Kinetic Evaluation of the Production of Mead from a Non-Saccharomyces Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey
2.2. Yeast
2.3. The Honey-Based Culture Media
2.4. Preparation of the Inoculum
2.5. Evaluation of Fermentation in Different Culture Media to Produce Mead
2.6. Sensory Testing
2.7. Overall Yield and Kinetic Modeling of the Yeast Biomass, Substrate Consumption, and Ethanol Concentration
| Model | Equation | |
|---|---|---|
| Logistic model [36] | (6) | |
| Gompertz model, modified and re-parameterized by Norton [37] | (7) | |
2.8. Analytical Methods
2.8.1. Determinations Made to Monitor the Kinetics of Mead Production
2.8.2. Evaluation of the Physicochemical and Microbiological Characteristics of the Mead
2.8.3. Analysis of Mid-Infrared Spectroscopy
2.9. Establishment of the Parameters of the Kinetic Models and Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Fermentation in Different Culture Media
3.2. Sensory Test of the Final Products Obtained with M2
3.3. Comparison of the Sensorial Attributes of the Mead from M2–23 with Those of a Commercial Sample
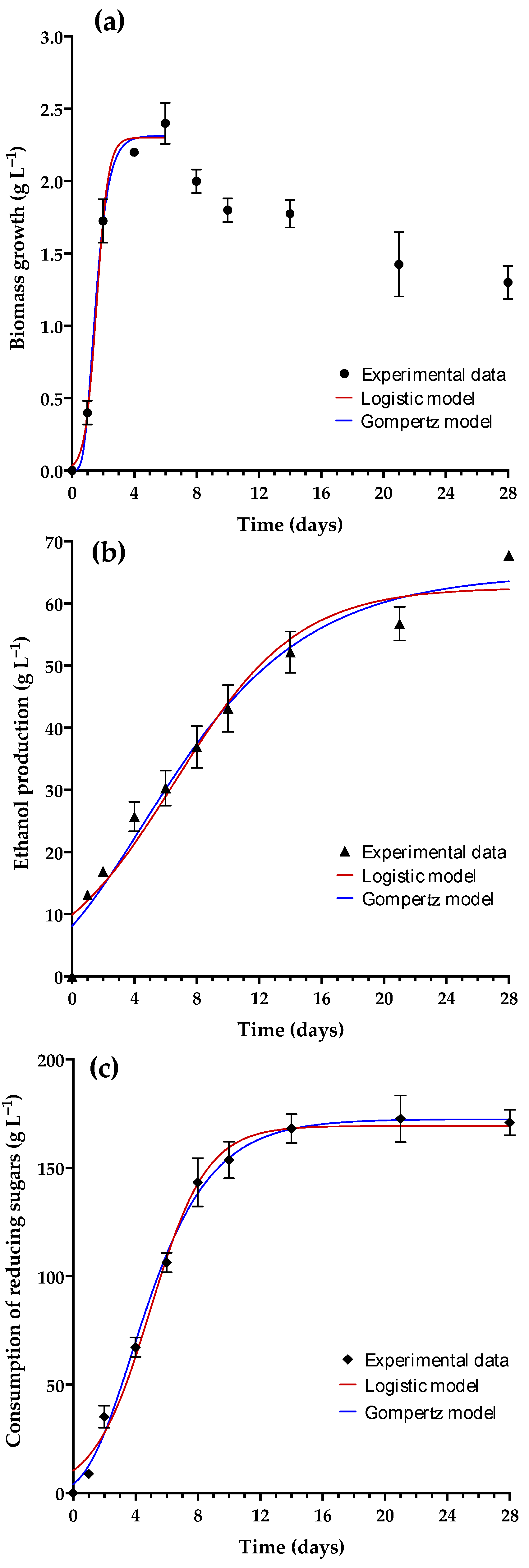
3.4. The Kinetic Parameters Used to Characterize the Mead Derived from M2–23
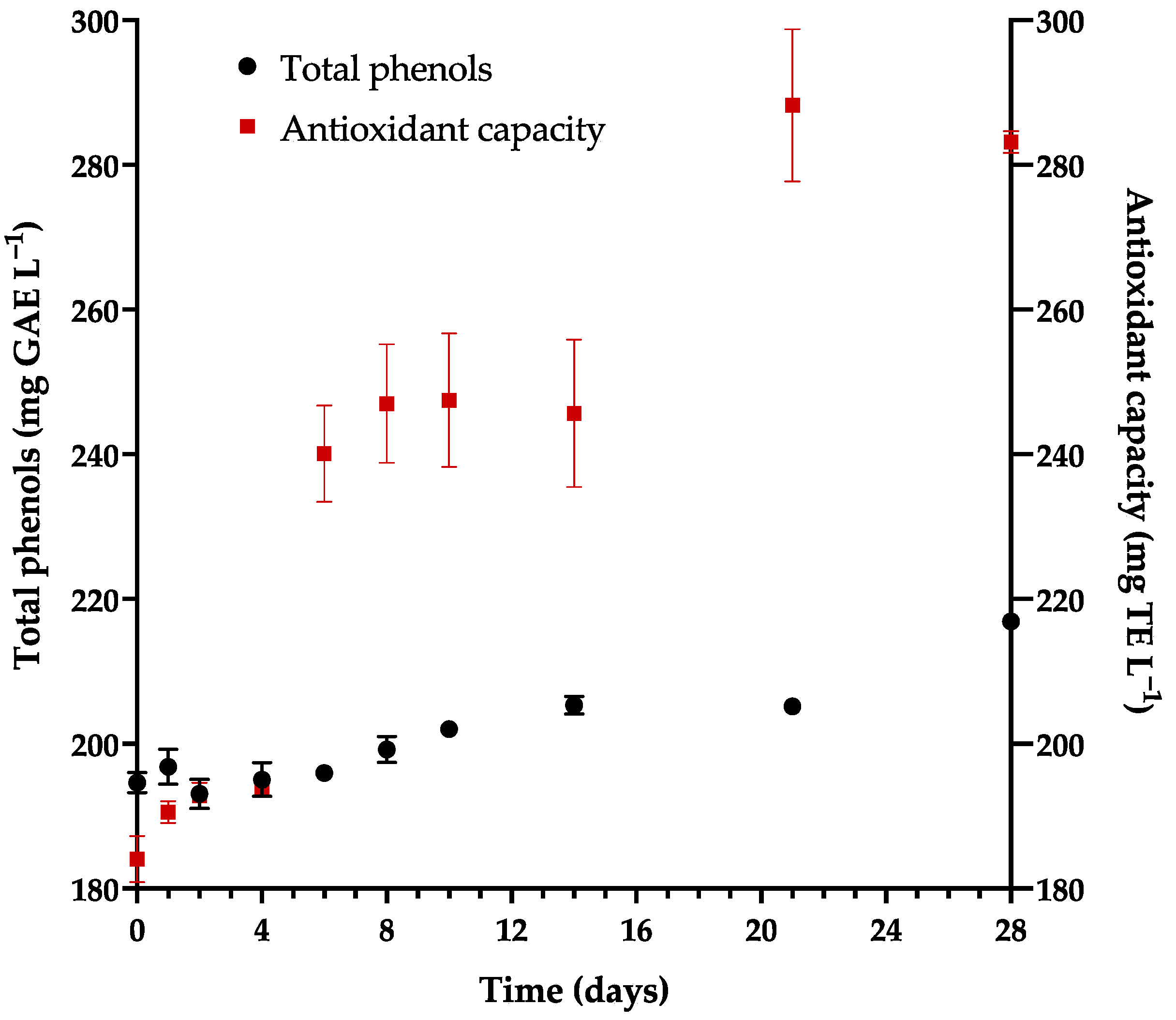
3.5. Kinetics of Antioxidants during Fermentation
3.6. Characteristics of Mead Derived from the M2–23 Medium
| Characteristic | Result | Specification/Reported Range | Reference |
|---|---|---|---|
| pH | 3.51 ± 0.015 | 2.49–4.2 | [73,74,75,76] |
| Density (g cm−3) | 1.012 ± 0.001 | 0.9757–1.293 | [73,77] |
| Ethanol (% v/v) | 8.57 ± 0.03 | 8–18 | [8,25,42] |
| Methanol (mg 100 mL−1 AA) | <DL | <300 | [78] |
| Higher alcohols (mg 100 mL−1 AA) | 122 ± 0.20 | 58–129 | [25,73] |
| Aldehydes (mg 100 mL−1 AA) | 13.46 ± 0.11 | 6.2–125.5 | [63,73] |
| Esters (mg 100 mL−1 AA) | 332.09 ± 0.23 | 24.72–317 | [8,79] |
| Total acidity (g TA L−1) | 2.812 ± 0.14 | 2.2–7.7 | [42,63,73,76] |
| Total sulfur dioxide (mg L−1) | 6.58 ± 0.02 | 5–275 | [76] |
| Free sulfur dioxide (mg L−1) | 5.35 ± 0.05 | 1–61 | [20,76] |
| Reducing sugars (g L−1) | 82.91 ± 6.16 | > 50 | [78] |
| Antioxidant capacity (mM TE L−1) | 1.13 ± 0.005 | 1.39–4.13 | [15,71,72] |
| Total phenols (mg GAE L−1) | 216.9 ± 0.51 | 210.5–303.2 | [15,71] |
| Lead (mg L−1) | <LQ | <0.5 | [78] |
