Effect of Specific Spoilage Organisms on the Degradation of ATP-Related Compounds in Vacuum-Packed Refrigerated Large Yellow Croaker (Larimichthys crocea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Specific Spoilage Organisms
2.2. Sample Processing
2.3. Preparation of Sterile Fish Fillets
2.4. Bacterial Inoculation
2.5. Total Viable Count (TVC)
2.6. Analysis of ATP-Related Compounds
2.7. Determination of Adenosine Deaminase (AMPD), Acid Phosphatase (ACP), Alkaline Phosphatase (ALP), 5′-Nucleotidase (5′-NT) and Xanthine Oxidase (XOD) Activity
2.8. Calculation of K-Value and Other Relevant Values
2.9. Statistical Analysis
3. Results
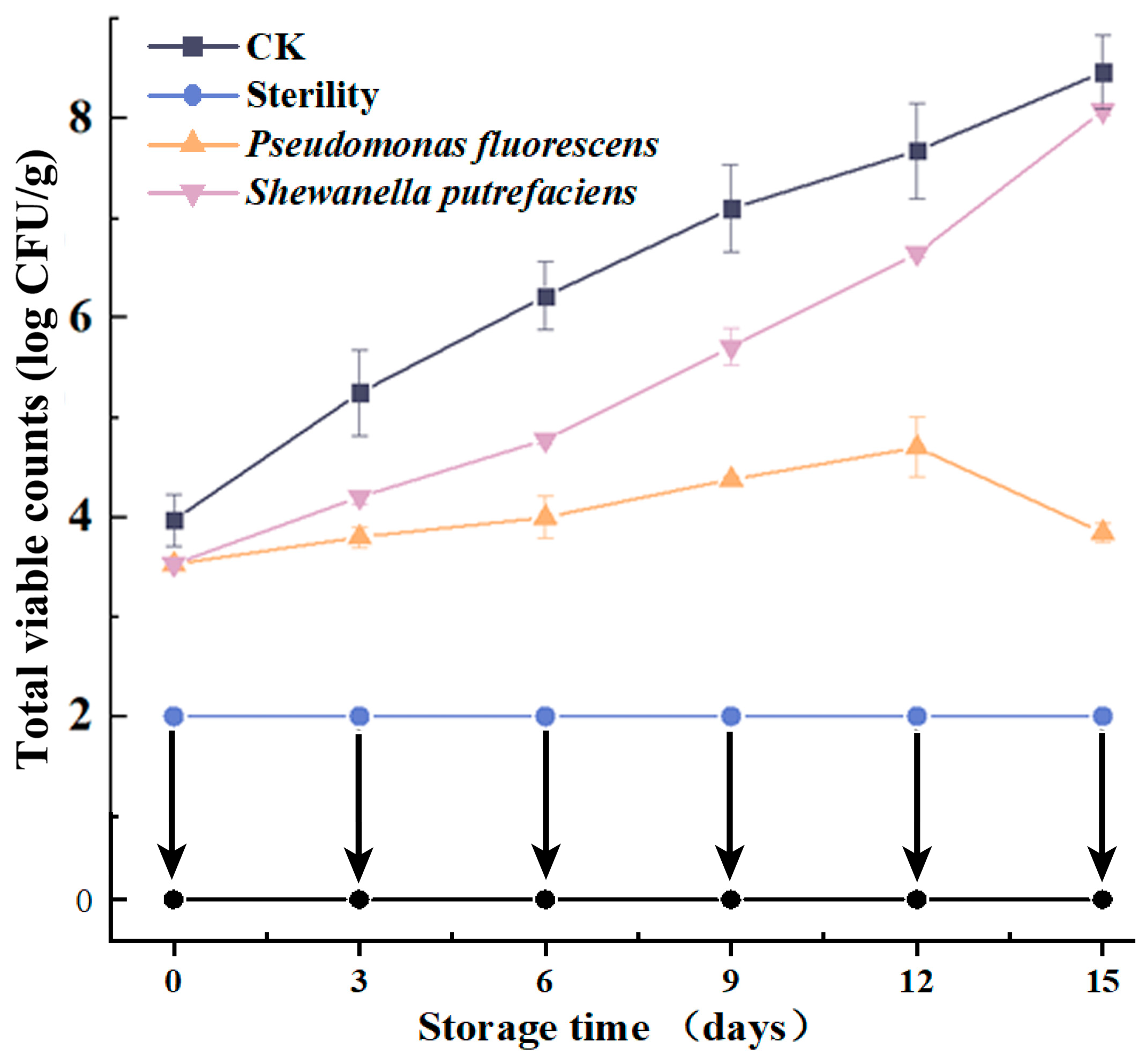
3.1. TVC
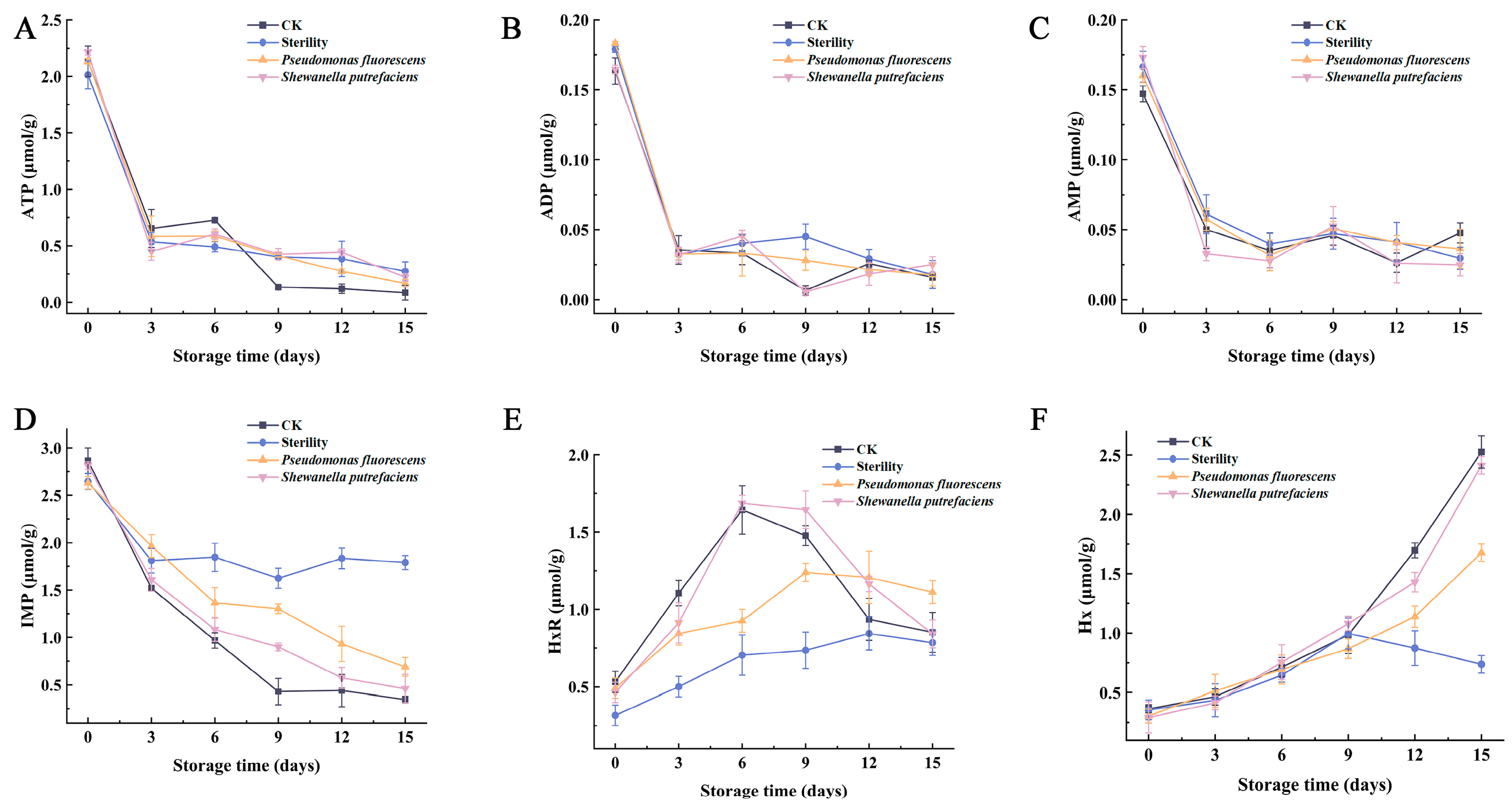
3.2. Changes in ATP-Related Components
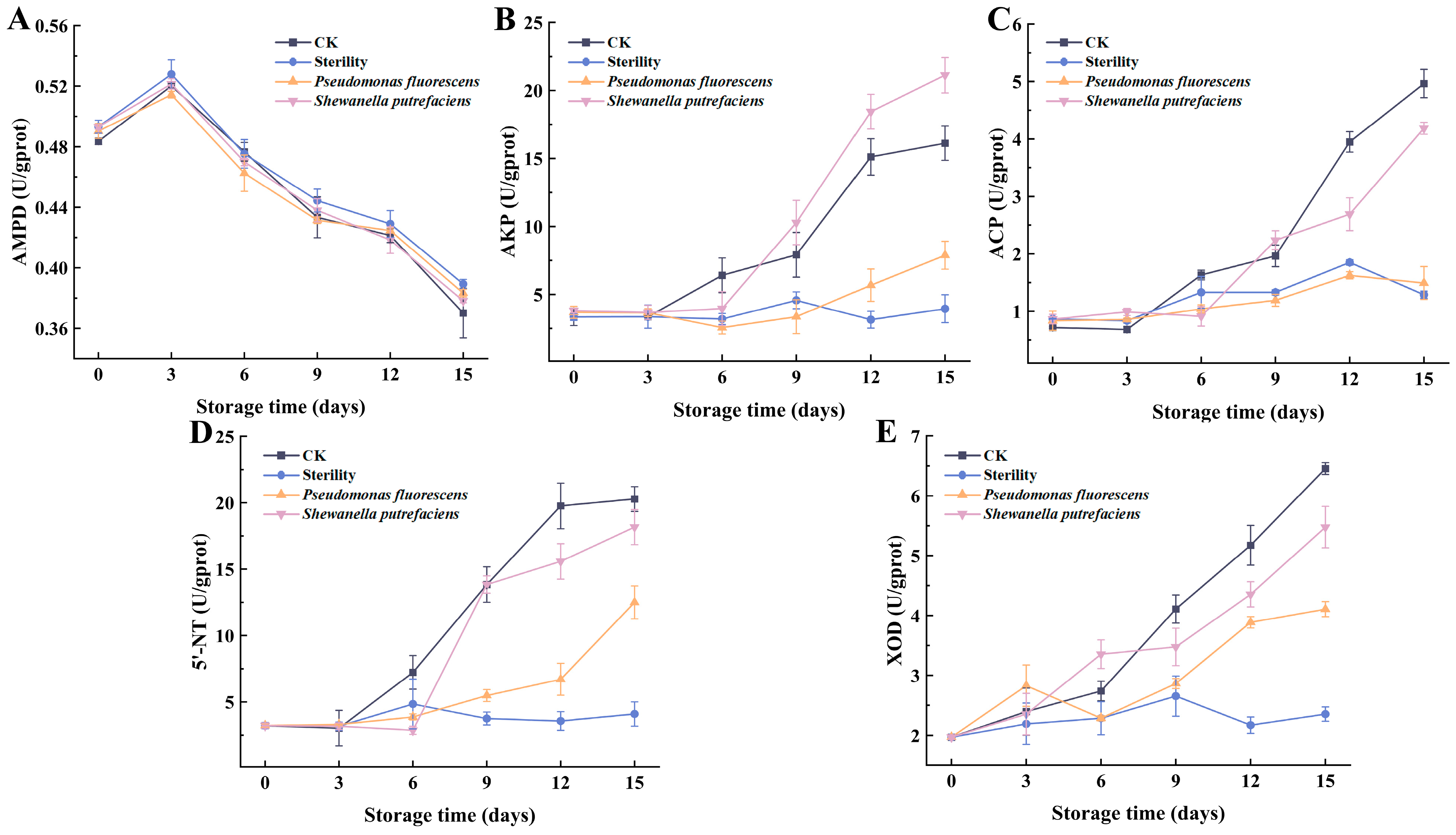
3.3. Changes in the Viability of Enzymes Involved in ATP Degradation
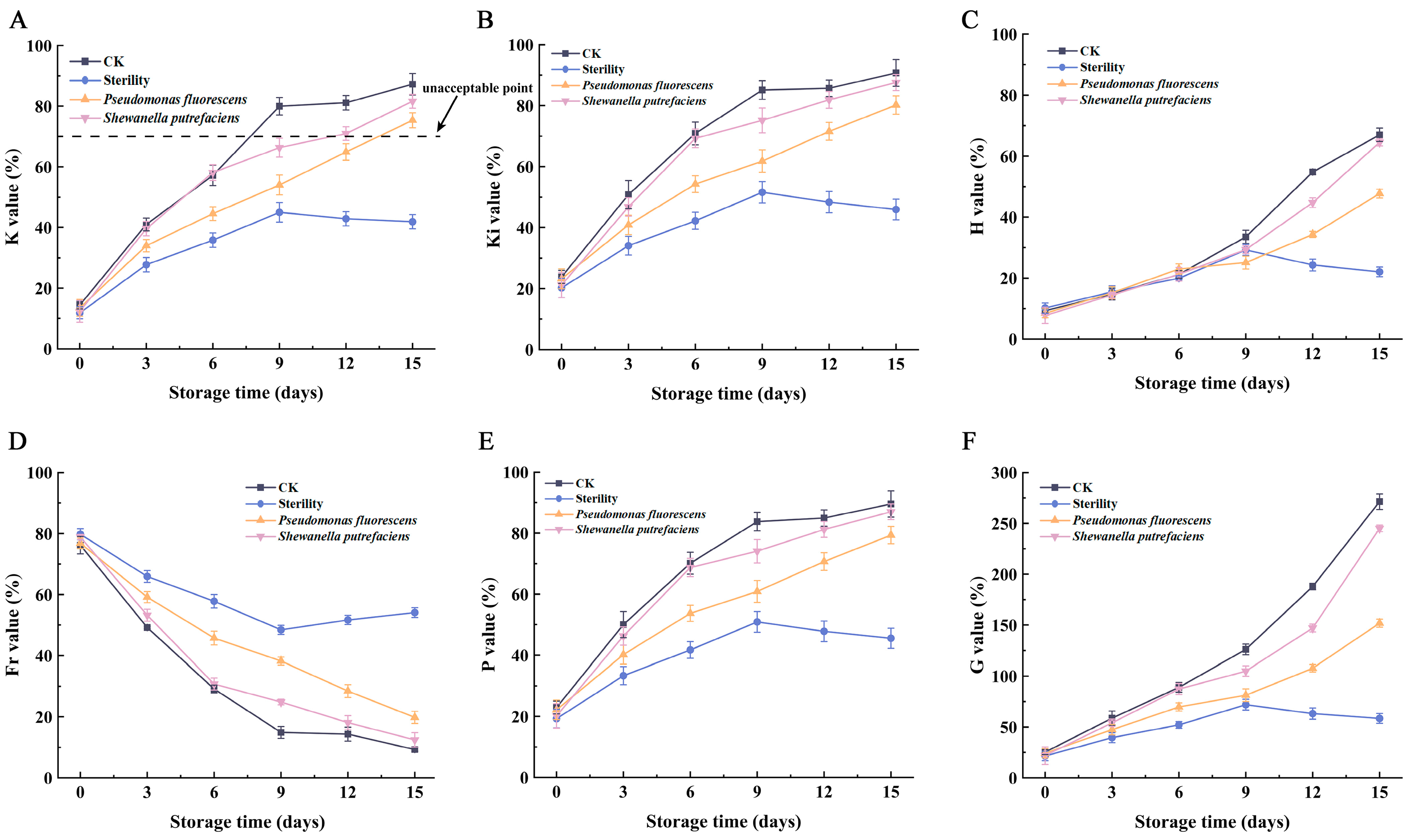
3.4. K Value and Other Relevant Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Zheng, Y.; Li, B.; Yang, X.; Guo, Q.; Liu, A. Prevention of quality characteristic decline in freeze-thawed cultured large yellow croaker (Larimichthys crocea) using flammulina velutipes polysaccharide. Food Sci. Nutr. 2023, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Liu, X.; Li, Y.; Zhang, L.; Hong, H.; Liu, J.; Luo, Y. Biochemical changes and amino acid deamination & decarboxylation activities of spoilage microbiota in chill-stored grass carp (Ctenopharyngodon idella) fillets. Food Chem. 2021, 336, 127683. