; Liu, S. Effects of konjac oligo-glucomannan on the physicochemical properties of frozen surimi from red gurnard (Aspitrigla cuculus). Food Hydrocoll. 2018, 89, 668–673. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Tu, Z.; Ma, D.; Wang, H.; Huang, X.; He, N. Effect of γ-irradiation on the physicochemical properties and structure of fish myofibrillar proteins. Radiat. Phys. Chem. 2015, 109, 70–72. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Portinaro, N.; Badalamenti, S.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation. BBA-Gen. Subj. 2015, 1850, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jantakoson, T.; Thavaroj, W.; Konno, K. Myosin and actin denaturation in frozen stored kuruma prawn Marsupenaeus japonicus myofibrils. Fish. Sci. 2013, 79, 341–347. [Google Scholar] [CrossRef]
- Ko, W.C.; Shi, H.; Chang, C.; Huang, Y.; Chen, Y.; Hsieh, C.W. Effect of adjustable parallel high voltage on biochemical indicators and actomyosin Ca2+-ATPase from tilapia (Orechromis niloticus). LWT-Food Sci. Technol. 2016, 69, 417–423. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Li-Chan, E.C.Y. Structural changes in natural actomyosin and surimi from ling cod (Ophiodon elongatus) during frozen storage in the absence or presence of cryoprotectants. J. Agric. Food Chem. 2001, 49, 4716–4725. [Google Scholar] [CrossRef]
- Zhou, A.; Benjakul, S.; Pan, K.; Gong, J.; Liu, X. Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodon nilotica) surimi during frozen storage. Food Chem. 2006, 96, 96–103. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Li, D.; Tan, Z.; Liu, Z.; Wu, C.; Liu, H.; Guo, C.; Zhou, D. Effect of hydroxyl radical induced oxidation on the physicochemical and gelling properties of shrimp myofibrillar protein and its mechanism. Food Chem. 2021, 351, 129344. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone—An emerging technology for the seafood industry. Braz. Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Lundi, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
| Samples | Hardness (g) | Elastic (mm) | Cohesion (g.s) | Adhesion (g) | Chewiness (mJ) | Reversibility (mm) |
|---|---|---|---|---|---|---|
| FF | 65.60 ± 6.25 a | 1.00 ± 0.08 a | 3.32 ± 0.06 a | 18.73 ± 3.66 b | 18.43 ± 4.03 c | 0.41 ± 0.01 a |
| TH | 44.65 ± 5.43 c | 0.96 ± 0.06 ab | 3.24 ± 0.05 b | 24.41 ± 4.38 b | 22.98 ± 5.20 bc | 0.38 ± 0.04 ab |
| LMH | 46.17 ± 5.47 c | 0.97 ± 0.05 ab | 3.25 ± 0.05 b | 22.77 ± 4.41 b | 21.33 ± 5.24 bc | 0.39 ± 0.03 ab |
| HIH | 57.62 ± 4.98 b | 0.98 ± 0.04 ab | 3.26 ± 0.02 b | 20.61 ± 3.05 b | 19.42 ± 3.29 bc | 0.40 ± 0.02 a |
| HMH | 40.78 ± 5.34 cd | 0.95 ± 0.05 ab | 3.23 ± 0.02 b | 26.58 ± 4.29 a | 23.08 ± 3.18 ab | 0.37 ± 0.03 ab |
| HOH | 39.30 ± 5.30 cd | 0.93 ± 0.06 ab | 3.21 ± 0.02 b | 27.19 ± 4.26 a | 24.72 ± 3.16 ab | 0.37 ± 0.03 ab |
| Control | 36.95 ± 6.26 d | 0.92 ± 0.07 b | 3.18 ± 0.05 c | 28.64 ± 4.63 a | 25.87 ± 4.53 a | 0.37 ± 0.02 b |
| Samples | L* | a* | b* | Thawing Loss (%) | Cooking Loss (%) | Centrifugal Loss (%) |
|---|---|---|---|---|---|---|
| FF | 56.07 ± 0.51 a | 2.78 ± 0.10 a | 1.49 ± 0.12 e | -- | 5.65 ± 0.65 d | 2.35 ± 0.32 e |
| TH | 47.65 ± 5.43 c | 0.96 ± 0.06 ab | 3.24 ± 0.05 b | 7.30 ± 0.55 d | 9.78 ± 0.45 b | 5.29 ± 0.85 c |
| LMH | 52.28 ± 0.91 b | 1.74 ± 0.05 b | 2.42 ± 0.13 d | 5.64 ± 0.49 e | 7.61 ± 1.82 c | 4.24 ± 0.09 cd |
| HIH | 54.71 ± 0.92 a | 2.06 ± 0.05 b | 2.08 ± 0.09 d | 5.61 ± 0.40 e | 6.81 ± 0.13 cd | 3.95 ± 0.23 d |
| HMH | 46.51 ± 0.97 d | 1.47 ± 0.14 c | 3.25 ± 0.05 b | 9.10 ± 0.60 c | 11.11 ± 0.25 b | 6.44 ± 0.34 b |
| HOH | 44.88 ± 0.98 c | 1.38 ± 0.14 d | 3.18 ± 0.05 b | 11.24 ± 0.52 b | 11.12 ± 0.60 b | 6.85 ± 0.76 a |
| Control | 42.38 ± 0.80 e | 1.13 ± 0.06 d | 3.52 ± 0.18 a | 12.60 ± 0.86 a | 13.22 ± 0.22 a | 7.70 ± 0.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Cao, S.; Cui, N.; Zhang, R.; Zhao, S.; Zhang, L.; Guan, S.; Xu, Y.; Yan, X.; Zhu, Z.; et al. Cryoprotective Activity of Different Characterized Fractions Isolated from Enzymatic Hydrolysates of Croceine Croaker (Pseudosciaena crocea). Foods 2024, 13, 1946. https://doi.org/10.3390/foods13121946
Xu Z, Cao S, Cui N, Zhang R, Zhao S, Zhang L, Guan S, Xu Y, Yan X, Zhu Z, et al. Cryoprotective Activity of Different Characterized Fractions Isolated from Enzymatic Hydrolysates of Croceine Croaker (Pseudosciaena crocea). Foods. 2024; 13(12):1946. https://doi.org/10.3390/foods13121946
Chicago/Turabian StyleXu, Zhe, ShengAo Cao, Na Cui, Rui Zhang, Shuang Zhao, Lijuan Zhang, Shuang Guan, Yikun Xu, Xu Yan, Zhixuan Zhu, and et al. 2024. "Cryoprotective Activity of Different Characterized Fractions Isolated from Enzymatic Hydrolysates of Croceine Croaker (Pseudosciaena crocea)" Foods 13, no. 12: 1946. https://doi.org/10.3390/foods13121946
APA StyleXu, Z., Cao, S., Cui, N., Zhang, R., Zhao, S., Zhang, L., Guan, S., Xu, Y., Yan, X., Zhu, Z., Tan, Z., & Li, T. (2024). Cryoprotective Activity of Different Characterized Fractions Isolated from Enzymatic Hydrolysates of Croceine Croaker (Pseudosciaena crocea). Foods, 13(12), 1946. https://doi.org/10.3390/foods13121946