| Arsenic (mg L−1) | <LQ | <0.5 | [78] |
| Fungi (CFUs mL−1) | Absent | Absent | [51] |
| Yeast (CFUs mL−1) | Absent | Absent | [51] |
| Total coliform bacteria (CFUs mL−1) | Absent | Absent | [52] |
| Aerobic mesophiles (CFUs mL−1) | Absent | Absent | [50] |
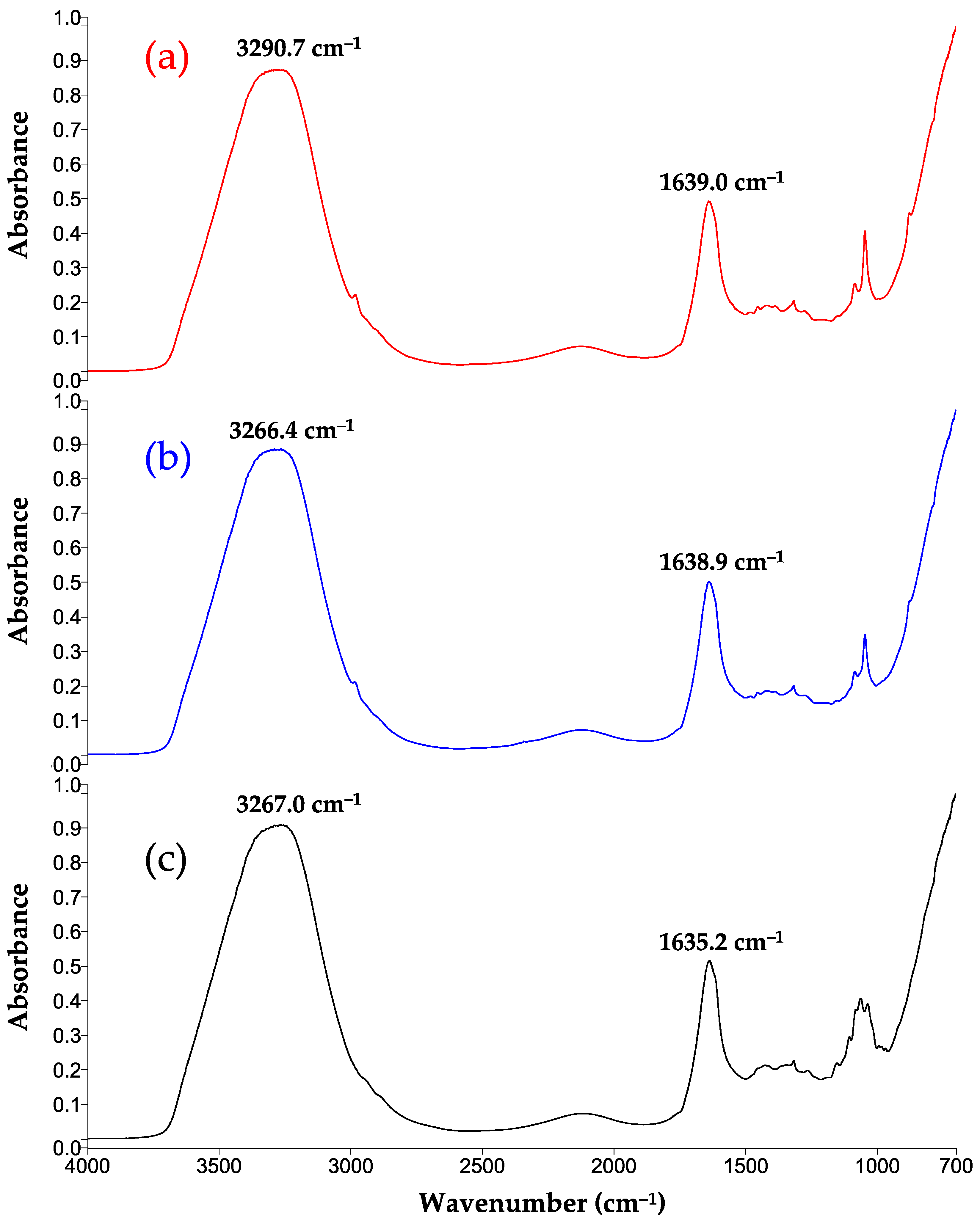
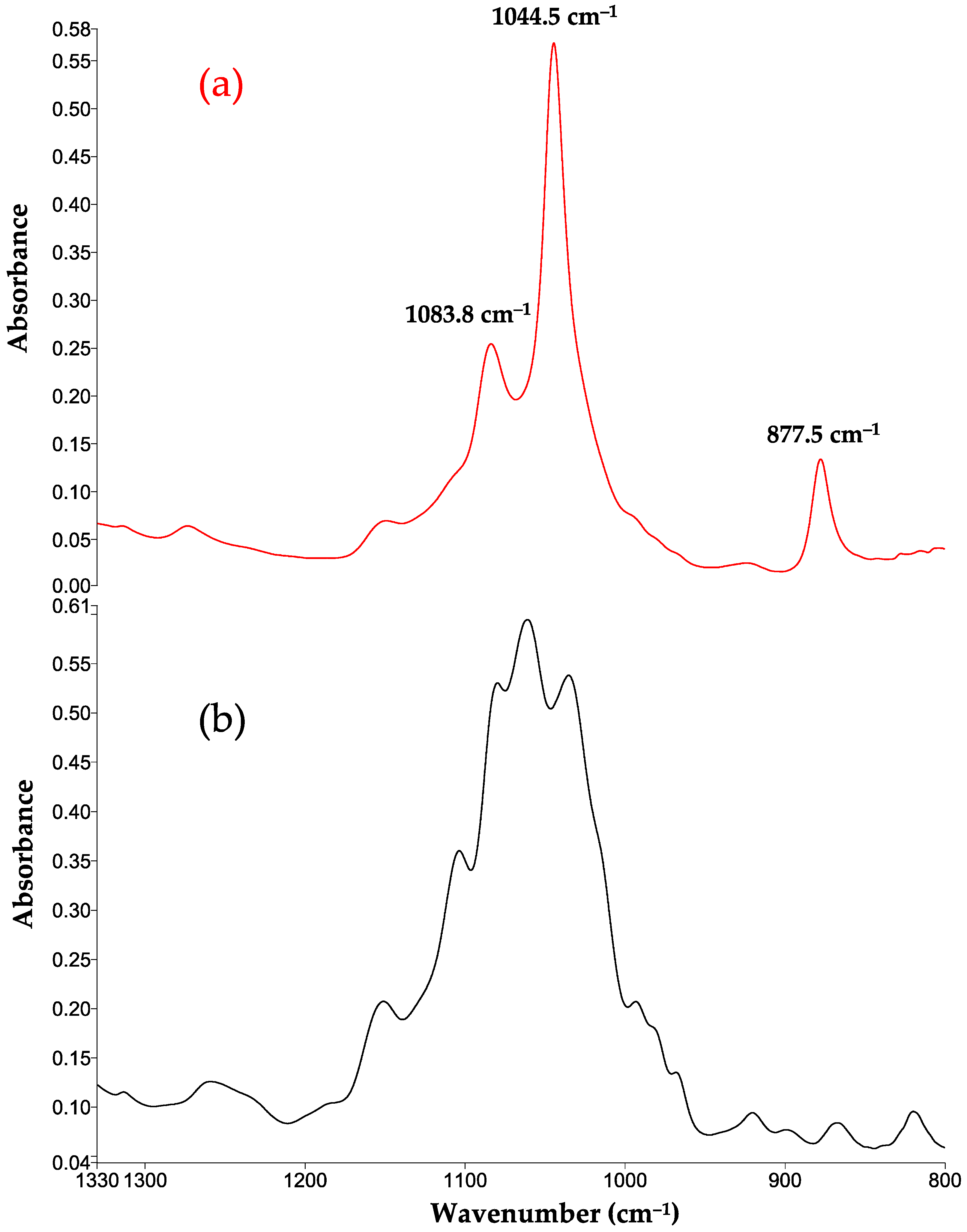
3.7. FTIR-MIR Spectra
| Functional Group | Origin of the Vibration | Position of the Bands a (cm−1) | ||
|---|---|---|---|---|
| M2–23 before Inoculation | The Present Mead | A Commercial Mead | ||
| O-H ν | Ethanol and water | 3267 | 3290.7 | 3266.4 |
| C-H ν | Alkyl groups (-CH3, -CH2) | - | 2984.4 | 2986.3 |
| C=O ν O-H δ | Organic acids Ethanol and water | 1635.2 | 1639.0 | 1638.9 |
| -O-CH2- δ | Esters | - | 1453.5 | 1454.4 |
| Aldehydes | 1424.4 | - | - | |
| O-H δ | In the C-OH group | - | 1418.9 | 1419.3 |
| C-H δ | Alkenes | 1316.0 | 1316.2 | 1316.3 |
| C-H ν C-O ν | Carbohydrates organic acids | 1261.7 | 1274 | 1272 |
| -C–O–C- ν | Carbohydrates such as glucose and fructose | 1152 | 1153 | 1151 |
| C–OH ν | Carbohydrates Organic acids | 1103.9 | - | - |
| CH-OH ν | Carbohydrates Ethanol | 1080.6 | 1083.8 | 1083.7 |
| CH2–OH ν | Carbohydrates | 1060.7 | - | - |
| C-OH δ | Ethanol | - | 1044.5 | 1044.8 |
| 1034.9 | - | - | ||
| α-pyranose ring sym | Carbohydrates | 993.2 | - | - |
| C-OH ν | Carbohydrates | 920 | - | - |
| C-C ν | Disaccharides | 899.1 | - | - |
| -CH3 δ | Ethanol | 877.5 | 878.7 | |
| C-H δ | Carbohydrates | 867.1 | - | - |
| 819.1 | 816.3 | - | ||
| α-pyranose ring | Disaccharides | 779.7 | 780.4 | 782.8 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey Antibacterial Activity: A Neglected Aspect of Honey Quality Assurance as Functional Food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J. Mead: The Oldest Alcoholic Beverage. In Traditional Foods: General and Consumer Aspects; Kristbergsson, K., Oliveira, J., Eds.; Integrating Food Science and Engineering Knowledge into the Food Chain; Springer: Boston, MA, USA, 2016; pp. 325–338. ISBN 978-1-4899-7648-2. [Google Scholar]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single Food Stuff Comprises Many Drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Leading Producers of Honey Worldwide 2021. Available online: https://www.statista.com/statistics/812172/global-top-producers-of-honey/ (accessed on 20 May 2023).