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Tian, L.; Liu, Y.; Wang, L.; Hong, H.; Luo, Y. Amino acid degradation and related quality changes caused by common spoilage bacteria in chill-stored grass carp (Ctenopharyngodon idella). Food Chem. 2023, 399, 133989. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhao, W.; Wen, W.; Chen, X.; Ma, Z.; Yu, G. Unraveling the effects of sulfamethoxazole on the composition of gut microbiota and immune responses in Stichopus variegatus. Front. Microbiol. 2022, 13, 1032873. [Google Scholar] [CrossRef]
- Zhuang, S.; Hong, H.; Zhang, L.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 252–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lv, X.; Liu, Z.; Ni, L. The spoilage and adhesion inhibitory effects of Bacillus subtilis against Shewanella and Pseudomonas in large yellow croaker (Pseudosciaena crocea). J. Food Sci. Technol. 2022, 42, e02721. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Liu, W.; Liao, T.; Huang, Y.; Zu, X. Changes in the microbiota of a vacuum-packed cooked bass product and the effects of cobalt irradiation on its quality during storage. LWT Food Sci. Technol. 2022, 172, 114199. [Google Scholar] [CrossRef]
- Chen, B.; Yan, Q.; Li, D.; Xie, J. Degradation mechanism and development of detection technologies of ATP-related compounds in aquatic products: Recent advances and remaining challenges. Crit. Rev. Food Sci. Nutr. 2023, 1–22. [Google Scholar] [CrossRef]