- Navrátil, M.; Šturdík, E.; Gemeiner, P. Batch and Continuous Mead Production with Pectate Immobilised, Ethanol-Tolerant Yeast. Biotechnol. Lett. 2001, 23, 977–982. [Google Scholar] [CrossRef]
- Starowicz, M.; Granvogl, M. Trends in Food Science & Technology an Overview of Mead Production and the Physicochemical, Toxicological, and Sensory Characteristics of Mead with a Special Emphasis on Flavor. Trends Food Sci. Technol. 2020, 106, 402–416. [Google Scholar] [CrossRef]
- Cavaco, T.; Figueira, A.C. Functional Properties of Honey and Some Traditional Honey Products from Portugal. In Functional Properties of Traditional Foods; Kristbergsson, K., Ötles, S., Eds.; Integrating Food Science and Engineering Knowledge into the Food Chain; Springer: Boston, MA, USA, 2016; pp. 339–352. ISBN 978-1-4899-7662-8. [Google Scholar]
- Sottil, C.; Salor-Torregrosa, J.M.; Moreno-Garcia, J.; Peinado, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Using Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus Wine Yeasts as Starter Cultures for Fermentation and Quality Improvement of Mead. Eur. Food Res. Technol. 2019, 245, 2705–2714. [Google Scholar] [CrossRef]
- Van Mullem, J.J.; Zhang, J.; Dias, D.R.; Schwan, R.F. Using Wild Yeasts to Modulate the Aroma Profile of Low-Alcoholic Meads. Braz. J. Microbiol. 2022, 53, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Ramalhosa, E.; Gomes, T.; Pereira, A.P.; Dias, T.; Estevinho, L.M. Chapter 4—Mead Production: Tradition versus Modernity. In Advances in Food and Nutrition Research; Jackson, R.S., Ed.; Speciality Wines; Academic Press: Cambridge, MA, USA, 2011; Volume 63, pp. 101–118. [Google Scholar]
- Mărgăoan, R.; Cornea-Cipcigan, M.; Topal, E.; Kösoğlu, M. Impact of Fermentation Processes on the Bioactive Profile and Health-Promoting Properties of Bee Bread, Mead and Honey Vinegar. Processes 2020, 8, 1081. [Google Scholar] [CrossRef]
- MarketWatch Mead Beverage Market Size, Share, Demands, Growth Analysis, Company Profiles, Revenue and Forecast by 2028. Available online: https://www.marketwatch.com/press-release/mead-beverage-market-size-share-demands-growth-analysis-company-profiles-revenue-and-forecast-by-2028-2023-03-14 (accessed on 20 May 2023).
- Amorim, T.S.; Lopes, S.D.B.; Bispo, J.A.C.; Bonafe, C.F.S.; de Carvalho, G.B.M.; Martínez, E.A. Influence of Acerola Pulp Concentration on Mead Production by Saccharomyces cerevisiae AWRI 796. LWT 2018, 97, 561–569. [Google Scholar] [CrossRef]
- Balogu, T.V.; Towobola, O. Production and Quality Analysis of Wine from Honey and Coconut Milk Blend Using Saccharomyces cerevisiae. Fermentation 2017, 3, 16. [Google Scholar] [CrossRef]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of Mead Wort Heat Treatment on the Mead Fermentation Process and Antioxidant Activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef] [PubMed]
- Jose-Salazar, J.A.; Morales-Barrera, L.; Andrade-Velásquez, A.; Hernández-Rodríguez, D.; Melgar-Lalanne, G. Effect of Thermal and Non-Thermal Preservation Methods on Bioactivity and Quality of Apis mellifera Honey for Its Use as Ingredient for Beverages. In Improving Health and Nutrition through Functional Foods: Benefits and Applications. Elsevier: Amsterdam, The Netherlands, Unpublished Work.
- Sroka, P.; Tarko, T.; Duda, A. The Impact of Furfural on the Quality of Meads. Molecules 2024, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.R. 125th Anniversary Review: Improvement of Higher Gravity Brewery Fermentation via Wort Enrichment and Supplementation. J. Inst. Brew. 2011, 117, 268–284. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sharma, R. Production Technology and Quality Characteristics of Mead and Fruit-Honey Wines: A Review. Nat. Prod. Rad. 2009, 8, 345–355. [Google Scholar]
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of Honey-Must Preparation and Alcoholic Fermentation by Saccharomyces cerevisiae for Mead Production. Int. J. Food Microbiol. 2010, 144, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Mendes-Ferreira, A.; Estevinho, L.M.; Mendes-Faia, A. Improvement of Mead Fermentation by Honey-Must Supplementation. J. Inst. Brew. 2015, 121, 405–410. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of Nitrogen Nutrition for Grapes, Fermentation and Wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Schwarz, L.V.; Marcon, A.R.; Delamare, A.P.L.; Echeverrigaray, S. Influence of Nitrogen, Minerals and Vitamins Supplementation on Honey Wine Production Using Response Surface Methodology. J. Apic. Res. 2021, 60, 57–66. [Google Scholar] [CrossRef]
- Matraxia, M.; Alfonzo, A.; Prestianni, R.; Francesca, N.; Gaglio, R.; Todaro, A.; Alfeo, V.; Perretti, G.; Columba, P.; Settanni, L.; et al. Non-Conventional Yeasts from Fermented Honey by-Products: Focus on Hanseniaspora uvarum Strains for Craft Beer Production. Food Microbiol. 2021, 99, 103806. [Google Scholar] [CrossRef]
- Li, R.; Sun, Y. Effects of Honey Variety and Non-Saccharomyces cerevisiae on the Flavor Volatiles of Mead. J. Am. Soc. Brew. Chem. 2019, 77, 40–53. [Google Scholar] [CrossRef]
- Nieto-Sarabia, V.L.; Ballinas-Cesatti, C.B.; Melgar-Lalanne, G.; Cristiani-Urbina, E.; Morales-Barrera, L. Isolation, Identification, and Kinetic and Thermodynamic Characterization of a Pichia kudriavzevii Yeast Strain Capable of Fermentation. Food Bioprod. Process. 2022, 131, 109–124. [Google Scholar] [CrossRef]
- Nieto-Sarabia, V.L.; Melgar-Lalanne, G.; Ballinas-Cesatti, C.B.; García-García, F.A.; Jose-Salazar, J.A.; Flores-Ortiz, C.M.; Cristiani-Urbina, E.; Morales-Barrera, L. Brewing a Craft Belgian-Style Pale Ale Using Pichia kudriavzevii 4A as a Starter Culture. Microorganisms 2023, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungus 2023, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, V.; Radecka, D.; Aerts, G.; Verstrepen, K.J.; Lievens, B.; Thevelein, J.M. Phenotypic Landscape of Non-Conventional Yeast Species for Different Stress Tolerance Traits Desirable in Bioethanol Fermentation. Biotechnol. Biofuels 2017, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A.S.; Ilyina, A.; Ramos-González, R.; Aguilar, C.N.