- Yan, Q.; Guo, M.; Chen, B.; Zhang, C.; Li, D.; Xie, J. Molecular characterization of spoilage microbiota in high CO2 refrigerated large yellow croaker (Larimichthys crocea) fillets using metagenomic and metabolomic approaches. Food Biosci. 2023, 56, 103227. [Google Scholar] [CrossRef]
- Huang, H.; Xv, Z.; Yang, J.; Wu, J.; Li, Y.; Li, Q.; Sun, T. Preparation, characterization of basil essential oil liposomes unidirectional single-conducting water sustained-release pads and their preservation properties to Lateolabrax japonicus fillets. Food Chem. 2024, 440, 137825. [Google Scholar] [CrossRef]
- Li, Y.; Lei, Y.; Tan, Y.; Zhang, J.; Hong, H.; Luo, Y. Efficacy of freeze-chilled storage combined with tea polyphenol for controlling melanosis, quality deterioration, and spoilage bacterial growth of Pacific white shrimp (Litopenaeus vannamei). Food Chem. 2022, 370, 130924. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, S. Pullulan suppresses the denaturation of myofibrillar protein of grass carp (Ctenopharyngodon idella) during frozen storage. Int. J. Biol. Macromol. 2018, 112, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Yuan, C.; Ding, T.; Chen, S.; Hu, Y. Developing a new spoilage potential algorithm and identifying spoilage volatiles in small yellow croaker (Larimichthys polyactis) under vacuum packaging condition. LWT Food Sci. Technol. 2019, 106, 209–217. [Google Scholar] [CrossRef]
- Li, Y.; Jia, S.; Hong, H.; Zhang, L.; Zhuang, S.; Sun, X.; Liu, X.; Luo, Y. Assessment of bacterial contributions to the biochemical changes of chill-stored blunt snout bream (Megalobrama amblycephala) fillets: Protein degradation and volatile organic compounds accumulation. Food Microbiol. 