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; González, M.L.C.; Aguilar, M.; Ballesteros, M.; Oliva, J.M.; et al. Ethanol Production from Banana Peels at High Pretreated Substrate Loading: Comparison of Two Operational Strategies. Biomass Conv. Bioref. 2021, 11, 1587–1596. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, J.; Yang, H.; Lei, X.; Jiang, J.; Song, Y.; Qin, Y.; Liu, Y.-L. Fungal Dynamic during Apricot Wine Spontaneous Fermentation and Aromatic Characteristics of Pichia kudriavzevii for Potential as Starter. Food Chem. X 2023, 19, 100862. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mandal, S.D.; Nath, N.; Kaipeng, D.L.; Rahaman, L.; Nachimuthu, S.K.; Sharma, B.K. Isolation and Partial Evaluation of a Potential Indigenous Yeast Strain Pichia kudriavzevii from a Traditional Rice Beer—“Gora” Prepared by the Koloi Tribes of Tripura. Adv. Microbiol. 2019, 9, 824–841. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of Non-Saccharomyces Yeasts for Orange Wine Fermentation Based on Their Enological Traits and Volatile Compounds Formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguyen, T.T.H.; Jin, J.; Lim, J.; Lee, J.; Piao, M.; Mok, I.-K.; Kim, D. Brewing of Glucuronic Acid-Enriched Apple Cider with Enhanced Antioxidant Activities through the Co-Fermentation of Yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and Bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of Non-Saccharomyces Yeasts in Cider Fermentation: Importance of the Nutrients Addition to Obtain an Efficient Fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Hu, J.; Zhao, G. Fermentation Kinetics of Different Sugars by Apple Wine Yeast Saccharomyces cerevisiae. J. Inst. Brew. 2004, 110, 340–346. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The Use of Gompertz Models in Growth Analyses, and New Gompertz-Model Approach: An Addition to the Unified-Richards Family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Economía. Alimentos Determinación de pH En Alimentos y Bebidas No Alcohólicas–Método Potenciométrico–Método de Prueba. NMX-F-317-NORMEX-2013|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2013. [Google Scholar]
- Noriega-Medrano, L.J.; Vega-Estrada, J.; Ortega-López, J.; Ruiz-Medrano, R.; Cristiani-Urbina, E.; Montes-Horcasitas, M. del C. Alternative Non-Chromatographic Method for Alcohols Determination in Clostridium acetobutylicum Fermentations. J. Microbiol. Methods 2016, 126, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Worthington. Worthington Enzyme Handbook: Enzymes, Enzymes Reagents and Related Biochemicals; Biochemical Corporation: Lakewood, NJ, USA, 1972. [Google Scholar]
- Burgos Montañez, L.J. Cuantificación de azúcares reductores del sustrato en residuos de piña con el método del ácido 3,5-dinitrosalicílico. Rev. Investig. 2020, 13, 57–66. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Estevinho, L.M.; Mendes-Faia, A. Mead Production: Fermentative Performance of Yeasts Entrapped in Different Concentrations of Alginate. J. Inst. Brew. 2014, 120, 575–580. [Google Scholar] [CrossRef]
- Secretaría de Economía. Bebidas Alcohólicas-Determinación Del Contenido Alcohólico (Por Ciento de Alcohol En Volumen a 20 °C (% Alc. Vol.)-Métodos de Ensayo (Prueba) NMX-V-013-NORMEX-2019|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2014. [Google Scholar]
- Secretaría de Economía. Norma Oficial Mexicana. Bebidas Alcohólicas.-Determinación de Densidad Relativa. NOM-V-32-S-1980. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 1980. [Google Scholar]
- Secretaría de Economía. Bebidas Alcohólicas-Determinación de Dióxido de Azufre (SO2) Libre y Total-Métodos de Ensayo (Prueba). NMX-V-027-NORMEX-2019|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2020. [Google Scholar]
- Secretaría de Economía. Bebidas Alcohólicas-Determinación de Acidez Total, Acidez Fija y Acidez Volátil-Métodos de Prueba. NMX-V-015-NORMEX-2014|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2015. [Google Scholar]
- Adu, J.K.; Amengor, C.D.K.; Orman, E.; Ibrahim, N.M.; Ifunanya, M.O.; Arthur, D.F. Development and Validation of UV-Visible Spectrophotometric Method for the Determination of 5-Hydroxymethyl Furfural Content in Canned Malt Drinks and Fruit Juices in Ghana. J. Food Qual. 2019, 2019, e1467053. [Google Scholar] [CrossRef]
- Secretaría de Economía. Bebidas Alcohólicas-Determinación de Aldehídos, Ésteres, Metanol, y Alcoholes Superiores-Métodos En Ensayo (Prueba). NMX-V-005-NORMEX-2018|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2018. [Google Scholar]
- Secretaría de Economía. Bebidas alcohólicas-Determinación de metales como cobre (Cu), plomo (Pb), arsénico (As), zinc (Zn), hierro (Fe), calcio (Ca), mercurio (Hg), cadmio (Cd), por absorción atómica-Métodos de ensayo (prueba). NMX-V-050-NORMEX-2010|NORMEX. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2011. [Google Scholar]
- Secretaría de Salud Norma Oficial Mexicana. Método Para La Cuenta de Bacterias Aerobias En Placa. NOM-092-SSA1-1994. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 1995. [Google Scholar]
- Secretaría de Salud. Norma Oficial Mexicana. Método Para La Cuenta de Mohos y Levaduras En Alimentos. NOM-111-SSA1-1994. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 1995. [Google Scholar]
- Secretaría de Salud Proyecto de Norma Oficial Mexicana. Método Para La Cuenta de Microorganismos Coliformes Totales En Placa. NOM-113-SSA1-1994. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 1994. [Google Scholar]
- Espinosa Manfugás, J. Evaluación Sensorial de los Alimentos; Editorial Universitaria (Cuba): Ciudad de la Habana, Cuba, 2020; ISBN 978-959-16-0539-9. [Google Scholar]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of Initial pH on Growth Characteristics and Fermentation Properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, H.S.; Babbar, N.; Sandhu, S.K.; Dhaliwal, S.S.; Kaur, U.; Chadha, B.S.; Bhargav, V.K. Ethanol Production from Alkali-Treated Rice Straw via Simultaneous Saccharification and Fermentation Using Newly Isolated Thermotolerant Pichia kudriavzevii HOP-1. J. Ind. Microbiol. Biotechnol. 2012, 39, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia kudriavzevii through Solid-State Fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Gencturk, E.; Ulgen, K.O. Understanding HMF Inhibition on Yeast Growth Coupled with Ethanol Production for the Improvement of Bio-Based Industrial Processes. Process Biochem. 2022, 121, 425–438. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Mead Production: Effect of Nitrogen Supplementation on Growth, Fermentation Profile and Aroma Formation by Yeasts in Mead Fermentation. J. Inst. Brew. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Díaz-Nava, L.E.; Montes-Garcia, N.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Effect of Carbon Sources on the Growth and Ethanol Production of Native Yeast Pichia kudriavzevii ITV-S42 Isolated from Sweet Sorghum Juice. Bioprocess Biosyst. Eng. 2017, 40, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Yuangsaard, N.; Yongmanitchai, W.; Yamada, M.; Limtong, S. Selection and Characterization of a Newly Isolated Thermotolerant Pichia kudriavzevii Strain for Ethanol Production at High Temperature from Cassava Starch Hydrolysate. Antonie Leeuwenhoek 2013, 103, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Chromatographic Analysis of Sugars Applied to the Characterisation of Monofloral Honey. Anal. Bioanal. Chem. 2004, 380, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, M.; Patsalou, M.; Stavrinou, S.; Vyrides, I. High Temperature Alcoholic Fermentation of Orange Peel by the Newly Isolated Thermotolerant Pichia kudriavzevii KVMP10. Lett. Appl. Microbiol. 2016, 62, 75–83. [Google Scholar] [CrossRef]