2020, 91, 103495. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, L.; Song, S.; Wang, Z.; Kong, C.; Luo, Y. The role of microorganisms in the degradation of adenosine triphosphate (ATP) in chill-stored common carp (Cyprinus carpio) fillets. Food Chem. 2017, 224, 347–352. [Google Scholar] [CrossRef]
- Wang, R.; Hu, X.; Agyekumwaa, A.K.; Li, X.; Xiao, X.; Yu, Y. Synergistic effect of kojic acid and tea polyphenols on bacterial inhibition and quality maintenance of refrigerated sea bass (Lateolabrax japonicus) fillets. LWT Food Sci. Technol. 2021, 137, 110452. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiao, X.; Yan, B.; Zhang, N.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Changes in physicochemical properties of silver carp (Hypophthalmichthys molitrix) surimi during chilled storage: The roles of spoilage bacteria. Food Chem. 2022, 387, 132847. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J.; Pan, D.; Wang, C. Removal of substrate inhibition of Acinetobacter baumannii xanthine oxidase by point mutation at Gln-201 enables efficient reduction of purine content in fish sauce. Food Chem. X 2023, 17, 100593. [Google Scholar] [CrossRef]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Jia, S.; Zhang, J.; Li, K.; Luo, Y. The roles of bacteria in the biochemical changes of chill-stored bighead carp (Aristichthys nobilis): Proteins degradation, biogenic amines accumulation, volatiles production, and nucleotides catabolism. Food Chem. 2018, 255, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wen, F.; Saiyi, Z.; Ma, C.; Li, P.; Kang, Z.; Peng, Z.; Zhu, M. Quality evaluation of tray-packed tilapia fillets stored at 0 °C based on sensory, microbiological, biochemical and physical attributes. Afr. J. Biotechnol. 