- Garg, N. Chapter 8—Technology for the Production of Agricultural Wines. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 463–486. ISBN 978-0-12-800850-8. [Google Scholar]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Francis, I.L.; Henschke, P.A. Comparison of Inorganic and Organic Nitrogen Supplementation of Grape Juice–Effect on Volatile Composition and Aroma Profile of a Chardonnay Wine Fermented with Saccharomyces cerevisiae Yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Šmogrovičová, D.; Nádaský, P.; Tandlich, R.; Wilhelmi, B.S.; Cambray, G. Analytical and Aroma Profiles of Slovak and South African Meads. Czech J. Food Sci. 2012, 30, 241–246. [Google Scholar] [CrossRef]
- García, G.; Moreno, J.F.; Bernal, T.; Posso, F.; Delgado-Noboa, J. Kinetic Modeling of Mead Production. J. Am. Soc. Brew. Chem. 2024, 82, 170–178. [Google Scholar] [CrossRef]
- Cuenca, M.; Blanco, A.; Quicazán, M.; Zuluaga-Domínguez, C. Optimization and Kinetic Modeling of Honey Fermentation for Laboratory and Pilot-Scale Mead Production. J. Am. Soc. Brew. Chem. 2022, 80, 248–257. [Google Scholar] [CrossRef]
- Ferla, L.T.; de Albuquerque Vassalli, I.; Silva, M.V.G.; Freitas, F.P.M.; Teixeira, P.O.; de Almeida, E.L.M.; Eller, M.R. Co-Cultures of Saccharomyces cerevisiae Strains JP14 and IM8 as Strategy for High-Quality Mead Production. Eur. Food Res. Technol. 2024, 250, 1093–1101. [Google Scholar] [CrossRef]
- Głód, B.K.; Piszcz, P. Changes in the Antioxidative Properties of Honeys during Their Fermentation. Open Chem. 2021, 19, 600–603. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and Herbal Meads–Chemical Composition and Antioxidant Properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fortes, J.P.; Franco, F.W.; Somacal, S.; Sautter, C.K. Meads with Brazilian Honey from Different Botanical Origins. Pesqui. Agropecuária Bras. 2023, 58, e03328. [Google Scholar] [CrossRef]
- Akalın, H.; Bayram, M.; Anlı, R.E. Determination of Some Individual Phenolic Compounds and Antioxidant Capacity of Mead Produced from Different Types of Honey. J. Inst. Brew. 2017, 123, 167–174. [Google Scholar] [CrossRef]
- Morales, E.; Alcarde, V.E.; de Franceschi de ANGELIS, D. Mead Features Fermented by Saccharomyces cerevisiae (Lalvin K1-1116). Afr. J. Biotechnol. 2013, 12, 199–204. [Google Scholar] [CrossRef]
- Cavalcante da Silva, S.M.P.; de Carvalho, C.A.L.; Sodré, G.D.S.; Estevinho, L.M. Production and Characterization of Mead from the Honey of Melipona scutellaris Stingless Bees. J. Inst. Brew. 2018, 124, 194–200. [Google Scholar] [CrossRef]
- Cuéllar, J.L.T.; Salamanca, G. Evaluación del proceso fermentativo en la producción de hidromieles monoflorales colombianas. Rev. Colomb. Investig. Agroindustriales 2016, 3, 6–14. [Google Scholar] [CrossRef]
- Senn, K.; Cantu, A.; Heymann, H. Characterizing the Chemical and Sensory Profiles of Traditional American Meads. J. Food Sci. 2021, 86, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; León, L.; Torres, Y.; Cano, N.; Herrera, A.; Cuenca, M. Evaluación y selección de levadura comercial para el proceso de fermentación alcohólica de hidromiel. Publicaciones Investig. 2019, 13, 6. [Google Scholar] [CrossRef]
- Secretaría de Economía. Norma Oficial Mexicana. Bebidas Alcohólicas-Denominación, Especificaciones Fisicoquímicas, Información Comercial y Métodos de Prueba. NOM-199-SCFI-2017. In Diario Oficial de la Federación; Dirección General de Normas: Ciudad de México, Mexico, 2017. [Google Scholar]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. High-Cell-Density Fermentation of Saccharomyces cerevisiae for the Optimisation of Mead Production. Food Microbiol. 2013, 33, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, T.; Lü, H.; Yu, Z.; Li, X. Effect of Added Sulphur Dioxide Levels on the Fermentation Characteristics of Strawberry Wine. J. Inst. Brew. 2016, 122, 446–451. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Cuenca, M.; Ciesa, F.; Romano, A.; Robatscher, P.; Scampicchio, M.; Biasioli, F. Mead Fermentation Monitoring by Proton Transfer Reaction Mass Spectrometry and Medium Infrared Probe. Eur. Food Res. Technol. 2016, 242, 1755–1762. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Teter, A.; Florek, M.; Matwijczuk, A.; Niemczynowicz, A.; Matwijczuk, A.; Czernel, G.; Skałecki, P.; Gładyszewska, B. Use of Physicochemical, FTIR and Chemometric Analysis for Quality Assessment of Selected Monofloral Honeys. J. Apic. Res. 2023, 62, 863–872. [Google Scholar] [CrossRef]
- Fayolle, P.; Picque, D.; Perret, B.; Latrille, E.; Corrieu, G. Determination of Major Compounds of Alcoholic Fermentation by Middle-Infrared Spectroscopy: Study of Temperature Effects and Calibration Methods. Appl. Spectrosc. AS 1996, 50, 1325–1330. [Google Scholar] [CrossRef]
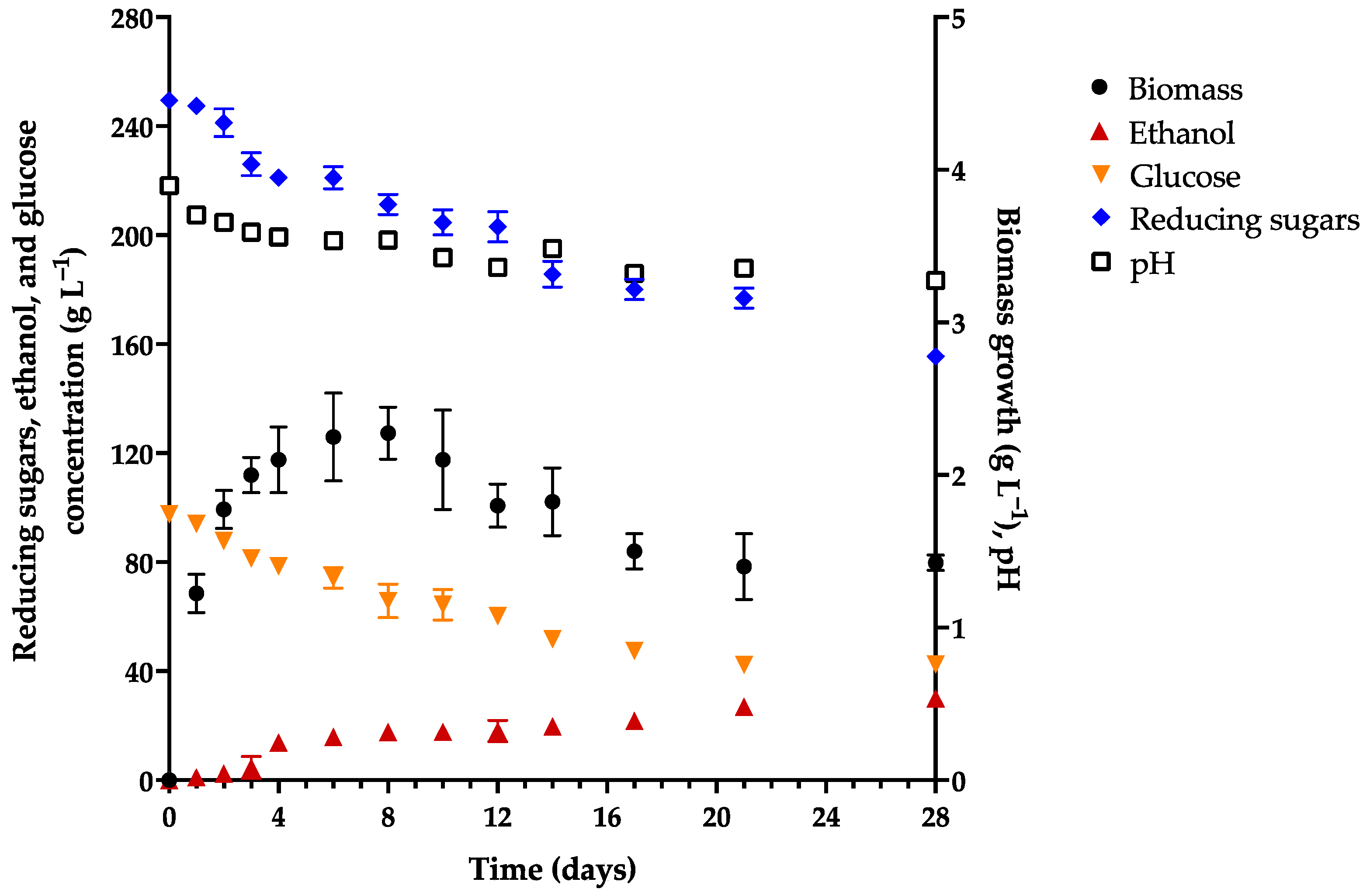
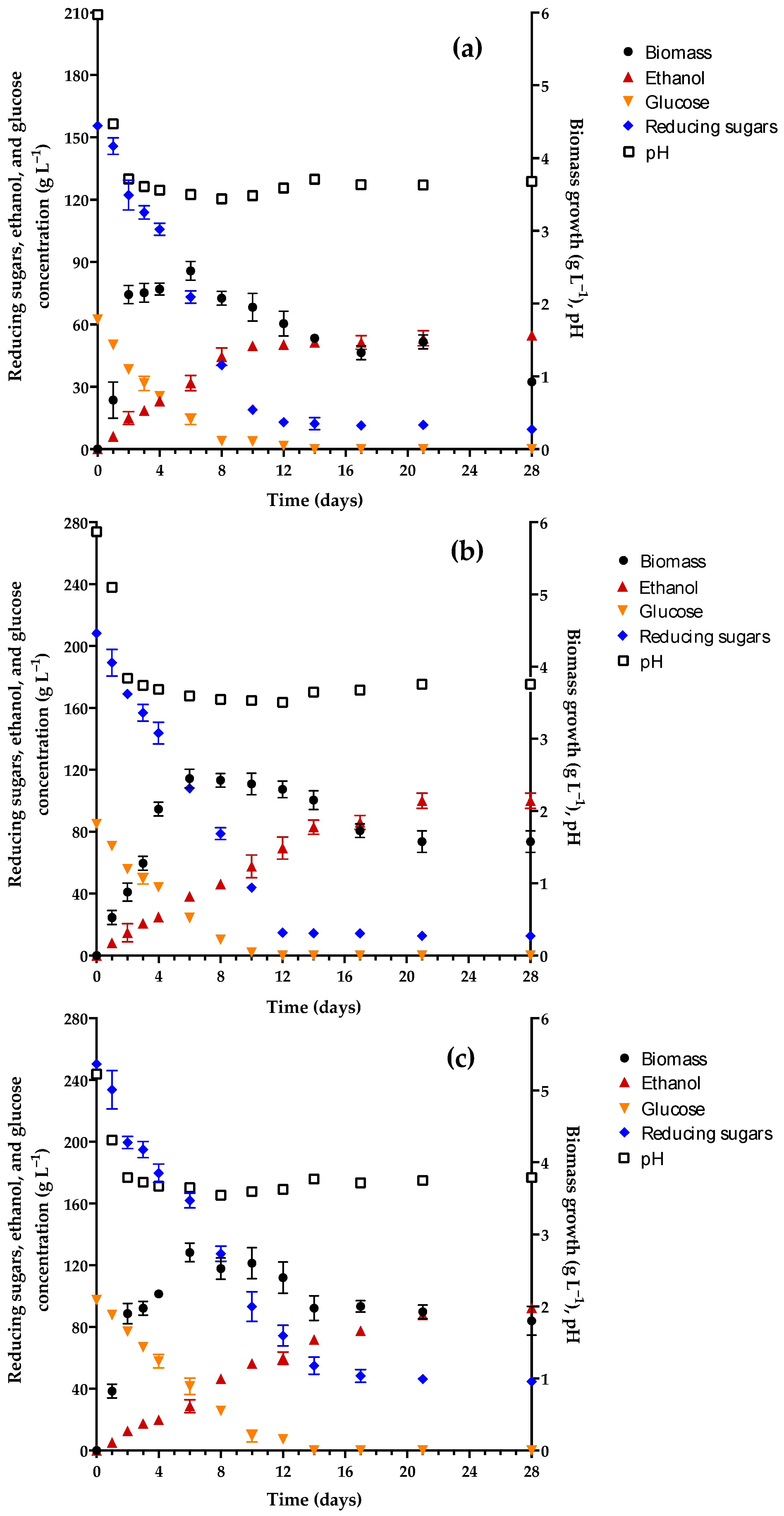
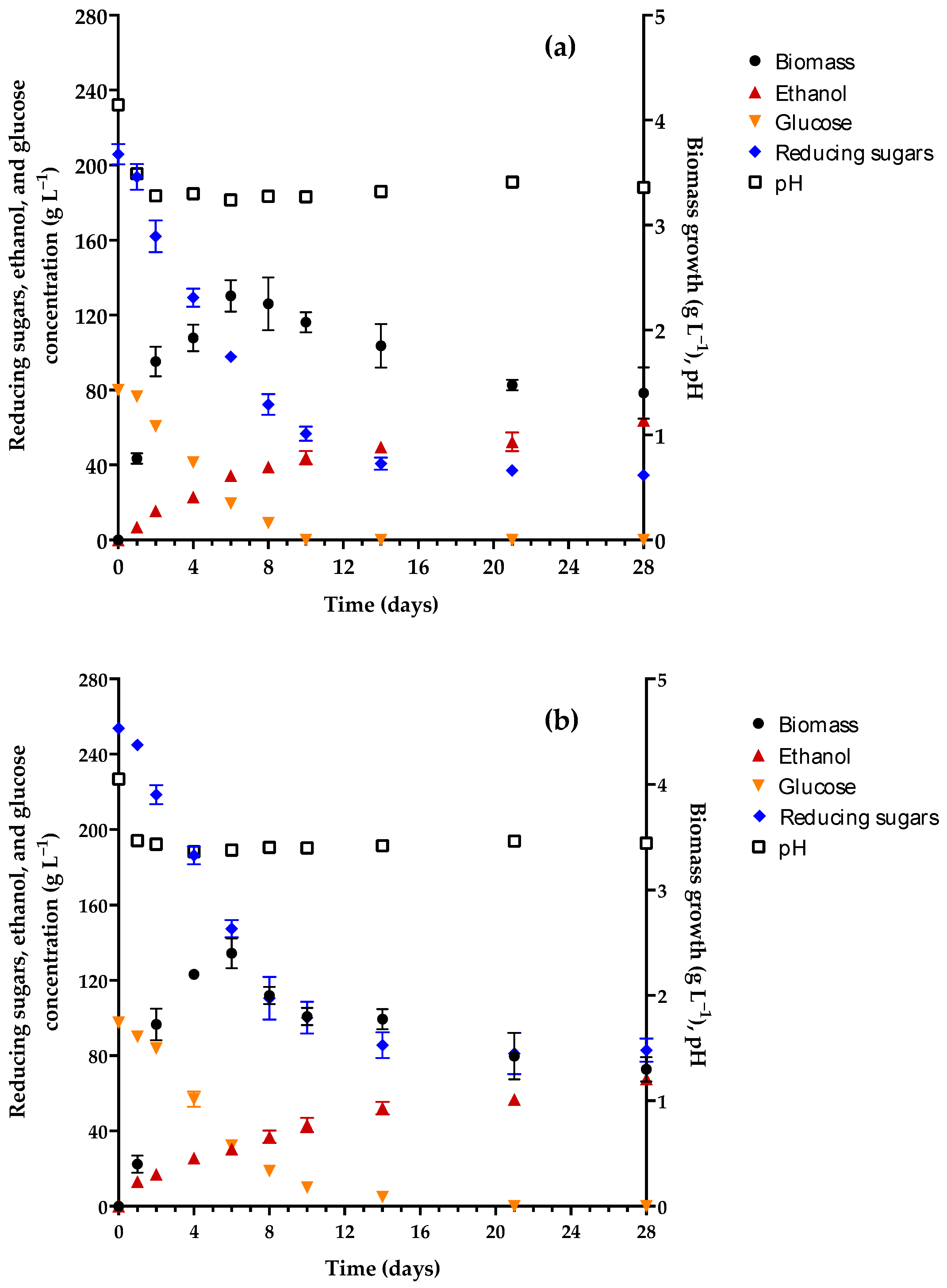

| Characteristic | Initial | Final |
|---|---|---|
| Ethanol (% v/v) | 0 | 3.80 ± 0.08 |
| Yeast assimilable nitrogen (mg YAN L−1) | 8.75 ± 3.50 | N.D. |
| Titratable acidity (g TA L−1) | 0.53 ± 0.09 a | 2.03 ± 0.29 b |
| 5-HMF (mg HMF L−1) | 46.66 ± 0.95 a | 43.94 ± 0.57 b |
| Characteristic | M1–15 | M1–19 | M1–23 | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| Ethanol (% v/v) | 0 | 7.14 ± 0.50 A | 0 | 12.67 ± 0.62 B | 0 | 11.12 ± 0.21 C |
| Yeast assimilable nitrogen (mg YAN L−1) | 553.00± 5.1 a | 498.75 ± 8.0 bA | 563.50 ± 4.4 a | 511.00 ± 5.2 bAB | 584.5 ± 4.04 a | 533.75 ± 6.71 bB |
| Titratable acidity (g TA L−1) | 1.24 ± 0.08 a | 2.325 ± 0.19 bA | 1.28 ± 0.09 a | 2.55 ± 0.27 bA | 1.54 ± 0.08 a | 3.11 ± 0.08 bB |
| 5-HMF (mg L−1) | 44.57 ± 0.69 a | 37.57 ± 1.84 bA | 53.34 ±1.90 a | 45.72 ± 1.99 bB | 56.39 ± 1.47 a | 52.21 ± 1.58 bC |
| Characteristic | M2–19 | M2–23 | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Ethanol (% v/v) | 0 | 8.040 ± 0.33 A | 0 | 8.574 ± 0.03 B |
| Yeast assimilable nitrogen (mg YAN L−1) | 197.4 ± 5.86 a | 152.25 ± 3.5 bA | 211.75 ± 3.50 a | 164.50 ± 4.04 bB |
| Titratable acidity (g TA L−1) | 1.09 ± 0.08 a | 2.36 ± 0.14 bA | 1.16 ± 0.08 a | 2.81 ± 0.14 bB |
| 5-HMF (mg L−1) | 49.74 ± 1.07 a | 47.486 ± 0.57 bA | 59.422 ± 2.35 a | 57.683 ± 0.54 aB |
| Logistic Model | |||||
|---|---|---|---|---|---|
| Biomass Growth | Ethanol Production | Consumption of Reducing Sugars | |||
| XM (g L−1) | 2.30 ± 0.04 | PM (g L−1) | 62.57 ± 1.89 | SM (g L−1) | 169.3 ± 2.32 |
| X0 (g L−1) | 0.033 ± 0.01 | P0 (g L−1) | 9.87 ± 1.17 | S0 (g L−1) | 10.31 ± 1.52 |
| k (days) | 2.67 ± 0.224 | k (days) | 0.254 ± 0.02 | k (days) | 0.552 ± 0.03 |
| R2 | 0.988 | R2 | 0.9462 | R2 | 0.985 |
| SS | 0.229 | SS | 880.6 | SS | 2592 |
| Sy.x | 0.116 | Sy.x | 4.879 | Sy.x | 8.37 |
| RMSE | 0.109 | RMSE | 4.752 | RMSE | 8.153 |
| Gompertz Model | |||||
| Biomass Growth | Ethanol Production | Consumption of Reducing Sugars | |||
| XM (g L−1) | 2.31 ± 0.04 | PM (g L−1) | 64.83 ± 2.02 | SM (g L−1) | 172.40 ± 2.19 |
| X0 (g L−1) | 0.00 ± 0.00 | P0 (g L−1) | 8.06 ± 1.12 | S0 (g L−1) | 4.17 ± 1.12 |
| k (days) | 1.76 ± 0.15 | k (days) | 0.17 ± 0.01 | k (days) | 0.35 ± 0.02 |
| R2 | 0.988 | R2 | 0.959 | R2 | 0.989 |
| SS | 0.213 | SS | 663.3 | SS | 1940 |
| Sy.x | 0.112 | Sy.x | 4.234 | Sy.x | 7.241 |
| RMSE | 0.106 | RMSE | 4.124 | RMSE | 7.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose-Salazar, J.A.; Ballinas-Cesatti, C.B.; Hernández-Martínez, D.M.; Cristiani-Urbina, E.; Melgar-Lalanne, G.; Morales-Barrera, L. Kinetic Evaluation of the Production of Mead from a Non-Saccharomyces Strain. Foods 2024, 13, 1948. https://doi.org/10.3390/foods13121948
Jose-Salazar JA, Ballinas-Cesatti CB, Hernández-Martínez DM, Cristiani-Urbina E, Melgar-Lalanne G, Morales-Barrera L. Kinetic Evaluation of the Production of Mead from a Non-Saccharomyces Strain. Foods. 2024; 13(12):1948. https://doi.org/10.3390/foods13121948
Chicago/Turabian StyleJose-Salazar, Jorge Alberto, Christian Bryan Ballinas-Cesatti, Diana Maylet Hernández-Martínez, Eliseo Cristiani-Urbina, Guiomar Melgar-Lalanne, and Liliana Morales-Barrera. 2024. "Kinetic Evaluation of the Production of Mead from a Non-Saccharomyces Strain" Foods 13, no. 12: 1948. https://doi.org/10.3390/foods13121948