2010, 9, 692–701. [Google Scholar] [CrossRef]
- Hernández-Cázares, A.S.; Aristoy, M.-C.; Toldrá, F. Nucleotides and their degradation products during processing of dry-cured ham, measured by HPLC and an enzyme sensor. Meat Sci. 2011, 87, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Husak, V.V.; Storey, K.B. Regulation of AMP-deaminase activity from white muscle of common carp Cyprinus carpio. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 362–369. [Google Scholar] [CrossRef]
- Saito, T.; Arai, K.I.; Matsuyoshi, M. A new method for estimating the freshness of fish. Nippon Suisan Gakkaishi 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Lou, X.; Wen, X.; Chen, L.; Shu, W.; Wang, Y.; Hoang, T.T.; Yang, H. Changes in texture, rheology and volatile compounds of golden pomfret sticks inoculated with Shewanella baltica during spoilage. Food Chem. 2023, 404, 134616. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Karube, I.; Matsuoka, H.; Suzuki, S.; Watanabe, E.; Toyama, K. Determination of fish freshness with an enzyme sensor system. J. Agric. Food Chem. 1984, 32, 314–319. [Google Scholar] [CrossRef]
- Gill, T.A.; Thompson, J.W.; Gould, S.; Sherwood, D. Characterization of quality deterioration in yellowfin tuna. J. Food Sci. 1987, 52, 580–583. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Öksüz, A.; Shahidi, F.; Alexis, M. Comparison of freshness quality of cultured and wild sea bass (Dicentrarchus labrax). J. Food Sci. 2002, 67, 3220–3226. [Google Scholar] [CrossRef]
- Dai, W.; Yan, C.; Ding, Y.; Wang, W.; Gu, S.; Xu, Z.; Zhou, X.; Ding, Y. Effect of a chitosan coating incorporating epigallocatechin gallate on the quality and shelf life of bighead carp (Aristichthys nobilis) fillets during chilled storage. Int. J. Biol. Macromol. 2022, 219, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Yan, Q.; Xu, T.; Li, D.; Xie, J. Effect of Specific Spoilage Organisms on the Degradation of ATP-Related Compounds in Vacuum-Packed Refrigerated Large Yellow Croaker (Larimichthys crocea). Foods 2024, 13, 1989. https://doi.org/10.3390/foods13131989
Chen B, Yan Q, Xu T, Li D, Xie J. Effect of Specific Spoilage Organisms on the Degradation of ATP-Related Compounds in Vacuum-Packed Refrigerated Large Yellow Croaker (Larimichthys crocea). Foods. 2024; 13(13):1989. https://doi.org/10.3390/foods13131989
Chicago/Turabian StyleChen, Bohan, Qi Yan, Tiansheng Xu, Dapeng Li, and Jing Xie. 2024. "Effect of Specific Spoilage Organisms on the Degradation of ATP-Related Compounds in Vacuum-Packed Refrigerated Large Yellow Croaker (Larimichthys crocea)" Foods 13, no. 13: 1989. https://doi.org/10.3390/foods13131989
APA StyleChen, B., Yan, Q., Xu, T., Li, D., & Xie, J. (2024). Effect of Specific Spoilage Organisms on the Degradation of ATP-Related Compounds in Vacuum-Packed Refrigerated Large Yellow Croaker (Larimichthys crocea). Foods, 13(13), 1989. https://doi.org/10.3390/foods13131989






